Abstract
Background & Aims
Parenteral methotrexate induces clinical remission but not endoscopic improvement of mucosal inflammation in patients with ulcerative colitis (UC). We performed a randomized, placebo-controlled trial to assess the efficacy of parenteral methotrexate in maintaining steroid-free response or remission in patients with UC following induction therapy with methotrexate and steroids.
Methods
We performed a 48-week trial, from February 2012 through May 2016, of 179 patients with active UC (Mayo score 6–12 with endoscopy subscore ≥2) despite previous conventional or biological therapy. The study comprised a 16-week open-label methotrexate induction period followed by a 32-week double-blind placebo-controlled maintenance period. Patients were given subcutaneous methotrexate (25 mg/week) and a 12-week steroid taper. At week 16, steroid-free responders were randomly assigned to groups that either continued methotrexate (25 mg/week, n=44) or were given placebo (n=40) until week 48. We compared the efficacy of treatment by analyzing the proportion of patients who remained relapse free and were in remission at week 48 without use of steroids or other medications to control disease activity.
Results
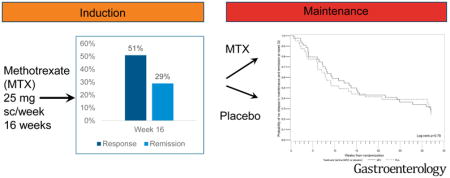
Ninety-one patients (51%) achieved response at week 16, and 84 patients were included in the maintenance period study. During this period, 60% of patients in the placebo group (24/40) and 66% in the methotrexate group (29/44) had a relapse of UC (P=.75). At week 48, 30% of patients in the placebo group (12/40) and 27% of patients in the methotrexate group (12/44) were in steroid-free clinical remission without need for additional therapies (P=.86). No new safety signals for methotrexate were detected.
Conclusions
Parenteral methotrexate (25 mg/week) was not superior to placebo in preventing relapses of UC in patients who achieved steroid-free response during induction therapy.
ClinicalTrials.gov ID no
Keywords: inflammatory bowel diseases, immunosuppressive agent, active ulcerative colitis, IBD therapy
Graphical Abstract
Introduction
Ulcerative colitis (UC) is a recurrent, chronic inflammatory bowel disease affecting the colon and leading to clinical symptoms, including fecal urgency, bloody diarrhea, abdominal pain, weight loss and fatigue. For patients with mild-moderate UC despite therapy with aminosalicylates, the therapeutic options include biologics or thiopurines. While thiopurines are markedly less expensive than the biologic drugs, they are associated with potentially serious side effects including pancreatitis, leukopenia, and lymphoma.1–3 Biological therapies, including anti-TNF agents (infliximab, adalimumab, golimumab) or anti-adhesion molecules (vedolizumab) are effective, but are expensive and also not without risks.4
MTX is an orally, subcutaneously, intramuscularly, or intravenously administered folate antagonist that was developed in 1948 for the treatment of leukemia. MTX targets thymidylate biosynthesis and the enzyme thymidylate synthase.5, 6 Methotrexate is converted intracellularly to MTX-polyglutamates and reduces cell proliferation, increases the rate of apoptosis of T cells, raises anti-inflammatory endogenous adenosine concentrations and alters cytokine production. The rationale behind the use of high-dose MTX in cancer chemotherapy is the promotion of starvation of cancer cells by eliminating purine and pyrimidine precursors, thus leading to decreased cell proliferation due to insufficient DNA and RNA synthesis. Oral or parenteral low-dose MTX has significantly decreased toxicity compared to high dose therapy and is used in several autoimmune diseases, including polyangiitis, psoriasis and rheumatoid arthritis. However, the mechanisms of the therapeutic effects of low-dose therapy, in contrast to high-dose therapy, remain incompletely understood.7
In inflammatory bowel diseases (IBD), MTX’s clinical efficacy in inducing and maintaining clinical remission has been established for steroid-dependent Crohn’s disease (CD) in adults and also in children refractory or intolerant to thiopurine therapy.8–14 In contrast to CD, the role of MTX in UC is controversial. In the first prospective, placebo-controlled study, oral low dose (12.5 mg) MTX weekly was not more efficacious than placebo.15 Since then, however, observational studies have demonstrated effectiveness of MTX if given parenterally similar to the dosing used in CD (15–25 mg per week).16, 17 To more definitively elucidate the clinical value of MTX therapy in patients with mild-moderately active UC, two explanatory investigator-initiated clinical trials were conceptualized in the mid 2000’s. The French “Controlled, Randomized, Double-Blind, Multicenter Study Comparing Methotrexate vs Placebo in Steroid Dependent Ulcerative Colitis” (METEOR), assessed the efficacy of MTX in inducing steroid-free remission over a 16 week time period.18 In METEOR, MTX induced clinical remission without steroids in a significantly larger percentage of UC patients but failed to improve mucosal inflammation. This ambiguous outcome led to continued debate regarding the efficacy of MTX.19 We report here the results of the second trial, “Randomized, Double Blind, Prospective Trial Investigating the Efficacy of Methotrexate in Induction and Maintenance of Steroid-free Remission in Ulcerative Colitis” (MERIT-UC), which was designed to determine the efficacy of MTX in maintaining steroid-free response or remission. In MERIT-UC, participants received open label therapy with subcutaneous MTX over a 16-week period, and steroid-free responders were randomly assigned to either continue MTX 25 mg/week or placebo until week 48. The primary outcome was the proportion of patients who remained relapse free and were in remission at week 48 without use of steroids or other medications to control disease activity.
Methods
Trial design and oversight
The trial was conducted at 37 sites (see supplementary material for site list) across the United States. Patients were recruited between February 2012 and May 2016. The study was designed by investigators of the Crohn’s and Colitis Foundation’s Clinical Research Alliance (CRA) and the majority of centers were affiliated with the CRA.20 The institutional review board at each participating institution approved the protocol and all the patients provided written informed consent before entering the trial. All trial data were collected using an electronic data management system located at the Bioinformatics Core of the Center for Gastrointestinal Biology and Disease. Two independent clinical research monitors tracked and monitored the data collection remotely and through site visits throughout the study. A data and safety monitoring board advised the investigators and the funding agency (National Institute of Diabetes and Digestive Kidney Diseases) throughout the trial. All authors had access to the study data and reviewed and approved the final manuscript. The trial was registered under ClinicalTrials.gov ID NCT01393405.
Patients
Eligible patients were 18 to 70 years of age and had active UC, defined by a Mayo Score of at least 6 points or higher and moderate-to severe active disease on sigmoidoscopy (Mayo endoscopic subscore ≥ 2) extending more than 15 cm from the anal verge. The Mayo Scoring system ranges from 0 – 12 points and is a composite index consisting of three clinical and one endoscopic variables (stool frequency, rectal bleeding, physician’s global assessment and endoscopy evaluation).21 The endoscopic subscore was assigned by the site endoscopist using standardized Mayo endoscopic score photographs, which had been distributed to all sites. In addition to the Mayo score, at least one of the following criteria had to be met for entry in the study: a) failure of 5-ASA therapy; b) steroid-dependent UC, defined as a partial or complete clinical response to treatment with prednisone 40–60 mg/day and relapse within 30 days after completion of prednisone treatment or relapse with attempted dose reduction of prednisone resulting in the use of prednisone at doses of 15–25 mg/day for at least 6 months21; c) intolerance or failure of azathioprine/6-MP therapy; d) primary failure or loss of response to an anti-TNF therapy; or e) primary failure or loss of response to vedolizumab.
Exclusion criteria included failure to respond to 40 mg of prednisone/day or higher in the last 2 weeks or failure of cyclosporine therapy in the last 6 months before inclusion in the trial. Other exclusion criteria were known infection with Clostridium difficile at screening visit, pre-existing renal dysfunction (creatinine >1.5 mg/dl), a serum albumin < 2.5 g/dl at baseline, low serum folate levels defined as a level of >10% below the lower limit of the normal range at baseline, white blood count < 3.0 x109/L, platelet count < 100 x109/L or elevation of AST or ALT > 1.5 times above the upper limit of the normal range at baseline, known hepatic disease, current hepatitis B or C infection or known non-alcoholic fatty liver disease (NAFLD), obesity defined by a body mass index (BMI) >35, pre-existing chronic lung disease other than a well-controlled asthma, interstitial lung disease of unknown cause, known previous or concurrent malignancy (other than that considered surgically cured, with no evidence for recurrence for 5 years), existing pregnancy, lactation, or planned pregnancy (men and women) within the next 12 months, high alcohol consumption (defined as more than seven drinks per week), refusal to use contraceptives in females of childbearing potential or in males with a child-fathering potential, and the use of non-steroidal anti-inflammatory medications (NSAIDs) as long-term treatment, defined as use for at least 4 days a week each month.
Patients could continue oral and/or rectal therapy with mesalamine or corticosteroids but had to be on a stable regimen for at least 2 weeks before screening. The following medications had to be discontinued before inclusion: thiopurines for at least 2 weeks; anti-TNF agents, vedolizumab and investigational agents for at least 4 weeks before inclusion in the study. Due to known interference with the metabolism of methotrexate, use of probenecid, trimethoprim/sulfamethoxazole, sulfasalazine, acitrecin and streptozocin were not allowed in the trial.
Randomization and treatment
The study included an open label induction period of 16 weeks and a randomized, placebo-controlled maintenance period of 32 weeks. At the baseline visit in the induction period, every patient was started on MTX 25 mg once weekly subcutaneously, oral folic acid 1 mg daily, and a predefined prednisone-tapering schedule for 10–12 weeks starting at either 40 mg or 20 mg at the discretion of the investigator. This assured a minimum of 4 weeks without prednisone prior to randomization in the maintenance period. A MTX dose reduction to 15 mg once weekly was allowed in the case of MTX-associated side effects. All patients meeting the predefined criteria for clinical response or remission at week 16 were randomly assigned, in a 1:1 ratio using a permuted block design with a fixed block size of 4 stratified by site, to receive MTX 25 mg once weekly and 1 mg folic acid daily or an identical-appearing placebo once weekly and 1 mg folic acid daily in the maintenance period. Due to the yellow color of methotrexate, placebo was prepared and packaged into matching 2 mL vials for subcutaneous injection using diluted Infuvit® Adult (multiple vitamins for infusion; Baxter Healthcare Corporation; Deerfield, IL; USA). All patients received 2.4 g mesalamine daily in the maintenance period. For patients experiencing nausea following MTX administration, promethazine (25 mg) was provided to be taken prior to each MTX injection. In the case of inadequate response or intolerance of promethazine, ondansentron (4 mg) was dispensed with the instruction to take 1 tablet prior each MTX injection. MTX (Hospira; Lake Forest, IL,USA), folic acid (Amneal/Akyma Pharmaceuticals, Bridgewater, NJ,USA), mesalamine (Asacol-HD, Warner Chilcott, Rockaway, NJ; USA), promethazine (Actavis Parsippany-Troy Hills, NJ, USA) and ondansentron (Sandoz, West Princeton, NJ, USA) were all purchased or prepared by the University of Pennsylvania Investigational Drug Service (IDS) and mailed directly to the patients in q8 weekly intervals.
Screening, baseline and follow-up
Screening procedures were performed 7 to 28 days before the baseline visit. These included: clinical examination, complete blood count, serum concentrations of folate, C-reactive protein, liver transaminases (LFTs), creatinine and albumin, serum pregnancy test for women of childbearing potential, hepatitis B virus serologies, Clostridium difficile toxin and chest-x-ray. Safety laboratory evaluations (CBC, LFT’s, creatinine) were performed at week 0, 2, 4 and then every 4 weeks in the induction and maintenance period. Fecal calprotectin (FCP) was measured at screening, week 16 and week 48 or earlier in the setting of an early withdrawal visit in the induction or maintenance period. All laboratory analyses were centrally performed by Quintiles (Quintiles Laboratories, Atlanta, Georgia). FCP was analyzed using an enzyme-linked immunosorbent assay (PhiCal, Trieste, Italy) with an analytic sensitivity of 6.25 mg/mL. The amount of FCP is expressed as milligrams (mg) / kilogram (kg) stool. The severity of mucosal inflammation was evaluated by sigmoidoscopy and scored by the local investigator using the endoscopic Mayo scoring system. Sigmoidoscopy was performed at week 0 (baseline) and week 48, or earlier if an early withdrawal visit in the maintenance period was required. The total Mayo score including the endoscopic Mayo score was determined at week 0 (baseline) and at the final sigmoidoscopy of the maintenance period. The clinical Mayo score was assessed at week 4, 12, 16 and then every 8 weeks during the maintenance period.
Definitions of outcome measures
The partial Mayo Score was used to measure outcomes at week 16 and determine eligibility for randomization.21 Clinical response was defined as a reduction from baseline in the partial Mayo score of ≥ 2 points and at least 25%, with an accompanying decrease in the rectal bleeding subscore of ≥ 1 point or an absolute rectal bleeding subscore of 0–1 point and a partial Mayo score ≤5. Clinical remission was defined as a partial Mayo score of ≤ 2 points with no individual subscore exceeding 1 point.
Primary and secondary endpoints
The primary outcome was relapse-free survival in the maintenance period, which was comprised of two components, both of which needed to be met to be categorized as relapse-free: (1) week 48 total Mayo score not exceeding 2 points, with all individual subscores not exceeding 1 point and (2) a numerically stable partial Mayo score without an increase of 3 or more points throughout 32 weeks of maintenance therapy compared to week 16 randomization partial Mayo score and no use of steroids or other immunosuppressive medications (anti-TNF agents, vedolizumab, thiopurines, cyclosporine, tacrolimus) to control disease activity throughout the 32 week maintenance period. Any patients for whom outcomes could not be assessed for any reason were classified as failures (relapses) with the event time assigned as the date that the patient was withdrawn from the study.
Six secondary outcomes were also defined: (1) mucosal healing, defined as an absolute subscore for endoscopy no more than 1 at week 48; (2) relapse of disease, defined as an increase of 3 or more points in the partial Mayo score with an absolute partial Mayo score ≥ 4 or re-treatment with steroids during maintenance; (3) steroid-free clinical remission or steroid-free clinical response and FCP levels <250 mg/kg stool at week 16 of the induction period in the subgroup of patients with FCP ≥250 mg/kg at screening; (4) steroid-free clinical remission and FCP levels of ≤ 50 mg/kg at week 16 of the induction period in the subgroup of patients with FCP ≥250 mg/kg at screening; (5) steroid-free clinical remission or steroid-free clinical response and stool FCP levels <250 mg/kg at week 48 in the subgroup of patients with FCP ≥250 mg/kg at screening; (6) steroid-free clinical remission and FCP levels of ≤ 50 mg/kg at week 48 in the subgroup of patients with FCP ≥250 mg/kg at screening.
Adverse events (AE)
AE’s were evaluated by the treating physicians and a medical monitor who were both unaware of the patients’ treatment assignments. The categorization of the AE’s was based on the Medical Dictionary for Regulatory Activities (MedDRA) and classified with respect to the likelihood of a causal relation to the study drug and severity according to the standard criteria of the World Health Organization. Grade of 0 indicates the absence of toxic effects, grade of 1 the presence of mild effects, grade of 2 the presence of moderate effects, grade of 3 the presence of severe effects, and grade of 4 the presence of life-threatening effects.
Sample size calculation
Based on the data from the uncontrolled and controlled MTX studies and the few available placebo-controlled maintenance studies in UC when this study was initially planned in 2007/2008, we hypothesized a success rate between 15%–40% in the placebo group and an absolute difference of 25% in the primary outcome between the study groups.9, 17, 22, 23 Using a 2-sided type I error rate of 5%, we estimated that for n=80 with equal numbers in placebo and MTX, we had 80% power to detect between 22% and 26% difference in survival, depending on the survival proportion among placebo (15%–40%). Some more recent studies have proposed that a 10%–15% difference is clinically meaningful.24, 25 The study had approximately 35%–56% power to detect a 10%–15% difference in remission rates between groups with a type 1 error rate of 5%. Calculations were performed using Stata v15.0, Austin, TX, and Schoenfeld's logrank test method. 26
Statistical analyses
Baseline characteristics were analyzed with the use of descriptive statistics. Fisher's exact and chi-square tests were used to compare categorical variables across groups. Continuous variables were analyzed using Student's t-tests or Wilcoxon rank-sum tests (for non-normally distributed variables). Kaplan-Meier analysis with log rank testing was utilized to compare the proportion of patients in each group who remained free of a relapse during the 32 weeks of follow-up in the maintenance period. In secondary analyses to adjust for potential confounders, we used Cox proportional hazards modeling to compare the proportions of patients in each group who remained free of a relapse during the 32 weeks of follow-up in the maintenance period. All analyses were performed in accordance with the intention-to-treat principle. A p-value of ≤ 0.05 less was considered statistically significant. All statistical analyses were performed with SAS software package version 9.4 (SAS Institute Inc, Cary, NC USA).
Results
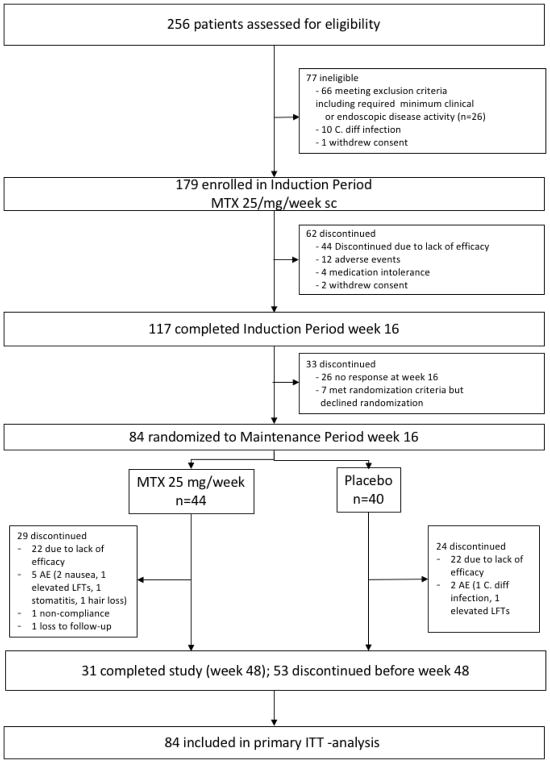
Between February 2012 and May 2016 256 patients were screened at 37 sites across the United States. A total of 77 patients failed screening, mostly due to insufficient disease activity and 179 patients were included in the induction period (Table 1, Figure 1). At week 16, 91/179 (51%) of the patients achieved a steroid-free clinical response and of these 52/179 (29%) were in steroid-free clinical remission. Patients with previous therapeutic failure of thiopurines only or biologics only achieved remission in week 16 more frequently when compared to patients with previous failure of mesalamine only or a combination therapy of a biologic and a thiopurine (supplementary Table S1a). However, when analyzing the groups based on previous exposure to a biologic, no significant difference in rate of remission was observed (steroid free remission week 16 biologic naïve 30/98 (31%) vs biologic experienced 22/81 (27%), p=0.62). Ninety-five patients started with a steroid dose of 20 mg and tapered over 10–12 weeks vs 84, who started at 40 mg and were also tapered off over the same time period. No difference in rates of steroid free remission were observed between both groups (steroid free remission low dose steroid 27/95 (28%) vs high dose steroid 25/84 (30%); p=0.84). Of the 91 patients eligible for randomization into the maintenance period, seven patients declined randomization, all of whom were in steroid-free response but none in steroid-free remission.
Table 1.
Characteristics of patients in the open label induction period and the randomized maintenance period*.
| Induction | Maintenance | |||||
|---|---|---|---|---|---|---|
| Open label MTX (n=179) | Placebo (n=40) | MTX (n=44) | ||||
| Age (years, mean, range) | 42 | (19–74) | 45.1 | (21–69) | 42.1 | (25–66) |
| Sex (m: n ;%) | 110 | (61%) | 24 | (60%) | 22 | (50%) |
| Duration of disease (median, IQR) | 6 | (3–11) | 6.0 | (2–13.5) | 7.5 | (4–14) |
| Smoking history | ||||||
| Current | 8 | (4%) | 1 | (2%) | 3 | (7%) |
| Former | 59 | (33%) | 13 | (33%) | 16 | (36%) |
| Never | 112 | (63%) | 26 | (65%) | 25 | (57%) |
| Site of disease | ||||||
| Left colon (n/%) | 86 | (48%) | 17 | (43%) | 25 | (57%) |
| Pancolitis (n/%) | 93 | (52%) | 23 | (57%) | 19 | (43%) |
| Failed drugs before inclusion (n;%) | ||||||
| Mesalamine | 65 | (36%) | 16 | (40%) | 14 | (32%) |
| Azathioprine/6-MP | 33 | (18%) | 11 | (28%) | 7 | (16%) |
| Anti-TNF | 20 | (11%) | 4 | (10%) | 7 | (16%) |
| Anti-TNF and azathioprine/6-MP | 61 | (35%) | 9 | (23%) | 16 | (36%) |
| Vedolizumab | 8 | (4%) | 1 | (3%) | 1 | (2%) |
| Steroid dependency at baseline (week 0) | 66 | (37%) | 17 | (43%) | 13 | (30%) |
| Mayo endoscopy score week 0 (score 2/3; n;%) | 106/73 | (59%/41%) | 26/14 | (65%/35%) | 24/20 | (55%/45%) |
| Steroid taper (20/40mg; n;%) | 95/84 | (53%/47%) | 18/22 | (45%/55%) | 24/20 | (55%/45%) |
| Clinical Mayo score week 0 / 16 (median, IQR) | 6 | (5–7) | 2 | (0–3) | 1 | (0–3) |
| Calprotectin week 0 /16 (median, IQR) | 657 | (252–825) | 163 | (30–593) | 344 | (84–767) |
| CRP week 0/16 (median, IQR) | 0.3 | (0.1–0.7) | 0.2 | (0.2–0.7) | 0.3 | (0.1–0.8) |
| Hemoglobin week 0/16 (median, IQR) | 13.8 | (12.7–14.7) | 13.1 | (12.2–14.5) | 13.0 | (12.0–13.9) |
There were no significant differences between groups in the maintenance period. TNF denotes tumor necrosis factor, 6-MP denotes 6-mercaptopurine.
Figure 1.
Figure S1: Consort flow diagram of the MERIT-UC trial
Eighty-four patients were randomized at week 16 to either continuation with placebo (n=40) or MTX 25 mg weekly (n=44). Of these 25/40 (63%) and 27/44 (61%) of patients were in steroid free remission and 15/40 (37%) and 17/44 (39%) of patients were in steroid free response in the placebo and the MTX group, respectively. The baseline characteristics between the groups were similar (Table 1). Overall, 60% (24/40) and 66% (29/44) of patients in the placebo and the MTX group, respectively, discontinued the therapy before week 32 of the maintenance period (p=0.71; Figure 1). Reasons for discontinuation of therapy were lack of efficacy in 22 patients in each group. Numerically more patients experienced adverse events leading to discontinuation in the MTX vs the placebo group (5 vs 2) and 2 patients dropped out of the MTX group due to loss of follow-up or non-compliance with study medication. Thirty-one patients completed the week 32 visit of the maintenance period (week 48 of the trial).
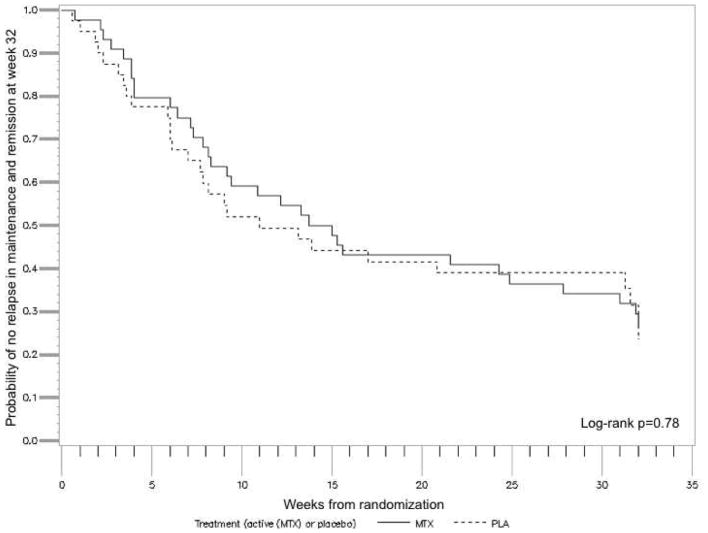
The primary outcome defined as relapse-free survival without need for additional therapies such as steroids, immunosuppressants, or biologics and remission at week 48 did not differ between the groups (p=0.78, Figure 2). The median time to loss of response was 71 days on placebo and 101 days on MTX. Thirty-one patients (16 and 15 in the placebo and MTX group, respectively) completed the week 48 visit. Of these 30% (12/40) of patients in the placebo group vs 27% (12/44) in the MTX group were in steroid-free remission with no need for additional therapies to treat UC activity over the maintenance period (p=0.91). Five patients were in steroid free response only (3/40 (8%) placebo vs 2/44 (5%) MTX; p=0.66) and 1 patient in each group experienced a relapse at week 48. There were no statistically significant differences in the key secondary outcomes (Table 2). Steroid dependence at inclusion, the type of prior failed therapy (anti-TNF naïve vs anti-TNF experienced), steroid taper in induction (low vs high dose), response or remission at week 16, disease location, severity or duration of disease or smoking status were not associated with the outcome at the end of induction at week 16 or at week 48. (supplemental material Tables S2–S8 and Figure S1). Additionally, when controlling for prior TNF use, there was no significant difference in relapse free survival when comparing MTX and placebo (Hazard Ratio 0.92, 95% CI 0.55 – 1.54).
Figure 2.
Kaplan-Meier survival plot of the probability of remaining relapse free during the maintenance period (week 0 – 32) and being in remission at week 32 following randomization (week 48 of the trial). 84 patients in steroid-free response to open label MTX 25 mg/week after a 16-week induction period with a 12-week steroid taper were randomized to placebo or continuing MTX therapy (25 mg/week) for 32 weeks.
Table 2.
Secondary outcomes following 32 weeks of therapy with methotrexate 25 mg weekly or placebo during the maintenance phase of the study.
| Placebo (n=40) | MTX (n=44) | P value | |
|---|---|---|---|
| Mucosal healing at week 48 | 15 (38%) | 13 (30%) | 0.36 |
| Relapse between week 16 and week 48 | 22 (55%) | 22 (50%) | 0.67 |
| Steroid-free clinical remission or response and FCP levels <250 mg/kg at week 48 in the subgroup of patients with FCP ≥250 mg/kg at screening* | 8 (20%) | 10 (23%) | 0.63 |
| Steroid-free clinical remission or response and FCP levels ≤50 mg/kg at week 48 in the subgroup of patients with FCP ≥250 mg/kg at screening* | 3 (8%) | 2 (5%) | 0.62 |
65 patients (placebo n=32, MTX n=33) at randomization had FCP at screening ≥ 250 mg/kg
FCP concentrations at screening, week 16 and week 48
At screening, the majority of the patients had FCP values above 250 mg/kg (≥250 mg/kg n=134; 75%). There were 32 (18%) and 13 (7%) patients with FCP values >50 – <250 mg/kg and ≤ 50 mg/kg, respectively (supplemental material Table S9). The median FCP concentrations in patients with steroid-free response or remission declined significantly from 657 mg/kg at screening to 185 mg/kg at week 16, compared to patients who failed the induction regimen (637 mg/kg at screening and 607 mg/kg at last visit; p=0.04). Among the 134 patients with FCP ≥250 mg/kg at screening, 93 (69%) had FCP levels of <250 mg/kg and 69 (51%) had FCP ≤50 mg/kg at week 16. Steroid free response or remission and a FCP <250 mg/kg was found in 56 (42%) patients while steroid-free remission and a FCP≤50 mg/kg was achieved in 54 (40%) patients at week 16.
Of the 84 patients randomized into the maintenance period, 24%, 33% and 43% had FCP concentrations of ≤50 mg/kg, >50 – <250 mg/kg and ≥250 mg/kg at week 16, respectively. Stratifying patients into these 3 different groups according to the FCP concentrations at week 16 showed similar results regarding clinical outcome during the maintenance period (Table 3).
Table 3.
Subgroup analysis of patients in the placebo and MTX groups with different FCP ranges entering the maintenance period at week 16 and clinical remission at week 48
| Clinical remission week 48 | |||
|---|---|---|---|
| FCP week 16 | Placebo n=40 | Methotrexate n=44 | p-value |
| <50 mg/kg; n=20 | 5/11 (45%) | 3/9 (33%) | 0.58 |
| >50 mg/kg and <250 mg/kg; n=28 | 4/15 (27%) | 5/13 (38%) | 0.50 |
| ≥250 mg/kg; n=36 | 3/14 (21%) | 4/22 (18%) | 0.81 |
Adverse events
The most common adverse event (AE) in the open label Induction period was nausea, which occurred in 20.1% of patients (Table 4). In the maintenance period, significantly more patients reported an AE in the MTX compared to the placebo group (p=0.03), but there were no significant differences in types of AE’s except a suggestion of a higher occurrence of nausea in the MTX group. Due to LFT elevation MTX was reduced to 15 mg in 3 patients (1 patient in induction, who was later on randomized to placebo and experienced a relapse and 2 patients in maintenance, of whom 1 finished the study in remission). None of the patients in the MTX group had a serious AE; one serious AE occurred in a patient in the placebo group who was hospitalized for a gastrointestinal infection and successfully treated with antibiotics and continued in the study.
Table 4.
Treatment emergent adverse events in >5% of patients in induction and maintenance period
| Induction period | Maintenance period | |||
|---|---|---|---|---|
| Methotrexate 25 mg/week | Placebo | Methotrexate 25 mg/week | p-value | |
| Patients evaluable for AE, n | 179 | 40 | 44 | |
| Total number of AE / SAE | 747 /17* | 111 / 1 | 137 / 0 | |
| Patients with AE or SAE, n (%) | 136 (76%) | 30 (75.0) | 41 (93.1) | 0.03 |
| Patients discontinued due to AE, n (%) | 12 (6.7) | 1 (2.5) | 5 (11.4) | 0.11 |
| Abdominal discomfort | 11 (6.1) | 10 (25.0) | 13(29.5) | 0.80 |
| Diarrhea | 8 (4.5) | 13 (32.5) | 14 (31.8) | 1.00 |
| Dizziness | 10 (5.6) | 3 (7.5) | 1 (2.3) | 0.34 |
| Elevated Liver enzymes | 6 (3.4) | 3 (7.5) | 5 (11.4) | 0.71 |
| Fatigue | 19 (10.6) | 5 (12.5) | 5 (11.4) | 1.00 |
| Nausea | 36 (20.1) | 5 (12.5) | 13 (29.5) | 0.07 |
| Pruritus | 2 (1.1) | 2 (5.0) | 0 (0.0) | 0.22 |
6 patients underwent colectomy due to therapy refractory disease course
Discussion
MERIT-UC is the first randomized, placebo-controlled study investigating the efficacy of subcutaneously applied MTX at a dose of 25 mg/week in patients who had previously responded to open label MTX. Despite a relatively large proportion of patients achieving steroid free response and remission during the open label induction phase, which in its magnitude was similar to the METEOR trial, MTX was not superior to placebo in maintaining these therapeutic effects.18 The METEOR trial provided uncertain results regarding the efficacy of MTX as an induction therapy for UC and the MERIT-UC outcome sheds further doubt on the efficacy of MTX for UC at the dose of 25mg once weekly administered subcutaneously.18
The only other prospective placebo-controlled maintenance study investigating MTX in UC by Oren et al., also failed to demonstrate a significantly better outcome in patients on MTX compared to placebo over a 9-month period.15 In contrast to the parenteral MTX dose of 25 mg/week in MERIT-UC, oral MTX was administered at a much lower dose of 12.5 mg weekly. Three additional uncontrolled prospective open label studies have investigated the effectiveness of methotrexate in UC and described overall favorable results.15, 27–29 Multiple issues have been raised with these studies including small sample size, an open label design, low doses of methotrexate, poorly designed inclusion criteria and other shortcomings.30, 31 The results of the MERIT-UC and METEOR studies highlight the importance of conducting multiple randomized, controlled trials to definitively establish or refute the efficacy of a therapy.
Our study complements the French METEOR study, which suggested clinical efficacy of MTX in inducing remission in patients with UC.18 The steroid-free remission in our trial at week 16 is in line with the 32% steroid-free remission rate reported in the METEOR trial. With caution, one can qualitatively compare these data to other recent studies. The steroid-free remission rates for azathioprine or infliximab monotherapy in the UC-SUCCESS trial were 24% and 22%, respectively.32 A recent meta-analysis of induction trials revealed a 10% remission rate for placebo-treated patients with active UC.33 Whether there could be enhanced short-term efficacy of MTX in combination with a steroid taper or only a prolonged efficacy of a standardized steroid taper alone is unknown. Similarly, steroid induction therapy as well as steroid administration before each infliximab dose has been hypothesized as a factor obscuring the hypothetical superior clinical efficacy of a combination therapy of MTX with IFX vs IFX monotherapy in the COMMIT (“Combination Of Maintenance Methotrexate-Infliximab Trial”) trial.34, 35
In METEOR, the complete Mayo score, which is a composite score of a clinical and endoscopic assessment, was not significantly different between MTX and placebo after 16 weeks of therapy.18 However, the results of the partial Mayo score alone without taking into account the endoscopy results suggested potential efficacy of MTX in UC with significant improvement of bowel frequency and absence of rectal bleeding. Carbonel, et al. speculated that the observed discrepancy in the METEOR trial may be due to the sites’ proficiency in assessing the degree of mucosal inflammation in the context of the Mayo endoscopic scoring system.18, 36 In contrast to our study, METEOR did not require endoscopically active inflammation for inclusion, which also may have influenced the outcome by favoring a placebo response. Similar to METEOR, our study did not have central reading, but the fact that 93% of patients had elevated FCP levels of > 50 mg/kg at inclusion indirectly corroborates the likely presence of significant inflammation in the majority of patients.
The MERIT-UC study design was based, in part, on the study by Feagan and the North American Crohn's Study Group Investigators, which demonstrated the effectiveness of MTX in the maintenance of remission in CD.9 In contrast to that study, in which fewer than 5% had experienced failure of thiopurine therapy before inclusion in the trial, nearly two-thirds of the randomized population in our study had previously failed therapies with thiopurines and/or biologics. However, thiopurine or mesalamine failure only and no exposure to biologics before inclusion in the trial was not associated with the likelihood of achieving steroid-free clinical remission at week 16 in our patient population.
Consistent with our a priori sample size calculations, nearly two-thirds of the randomized patients failed to achieve the primary outcome of steroid-free clinical and endoscopic remission at the end of the study without disease exacerbation or need for additional therapies during the maintenance period. This was similar to the results of Hawthorne’s landmark placebo-controlled withdrawal study among patients with UC treated with azathioprine for at least 6 months, where relapse rates were 59% and 39% for placebo and azathioprine, respectively.37 Moreover, in a recent meta-analysis of maintenance trials, the pooled estimate of maintenance of remission with placebo was 19% (95% CI11% to 30%)33. Thus, the lack of benefit observed relative to placebo is unlikely to be attributable to a higher than projected success rate among the placebo-treated patients.
FCP concentrations have been correlated with clinical outcomes and mucosal healing in UC clinical trials. In a meta-analysis by Lin et al. values >250 mg/kg as well as < 50 mg/kg were significantly correlated with mucosal inflammation and endoscopic healing, respectively.38 In our trial 75% of patients with an endoscopic subscore of 2 or 3 at screening had FCP stool concentrations ≥250 mg/kg, while 18% a FCP value of >50 mg/kg and <250 mg/kg and 7% had a FCP of ≤ 50 mg/kg. This is in line with a recent study describing the correlation of a single calprotectin value with a locally read Mayo endoscopy score of 2 or 3 in 194 patients in a clinical trial setting. 39 Seventy-seven percent of patients had FCP values > 250 mg/kg and 13% and 10% were found to have FCP values >50 mg/kg and <250 mg/kg and ≤50 mg/kg, respectively. Reduction in FCP concentrations and absolute values of FCP concentrations <250 mg/kg or <50 mg/kg at week 16 also correlated with clinical response at week 16, supporting the value of FCP as a biomarker for mucosal inflammation. Subgroup analyses of FCP concentrations in patients entering the placebo-controlled maintenance period revealed no differences in outcomes between placebo and MTX based on low vs high FCP concentrations at week 16, which corroborates the clinically observed ineffectiveness of MTX in maintaining remission even in the absence of a significant mucosal inflammation at entry of the maintenance period. More recently, a significant correlation of FCP concentrations <150 mg/kg with clinical and endoscopic remission was described.39 Post hoc analyses using FCP concentrations with a cutoff of <150 mg/kg for mucosal inflammation at week 16 yielded similar results (data not shown).
Over the 48-week trial period we did not detect any new safety signals for MTX at a parenteral dose of 25 mg/week. There was only one serious adverse event due to disease exacerbation with hospitalization, which occurred in the placebo group. However, significantly more adverse events, all graded as mild in severity, occurred in the MTX group and a numerically higher number of patients on MTX stopped therapy due to occurrence of an adverse event during the maintenance period. Overall a higher incidence of nausea appeared to be the primary cause of the difference in rates of adverse events.
This trial had a few limitations. The trial was powered to detect a 25% difference between placebo and MTX. As such, we cannot fully exclude a treatment effect of MTX in the range of 10%-15% compared to placebo. Given the current results, one would need to randomize 500–700 patients to conclusively exclude this possibility. However, given the overall similarity of the relapse rates and the multiple negative subgroup analyses, it is unlikely that a larger study would have come to a different conclusion. Due to financial constraints, we did not perform central reading of the sigmoidoscopies, which in the last 5 years has become standard for trials in patients with IBD.40 However, the sigmoidoscopy scoring did not play a major role for the outcome of the trial given that the majority of patients relapsed with the need of additional therapies before the final visit. We also did not assess the degree of mucosal healing by endoscopy before randomization at week 16, but used the biomarker FCP as a surrogate marker, which has been shown to correlate with endoscopic and clinical outcomes in similar trials.32, 39
In conclusion, MERIT-UC is the first randomized, placebo-controlled trial to provide evidence about the efficacy of parenteral MTX monotherapy in the maintenance of response or remission in patients with mild-moderate UC. Whereas MTX may have a limited efficacy to induce steroid-free response or remission in combination with a standardized steroid taper, MTX did not show better efficacy as a maintenance treatment than placebo in preventing relapse in patients with UC.
Supplementary Material
Acknowledgments
Source of Funding:
The study was funded by the National Institute of Diabetes and Digestive Kidney Diseases (NIDDK; U01DK092239). The Clinical Research Alliance is supported by the Crohn’s and Colitis Foundation. The Data Management Center of the Center for Gastrointestinal Biology and Disease is supported by the Crohn’s and Colitis Foundation and the National Institutes of Health (P30 DK034987).
Involvement of authors
Hans Herfarth1–5, Edward L Barnes3–5, John F Valentine2,4, John Hanson2,4, Peter DR Higgins1–4, Kim L Isaacs1–4, Susan Jackson2,6 Mark T Osterman1–4, Kristen Anton2,6, Anastasia Ivanova3, Millie D Long2,4,6, Chris Martin3 Robert S Sandler4,6, Bincy Abraham2,4, Raymond K Cross2,4, Gerald Dryden2,4, Monika Fischer2,4, William Harlan2,4, Campbell Levy2,4, Robert McCabe2,4, Steven Polyak2,4, Sumona Saha2,4, Emmanuelle Williams2,4, Vijay Yajnik2,4, Jose Serrano6, Bruce E Sands1–4, James D Lewis1–4
1. study concept and design; 2. acquisition of data; 3. analysis and interpretation of data; 4. drafting and critical revision of the manuscript; 5. statistical analysis; 6. technical or material support
Conflicts of Interest
Bincy Abraham has received consulting fees from Abbvie, Daiichi Sankyo , Diasoren, Janssen, Pfizer, Salix, Takeda, UCB. She has received research funding from Abbvie, Celgene, Genentech, Janssen, UCB.
Kristen Anton: No disclosures.
Edward Barnes has received consulting fees from Janssen and research support from TARGET Pharmasolutions.
Raymond K. Cross has received consulting fees from Abbvie, Janssen, Pfizer, and Takeda. He has received research funding from Abbvie.
Gerald Dryden has received consulting fees from AbbVie, Takeda, Janssen, Allergan, Gilead, Takeda. He has received research funding from AbbVie, Takeda, Janssen, Gilead, Eli Lilly, TiGenix, MedImmune.
Monika Fischer has received consulting fees from AbbVie and Finch Therapeutics.
John Hanson has received consulting fees from AbbVie and Target Pharmasolutions.
William Harlan: No disclosures.
Hans Herfarth has received consulting fees from Merck, Pfizer, Celltrion, Lycera, Boehringer-Ingelheim, Seres.
Peter Higgins has received consulting fees from Abbvie, Allergan, Amgen, Eli Lilly, GI Health Foundation, Janssen, Lycera, PRIME Medical Education, Takeda, and UCB. He has received research funding from Abbvie, Amgen, Arean, Ascentage, BCBS of Michigan, Buhlmann, Eli Lilly, Genentech, Janssen, Lycera, MedImmune, Nestle, Pfizer, Seres, Shire, Takeda, and UCB.
Kim Isaacs received consulting fees from Allergan and Johnson & Johnson.
Anastasia Ivanova: No disclosures.
Susan Jackson: No disclosures.
James Lewis has received consulting fees from Merck, Pfizer, Janssen, AbbVie, Immune Pharmaceuticals, AstraZeneca, Amgen, MedImmune, Nestle Health Science, Lilly, Samsung Bioepis, Johnson & Johnson Consumer Products, Takeda, Gilead, Celgene, and Bridge Biotherapeutics. He has received research funding from Takeda, Shire, Nestle Health Science. He has received non-grant research support from AbbVie.
Campbell Levy: No disclosures.
Millie Long has received consulting fees from Pfizer, AbbVie, Takeda, Theravance, UCB, Target Pharmasolutions. She has received research funding from Takeda.
Christopher Martin: No disclosures.
Robert McCabe received consulting fees from Janssen.
Mark Osterman has received consulting fees from Abbvie, Janssen, Lycera, Merck, Pfizer, Takeda, and UCB. He has received research funding from UCB.
Steven Polyak has received research funding from AbbVie, Celgene, Gilead, and Takeda.
Bruce Sands has received consulting fees and research grants from AbbVie, Pfizer, Amgen, Bristol-Myers Squibb, Celgene, Janssen, and Takeda, and has received consulting fees from Boehringer Ingelheim, Akros Pharma, Arena Pharmaceuticals, Forward Pharma, Immune Pharmaceuticals, Lilly, Synergy Pharmaceuticals, Theravance, Receptos, TiGenix, TopiVert Pharma, MedImmune, Vedanta Biosciences, Allergan, UCB Pharma, EnGene, Target PharmaSolutions, Lycera, Lyndra, Vivelix Pharmaceuticals, Oppilan Pharma, and Gilead.
Sumona Saha received consulting fees from UCB.
Robert Sandler: No disclosures.
Jose Serrano: No disclosures.
John Valentine received research funding from Pfizer, AbbVie, Celegene, Janssen, Roche/Genentech, Takada, UCB.
Emmanuelle Williams received consulting fees from Abbvie.
Vijay Yajnik is currently a fulltime employee of Takeda Pharmaceuticals. At the time of the study he worked at Massachusetts General Hospital and received consulting fees from Jansen, Takeda, Pfizer and NPS Pharmaceuticals.
MERIT-UC sites
| Investigator | Institution | Location of Institution |
|---|---|---|
| Abraham, Bincy, Kaur, Manreet | Baylor College of Medicine | Houston, TX |
| Afzali, Anita | Harborview Medical Center | Seattle, WA |
| Brinberg, Donald | Great Lakes Gastroenterology | Mentor, OH |
| Cangemi, John | Mayo Clinic, Florida | Jacksonville, FL |
| Cross, Raymond | University of Maryland | Baltimore, MD |
| Dryden, Gerald | University of Louisville | Louisville, KY |
| Ericson, Robert | Essentia Health Duluth Clinic | Duluth, MN |
| Fischer, Monika | IU Health, Indiana University | Indianapolis, IN |
| Flomenhoft, Deborah | University of Kentucky | Lexington, KY |
| Gerich, Mark | University of Colorado | Denver, CO |
| Grunkemeier, David | The Oregon Clinic | Portland, OR |
| Hanson, John Mohanty, Sanjib | Charlotte Gastroenterology and Hepatology | Chartlotte, NC |
| Hardi, Robert | Metropolitan Gastroenterology Group | Chevy Chase, MD |
| Harlan, William | Asheville Gastroenterology Associates NC | Asheville, NC |
| Herfarth, Hans Isaacs, Kim | University of North Carolina | Chapel Hill, NC |
| Hudesman, David | Beth Israel Medical Center | New York City, NY |
| Hwang, Caroline | University of Southern California | Los Angeles, CA |
| Katz, Jeffry | Case Western Reserve University | Cleveland, OH |
| Kaur, Nirmal | Henry Ford Health Systems | Novi, MI |
| Levy, Campbell | Dartmouth-Hitchcock Medical Center | Lebanon, NH |
| James Lewis Mark Osterman | Perelman School of Medicine at the University of Pennsylvania | Philadelphia, PA |
| Loftus, Edward | Mayo Clinic Rochester | Rochester, MN |
| McCabe, Robert | Minnesota Gastroenterology | Plymouth, MN |
| McGreal, Nancy | Duke University | Durham, NC |
| Lodhia, Nilesh | Medical University of South Carolina | Charleston, SC |
| Polyak, Steven | University of Iowa | Iowa City, IA |
| Regueiro, Miguel | University of Pittsburgh Medical Center | Pittsburgh, PA |
| Rubin, David | University of Chicago | Chicago, IL |
| Saha, Sumona | University of Wisconsin | Madison, WI |
| Sands, Bruce | Mt. Sinai School of Medicine | New York City, NY |
| Schwartz, David | Vanderbilt University | Nashville, TN |
| Shafran, Ira | Shafran Gastroenterology Center | Winter Park, FL |
| Valentine, John | University of Utah | Salt Lake City, UT |
| Williams, Emmanuelle Tinsley, Andrew | Penn State College of Medicine | Hershey, PA |
| Wolf, Douglas | Atlanta Gastroenterology Associates Massachusetts | Atlanta, GA |
| Yajnik, Vijay | General Hospital University of | Boston, MA |
| Zisman, Timothy | Washington | Seattle, WA |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mottet C, Schoepfer AM, Juillerat P, et al. Experts Opinion on the Practical Use of Azathioprine and 6-Mercaptopurine in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22:2733–2747. doi: 10.1097/MIB.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 2.Bressler B, Marshall JK, Bernstein CN, et al. Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensus. Gastroenterology. 2015;148:1035–1058 e3. doi: 10.1053/j.gastro.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Harbord M, Eliakim R, Bettenworth D, et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J Crohns Colitis. 2017;11:769–784. doi: 10.1093/ecco-jcc/jjx009. [DOI] [PubMed] [Google Scholar]
- 4.Lemaitre M, Kirchgesner J, Rudnichi A, et al. Association Between Use of Thiopurines or Tumor Necrosis Factor Antagonists Alone or in Combination and Risk of Lymphoma in Patients With Inflammatory Bowel Disease. Jama. 2017;318:1679–1686. doi: 10.1001/jama.2017.16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wessels JA, Huizinga TW, Guchelaar HJ. Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2008;47:249–55. doi: 10.1093/rheumatology/kem279. [DOI] [PubMed] [Google Scholar]
- 6.Brown PM, Pratt AG, Isaacs JD. Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nat Rev Rheumatol. 2016;12:731–742. doi: 10.1038/nrrheum.2016.175. [DOI] [PubMed] [Google Scholar]
- 7.Kremer JM. Still trying to understand methotrexate. J Rheumatol. 2014;41:2099–101. doi: 10.3899/jrheum.141081. [DOI] [PubMed] [Google Scholar]
- 8.Feagan BG, Rochon J, Fedorak RN, et al. Methotrexate for the treatment of Crohn's disease. The North American Crohn's Study Group Investigators. N Engl J Med. 1995;332:292–7. doi: 10.1056/NEJM199502023320503. [DOI] [PubMed] [Google Scholar]
- 9.Feagan BG, Fedorak RN, Irvine EJ, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn's disease. North American Crohn's Study Group Investigators. N Engl J Med. 2000;342:1627–32. doi: 10.1056/NEJM200006013422202. [DOI] [PubMed] [Google Scholar]
- 10.Alfadhli AA, McDonald JW, Feagan BG. Methotrexate for induction of remission in refractory Crohn's disease. Cochrane Database Syst Rev. 2005:CD003459. doi: 10.1002/14651858.CD003459.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Patel V, Macdonald JK, McDonald JW, et al. Methotrexate for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2009:CD006884. doi: 10.1002/14651858.CD006884.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Weiss B, Lerner A, Shapiro R, et al. Methotrexate treatment in pediatric Crohn disease patients intolerant or resistant to purine analogues. J Pediatr Gastroenterol Nutr. 2009;48:526–30. doi: 10.1097/MPG.0b013e318196df3e. [DOI] [PubMed] [Google Scholar]
- 13.Uhlen S, Belbouab R, Narebski K, et al. Efficacy of methotrexate in pediatric Crohn's disease: a French multicenter study. Inflamm Bowel Dis. 2006;12:1053–7. doi: 10.1097/01.mib.0000235103.47280.bb. [DOI] [PubMed] [Google Scholar]
- 14.Turner D, Grossman AB, Rosh J, et al. Methotrexate following unsuccessful thiopurine therapy in pediatric Crohn's disease. Am J Gastroenterol. 2007;102:2804–12. doi: 10.1111/j.1572-0241.2007.01474.x. quiz 2803, 2813. [DOI] [PubMed] [Google Scholar]
- 15.Oren R, Arber N, Odes S, et al. Methotrexate in chronic active ulcerative colitis: a double-blind, randomized, Israeli multicenter trial. Gastroenterology. 1996;110:1416–21. doi: 10.1053/gast.1996.v110.pm8613046. [DOI] [PubMed] [Google Scholar]
- 16.Herfarth HH, Kappelman MD, Long MD, et al. Use of Methotrexate in the Treatment of Inflammatory Bowel Diseases. Inflamm Bowel Dis. 2016;22:224–33. doi: 10.1097/MIB.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herfarth HH, Osterman MT, Isaacs KL, et al. Efficacy of methotrexate in ulcerative colitis: failure or promise. Inflamm Bowel Dis. 2010;16:1421–30. doi: 10.1002/ibd.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carbonnel F, Colombel JF, Filippi J, et al. Methotrexate Is Not Superior to Placebo for Inducing Steroid-Free Remission, but Induces Steroid-Free Clinical Remission in a Larger Proportion of Patients With Ulcerative Colitis. Gastroenterology. 2016;150:380–388 e4. doi: 10.1053/j.gastro.2015.10.050. [DOI] [PubMed] [Google Scholar]
- 19.Macaluso FS, Renna S, Cottone M, et al. The METEOR Trial: The Burial of Methotrexate in Ulcerative Colitis? Gastroenterology. 2016;151:211–2. doi: 10.1053/j.gastro.2016.02.085. [DOI] [PubMed] [Google Scholar]
- 20.Herfarth HH, Jackson S, Schliebe BG, et al. Investigator-Initiated IBD Trials in the United States: Facts, Obstacles, and Answers. Inflamm Bowel Dis. 2017;23:14–22. doi: 10.1097/MIB.0000000000000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D'Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763–86. doi: 10.1053/j.gastro.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 22.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–76. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 23.Ardizzone S, Maconi G, Russo A, et al. Randomised controlled trial of azathioprine and 5-aminosalicylic acid for treatment of steroid dependent ulcerative colitis. Gut. 2006;55:47–53. doi: 10.1136/gut.2005.068809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257–65. doi: 10.1053/j.gastro.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 25.Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous Golimumab Maintains Clinical Response in Patients With Moderate-to-Severe Ulcerative Colitis. Gastroenterology. 2014;146:96–109. doi: 10.1053/j.gastro.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Schoenfeld D. The asymptotic properties of nonparametric tests for comparing survival distributions. Biometrika. 1981;68:316–319. [Google Scholar]
- 27.Paoluzi OA, Pica R, Marcheggiano A, et al. Azathioprine or methotrexate in the treatment of patients with steroid-dependent or steroid-resistant ulcerative colitis: results of an open-label study on efficacy and tolerability in inducing and maintaining remission. Aliment Pharmacol Ther. 2002;16:1751–9. doi: 10.1046/j.1365-2036.2002.01340.x. [DOI] [PubMed] [Google Scholar]
- 28.Egan LJ, Sandborn WJ, Tremaine WJ, et al. A randomized dose-response and pharmacokinetic study of methotrexate for refractory inflammatory Crohn's disease and ulcerative colitis. Aliment Pharmacol Ther. 1999;13:1597–604. doi: 10.1046/j.1365-2036.1999.00667.x. [DOI] [PubMed] [Google Scholar]
- 29.Mate-Jimenez J, Hermida C, Cantero-Perona J, et al. 6-mercaptopurine or methotrexate added to prednisone induces and maintains remission in steroid-dependent inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2000;12:1227–33. doi: 10.1097/00042737-200012110-00010. [DOI] [PubMed] [Google Scholar]
- 30.Chande N, MacDonald JK, McDonald JW. Methotrexate for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007:CD006618. doi: 10.1002/14651858.CD006618.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, MacDonald JK, Vandermeer B, et al. Methotrexate for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2015;8:CD007560. doi: 10.1002/14651858.CD007560.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panaccione R, Ghosh S, Middleton S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146:392–400 e3. doi: 10.1053/j.gastro.2013.10.052. [DOI] [PubMed] [Google Scholar]
- 33.Jairath V, Zou G, Parker CE, et al. Systematic Review and Meta-analysis: Placebo Rates in Induction and Maintenance Trials of Ulcerative Colitis. J Crohns Colitis. 2016;10:607–18. doi: 10.1093/ecco-jcc/jjw004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narula N, Peyrin-Biroulet L, Colombel JF. Combination therapy with methotrexate in inflammatory bowel disease: time to COMMIT? Gastroenterology. 2014;146:608–11. doi: 10.1053/j.gastro.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 35.Feagan BG, McDonald JW, Panaccione R, et al. Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn's disease. Gastroenterology. 2014;146:681–688 e1. doi: 10.1053/j.gastro.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 36.Jharap B, Sandborn WJ, Reinisch W, et al. Randomised clinical study: discrepancies between patient-reported outcomes and endoscopic appearance in moderate to severe ulcerative colitis. Aliment Pharmacol Ther. 2015;42:1082–92. doi: 10.1111/apt.13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hawthorne AB, Logan RF, Hawkey CJ, et al. Randomised controlled trial of azathioprine withdrawal in ulcerative colitis. Bmj. 1992;305:20–2. doi: 10.1136/bmj.305.6844.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin JF, Chen JM, Zuo JH, et al. Meta-analysis: fecal calprotectin for assessment of inflammatory bowel disease activity. Inflamm Bowel Dis. 2014;20:1407–15. doi: 10.1097/MIB.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 39.Sandborn WJ, Panes J, Zhang H, et al. Correlation Between Concentrations of Fecal Calprotectin and Outcomes of Patients With Ulcerative Colitis in a Phase 2 Trial. Gastroenterology. 2016;150:96–102. doi: 10.1053/j.gastro.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Feagan BG, Sandborn WJ, D'Haens G, et al. The role of centralized reading of endoscopy in a randomized controlled trial of mesalamine for ulcerative colitis. Gastroenterology. 2013;145:149–157 e2. doi: 10.1053/j.gastro.2013.03.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.