Abstract
Age-associated structural and functional remodeling of the arterial wall produces a productive environment for the initiation and progression of hypertension and atherosclerosis. Chronic aging stress induces low grade pro-inflammatory signaling and causes cellular proinflammation in arterial walls, which triggers the structural phenotypic shifts characterized by endothelial dysfunction, diffuse intimal-medial thickening, and arterial stiffening. Microscopically, aged arteries exhibit an increase in arterial cell senescence, proliferation, invasion, matrix deposition, elastin fragmentation, calcification, and amyloidosis. These characteristic cellular and matrix alterations not only develop with aging but can also be induced in young animals under experimental proinflammatory stimulation. Interestingly, these changes can also be attenuated in old animals by reducing low grade inflammatory signaling. Thus, mitigating age-associated proinflammation and arterial phenotype shifts is a potential approach to retard arterial aging and prevent the epidemic of hypertension and atherosclerosis in the elderly.
Keywords: Aging, Chronic Stress, Proinflammation, Arterial Remodeling, Arterial Stiffening
1. Introduction
Aging is a major risk factor for the morbidity and mortality of cardiovascular disease. Systemic aging is defined as an age-related decline in physiological function primarily driven by chronic exposure to low levels of sterile inflammation, known as “proinflammation”, contributing to cellular senescence and pathological aging [1, 2]. Arterial aging is the cornerstone of systemic aging and is mainly driven by local proinflammation [3–5]. Age-associated proinflammatory cellular and matrix modifications are the foundation for an exponential increase in the pathogenesis of hypertension and atherosclerosis [3, 6].
Age-associated arterial proinflammation is mainly generated by vascular endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) [3]. Cellular proinflammation is tightly regulated by sympathetic nerve activity (SNA), renin angiotensin aldosterone system (RAS), and endothelin activities induced by physical and mental stress known as allostatic load [7–16]. This chronic stress is the cost that the organism pays for trying to maintain stability in a changing environment over a lifetime.
A comprehensive view of arterial aging is illustrated in Figure 1: at the molecular level, proinflammatory cytokines and chemokines accumulate within the arterial wall; and at the cellular level, vascular cells shift phenotypically to heterogenous phenotypes: a subset of VSMCs become senescent, while another subset of cells becomes more proliferative, invasive/migratory, secretory and more stiff; and the extracellular matrix demonstrates fibrosis, elastolysis, calcification, amyloidosis, and glycoxidation. Finally, at the tissue level, the proinflammation increases arterial intimal-medial thickening (IMT), endothelial dysfunction, arterial stiffening, and elevated blood pressure (BP). These tissue level changes comprise “proinflammatory arterial stiffness syndrome,” a clinical change that does not necessarily evolve into cardiovascular disease. Thus, inhibiting age-associated proinflammation may be a novel approach for maintaining a healthy vasculature and curbing the epidemic of cardiovascular disease in the aging population.
Figure 1. Age-associated proinflammatory signaling cascades at the molecular, cellular, and tissue levels in the arterial wall.
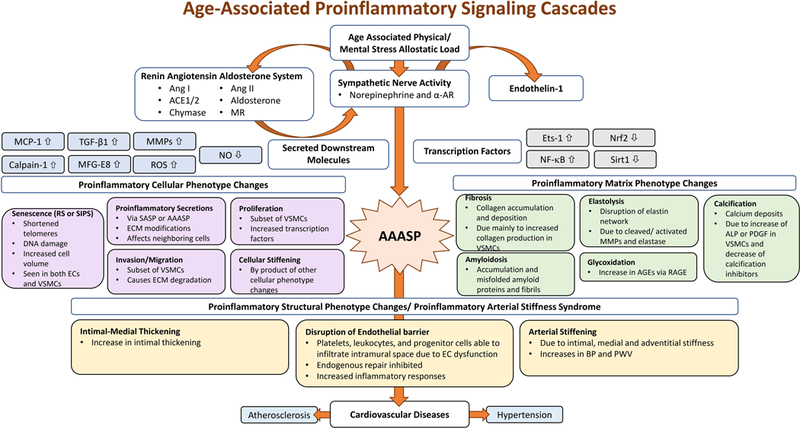
The age-associated vascular molecular cascades are triggered by changes due to physical/mental stress allostatic load on the organism. These molecular changes impact the interconnected RAAS and the SNA systems, thus, activating ET-1, and lead to the secretion of downstream molecules and transcription factors.
Abbreviations and acronyms: α-AR: alpha-adrenergic receptor; ACE1/2: angiotensin converting enzyme; AAASP: age-associated arterial secretory phenotype; AGEs: advanced glycoxidation end-products; ALP: alkaline phosphatase; Ang I: angiotensin I; Ang II: angiotensin II; BP: blood pressure; EC: endothelial cells; ECM: extracellular matrix; Ets-1: the v-ets erythroblastosis virus E26 oncogene homolog 1; MCP-1: monocyte chemo-attractant protein-1; MFG-E8: milk fat globule epidermal growth factor-8; MMPs: matrix metalloproteases; MR: mineralocorticoid receptors; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; Nrf2: NF-E2- related factor 2; NO: nitric oxide; PDGF: platelet derived growth factor; PWV: pulse wave velocity; RAGE: receptor for advanced glycoxidation end products; ROS: reactive oxygen species; RS: replicative senescence; SASP: senescence-associated secretory phenotype; Sirt1: silent information regulation 2 homolog 1; SIPS: stress-induced premature senescence; TGF-β1: transforming growth factor β1; SASP: senescence-associated secretory phenotype; VSMC: vascular smooth muscle cell.
2. Molecular Phenotypes in the Aging Arterial Wall
2.1. Age-associated Leading Stressors
We recognize that increases in the key molecules of SNA, the renin angiotensin aldosterone system (RAAS) and endothelin-1 (ET-1) activity are the leading proinflammatory stressors of arterial aging based upon recently published studies (Figure 1).
2.1.1. Norepinephrine
Aging increases SNA which is characterized by an increase of neurohormone norepinephrine secretion in the arterial wall and an upregulation of its receptor, alpha-adrenergic receptor (α-AR) [9, 10, 17, 18]. SNA is also interconnected with the RAAS system and ET-1. Increased SNA contributes to endothelial dysfunction, vasoconstriction, IMT, and BP increase and increases arterial proinflammation [8, 9, 14, 19].
2.1.2. Angiotensin II
Increased SNA triggers an activation of RAAS at both central and peripheral levels with aging [9, 12, 14, 19]. The transcription, translation and activity of angiotensin converting enzyme (ACE) markedly increases in the arterial wall with aging [20–26]. Chymase, an alternative angiotensin convertase, also increases in expression within the arterial adventitia [22]. Both ACE and chymase cleave angiotensin I (Ang I) into angiotensin II (Ang II). Consequently, Ang II protein is significantly elevated in the aging arterial wall [21–23, 25, 27–29], along with increased expression of the Ang II receptor, AT1 [21, 30]. The “aging-elevated” Ang II/AT1 expression in the arterial wall signals to the SNA subsequently creating an inflammatory environment which contributes to arterial remodeling.
2.1.3. Aldosterone
Aldosterone (Aldo) a component of RAAS and a downstream Ang II effector, is also regulated by the SNA, and is known as the sympathetic-adrenal system [9]. Aldo, secreted by the adrenal glands, binds to the mineralocorticoid receptors (MR). Aging increases the clustered zona glomerulosa cells of the adrenal glands and subsequently enhances the production and secretion of Aldo, called “age-related autonomous aldosteronism” [31, 32]. Further, aging also increases the local expression of MR in arterial walls and cells [33–35]. Elevated Aldo/MR signaling enhances extracellular signal–regulated kinases (ERK1/2) signaling, contributing to the proinflammatory phenotypic shift of VSMCs, thus promoting vasoconstriction, stiffening, and BP increase [33–37].
2.1.4. Endothelin-1
The endothelium, known as the body’s largest “endocrine gland”, produces ET-1, and is also regulated by the SNA [14, 38]. With advancing age, aortic proendothelin-1 (pro-ET-1) and activated matrix metalloproteinase type II (MMP-2) levels increase [39]. Pro-ET-1 can be cleaved into an active ET-1 peptide by either endothelial converting enzyme or MMP-2 within the arterial walls [39–41]. Consequently, active ET-1 levels are increased in aging [38, 39, 42]. ET-1 enhances inflammation by increasing the expression of the transcription factor, E26 transformation-specific proto-oncogene 1 (ets-1), in VSMCs with aging [39]. Aging increases the sensitivity of the ET receptor in aortic walls, further augmenting proinflammation [43].
2.2. Age-associated Key Secreted Downstream Molecules
The interrelationship of the SNA/RAAS/ET-1 signaling pathways promotes the proinflammatory response in the aged arterial wall leading to an increase in key downstream molecules (Figure 1). Aged cells, including senescent cells, modify their microenvironment via the secretions of a variety of bioactive factors known as the age-associated arterial secretory phenotype (AAASP), including the senescence associated secretory phenotype (SASP) [3].
2.2.1. Monocyte Chemoattractant Protein-1
Monocyte chemoattractant protein-1 (MCP-1) increases in aged arterial walls in associations with both SNA and Ang II signaling [44, 45]. ET-1 also promotes the secretion of MCP-1 in aged VSMCs [39]. Both mRNA and protein levels of MCP-1 are upregulated within the aged aortic wall [45–47]. Increases in MCP-1 protein expression are mainly localized to the thickened intima and promotes VSMC proinflammation [45–47]. MCP-1 is a key intermediary signaling molecule which connects the SNA/RAAS/ET-1 signaling pathway to proinflammatory cellular and matrix phenotype changes.
2.2.2. Transforming Growth Factor-β1
Transforming growth factor-β1 (TGF-β1)/TGF beta receptor type II (TβIIR) activation is a powerful fibrotic signaling cascade, that is closely mediated by Ang II/AT1 signaling [48–51]. The levels of secreted TGF-β1 protein from VSMCs are upregulated in aged rat aortae [49]. Increased collagen synthesis, secretion, and deposition is triggered by interactions of TGF-β1/TβIIR and p- SMAD-2/3 signaling [39, 48, 49, 52]. In addition, treating ECs with TGF-β1 peptide also increases the expression of collagen types I and III [53, 54]. Interestingly, MCP-1 has been shown to co-localize with TGF-β1 within the arterial wall to enhance the activity of TGF-β1 in VSMCs [55]. Thus, interactions of TGF-β1 and MCP-1 may play a significant role in age-associated arterial fibrosis.
2.2.3. Matrix Metalloproteinases
Matrix metalloproteinases (MMPs) degrade the extracellular matrix. MMP-2 is a downstream molecule of both Ang II and phenylephrine signaling in the arterial wall and cultured VSMCs [27]. Both the mRNA and protein levels of MMP-2/9 are upregulated in aged aortic walls [22, 27, 49, 55–57]. The increased ratio of the MMP activator, membrane-type1 matrix metalloproteinase, to MMP inhibitor, tissue inhibitor of MMP-2 potentially promotes MMP-2/9 activation with aging [21, 22, 58]. Activated MMP-2/9 is predominantly located to the thickened intima and the inner media of arteries [22, 58]. Notably, secreted activated MMP-2/9 from VSMCs is upregulated with aging, which mainly contributes to an increased activation of arterial MMP-2/9. Importantly, activated MMP-2 increases the bioavailability of proinflammatory vasoactive molecules, e.g., cleaves latent transforming protein-1 and pro-ET1, into activated TGF-β1 and ET-1 in the arterial wall or VSMCs [39–41, 49].
2.2.4. Calpain-1
Calpain-1 is a calcium-dependent intracellular protease, which modulates extracellular MMP-2 and TGF-β1 activity in the aged arterial wall or cultured VSMCs [29, 59]. The activity of calpain- 1 is significantly increased in both aged rat aortae and cultured aortic VSMCs [29] and facilitates activation of MMP-2 and TGF-β1 leading to fibrosis and calcification [59]. In contrast, the calpain-1 inhibitor, BDA-1, attenuates aortic calcification in aging klotho-deficient mice [60]. Ang II both activates and colocalizes with calpain-1 in the aged arterial wall and VSMCs [29]. Thus, calpain partially relays the proinflammatory signaling of Ang II in the aged arterial wall or cultured VSMCs.
2.2.5. Milk Fat Globule EGF-8
MFG-E8 is a highly-glycosylated protein enriched in milk fat globule containing EGF and blood clotting factor VIII. Aging not only increases MFG-E8 mRNA and protein levels but also increases its fragment medin in the arterial wall [61–63]. Treating aged VSMCs with MFG-E8 increases the proliferation and migration of VSMCs. Medin has a strong affinity to elastin fibers, promoting elastolysis and amyloidosis in the arterial wall [62–65]. In addition, Ang II induces MFG-E8 protein expression, which increases MCP-1 expression in VSMCs, promoting proinflammation [28]. Notably, MFG-E8 is also a well-known bridging molecule, that mediates the clearance of apoptotic cells (efferocytosis) by macrophages, retarding the growth and vulnerability of atherosclerotic plaques in mice with aging [66–68]. The complex role of MFG-E8 in the aged proinflamed arterial wall needs to be further explored.
2.2.6. Reactive Oxygen Species
Reactive oxygen species (ROS) are increased in the aged arterial wall or VSMCs. Nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) expression is the main source of production of arterial ROS. Further, levels of the anti-oxidant proteins, Cu/Zn SOD (SOD1), Mg SOD (SOD2), and extracellular matrix superoxide dismutase (ECM-SOD/SOD3) are downregulated during aging [69–72]. Therefore, aging creates an imbalance of NADPH oxidase and dismutase in the arterial wall, eventually augmenting an increase of ROS levels. This imbalance, along with increases in both Ang II and ET-1 enhances NADPH expression and the production of ROS [14, 25, 52, 69, 73–77]. Increased ROS modifies proinflammation, endothelial dysfunction, and arterial stiffening in the arterial wall with aging [25, 52, 72, 78–82].
2.2.7. Nitric Oxide and Bioavailability
Nitric oxide (NO), a small diffused signaling molecule, regulates arterial dilatation, stiffening and inflammation with aging [25, 69, 72, 74, 83–87]. Endothelial nitric oxide synthase (eNOS) activation determines the production of NO in the arterial wall. Expression of arterial eNOS is decreased and contributes to a reduction of NO production in the aged arterial wall [70, 84, 88– 90]. In addition, NO interacts with ROS to generate peroxynitrite (ONOO-). This ROS further decreases the bioavailability of NO, and impairs endothelium-dependent relaxation and enhances vasoconstriction and proinflammation [74, 90, 91].
2.3. Age-associated Transcription Factors
RAAS/SNA/ET-1 signaling contributes to the activation or inactivation of nuclear transcription factors that are key intermediary molecules that contribute to proinflammatory cellular and matrix phenotype changes (Figure 1).
2.3.1. Ets-1 and NF-κB
The major proinflammatory transcription factors, Ets-1 and NF-κB, are activated in the aged arterial wall. Increased Ets-1 activity is closely associated with increased transcription levels of ET-1, MCP-1, TGF-β1, and MMP-2 [39]. Increased NF-κB activity in old arterial cells promotes oxidative stress and triggers an inflammatory response [92–94].
2.3.2. Nrf-2 and SIRT1
The major anti-proinflammatory transcription factors, nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and sirtuin (silent mating type information regulation 2 homolog) 1 (S. cerevisiae) (SIRT1), are decreased in the aged arterial wall. Nrf2 protects against oxidative stress and its related cytotoxic effects and magnifies NF-κB activation [95]. SIRT1 is downregulated and inactivated with aging [71, 83, 87, 96, 97]. Decreased Sirt1 activity increases NADPH oxidase- dependent ROS production, senescence, inflammation, and enhances endothelial dysfunction in the aged arterial wall [83, 96–98].
2.4. Summary
With advancing age, both physical and mental stress increases due to continuous adaptations to changes in the living environment. Increased stress triggers the activation of both RAAS and SNA leading to ET-1 activation. These “leading proinflammatory signaling” events act on arterial cells by directly promoting the secretion or production of MCP-1, TGF-β1, MMPs, calpain-1, and MFG-E8, known as the AAASP, as well as the activation or inactivation of transcription factors; Ets-1, NF-kB, Nrf2, or Sirt1. These age-associated proinflammatory molecular phenotype alterations eventually lead to age-associated cellular and matrix phenotype changes. Further studies are needed to elucidate the finer details of the mutable events that facilitate the directionality and movement of the proinflammatory signaling cascade with aging as illustrated in Figure 1.
3. Cellular and Matrix Phenotypes in the Aging Arterial Wall
The structure and function of arterial cells and matrix are remodeled with advancing age through proinflammatory signaling. Changes in age-associated cellular and extracellular phenotypes are illustrated in Figure 1.
3.1. Age-associated Cellular Phenotypes
3.1.1. EC Senescence
The number of arterial ECs decreases with aging potentially due to either replicative senescence (RS) with telomere reduction and inactivation of telomerase, or stress-induced premature senescence (SIPS) without telomere involvement. Indeed, the number of both RS and SIPS ECs increases in the aged arterial wall [81, 85, 88, 99–102]. A subset of aged ECs appears with decreased mitotic frequency, and an increase in cellular volume, and shortened telomeres, while entering a RS state [103–105]. In addition to RS, the Ang II signaling cascade plays a significant role in the SIPS of ECs via a reduction of Sirt1 and ERK1/2/BCL2 signaling, and functional autophagy in addition to increased ROS production [81, 100–102].
3.1.2. VSMC Senescence
A subset of aged VSMCs appears enlarged and becomes senescent in the arterial wall [97, 98, 100, 106–110]. Oxidative stress and DNA damage cause VSMC senescence and are linked to shortened telomeres or SIPS triggered through Ang II signaling [82, 100, 111, 112]. Increased senescence is associated with an increase of p16 expression , a loss of SIRT1 expression, and an upregulation of miR-34a in VSMCs . In addition, the mutant lamin A, known as progerin, also drives the senescence of VSMCs [110, 113].
3.1.3. Proliferation
VSMC proliferation is increased in the aged arterial wall. Age-associated secretory molecules, such as MFG-E8, may promote a replicative subset of VSMCs [108, 114]. This subset of VSMCs have an increased proliferative capacity via MFG-E8 signaling, displaying increased ERK1/2 phosphorylation, 5-bromo-2’-deoxyuridine (BrdU) incorporation, as well as increased expression of proliferative cellular nuclear antigen (PCNA), cyclin-dependent kinase 4 (CDK4), and platelet derived growth factor (PDGF-BB) [115].
3.1.4. Invasion/Migration
VSMCs invasion is the ability to migrate or infiltrate neighboring tissue through vascular extracellular matrix. Interestingly, the invasive capacity of a VSMC subset is increased in the aged arterial wall, contributing to diffuse intimal thickening [3, 108, 114, 116]. Aging increases the signaling of Ang II which triggers the secretion of calpain-1, MFG-E8, or MCP-1 in VSMCs, and also activates MMP-2 [27–29]. Increased MMP-2 activation is the key molecule that drives the invasive capabilities of VSMCs via a breakdown of their basement membrane and surrounding extracellular matrix [21, 28, 29, 55]. Conversely, the invasive capacity of old VSMCs can be inhibited by the MMP-2 inhibitor, GM6001 [46, 55].
3.1.5. Stiffening
The stiffening of VSMCs is a central and mutable element to arterial aging [78, 117–121]. VSMCs derived from older animals demonstrate increased stiffness over similar cells derived from young adults [119, 122]. VSMC stiffness is highly sensitive to the microenvironmental molecules such as TGF-β1 and transglutaminase 2 [119, 123]. TGF-β1 serves as a specific modifier of age- associated VSMC stiffening through the clustering of mechanosensitive α5β1 and αvβ3 integrins [122]. Increased stiffness in aging is also dependent on the expression and organization of the VSMC cytoskeleton proteins along the arterial tree [123, 124]. Interestingly, a stiffened substrate reinforces VSMC stiffening [125]. The stiffness correlate with phenotypic changes of VSMCs [123]. Increased stiffening is converted into proinflammatory signaling in the aged arterial wall [126].
3.1.6. Proinflammatory Secretions
Aged vascular cells, including senescent cells, modify their microenvironment via the autonomous or non-autonomous secretion of a variety of bioactive factors. Vascular senescence associated secretory phenotype (SASP) are cells that promote proinflammation of neighboring cells [108, 114]. Interestingly, growing evidence indicates that aged primary isolated VSMCs from aortic walls, have a unique chemokine and cytokine proinflammatory profile, known as the age- associated arterial secretory phenotype (AAASP), that also drives proinflammation in neighboring cells[5, 92]. MMP-2, MCP-1, TGF-β1, MFG-E8, and tissue necrosis factor-alpha (TNF-α) are characteristic proteins secreted from old VSMCs with the AAASP [3, 92].
3.2. Age-associated Matrix Phenotype Changes
The extracellular matrix of arterial walls is modified by age-associated proinflammatory secretions of vascular cells through fibrosis, elastolysis, calcification, and amyloidosis [47, 85, 97, 99, 127].
3.2.1. Fibrosis
Fibrosis develops through an increase of collagen deposition in the arterial wall of aging rats [58, 75]. Collagen accumulation also significantly increases within the arterial walls of aged humans[128]. Secreted MMP-2 activates TGF-β1 and promotes VSMC collagen production [108, 114]. Collagen deposition within the interlamellar layers of the arterial wall plays a significant mechanical role in arterial stiffening [129].
3.2.2. Elastolysis
An intact interlamellar elastin layer is important for the health of large arteries. The aging interlamellar elastin network is disrupted and collapsed in elastolysis due to the cleavage by MMPs and elastase [58, 130–132]. Elastolysis is observed with an increase in the amounts of activated MMP-2/9 or elastase in the interlamellar elastin network [50, 55]. This age-associated destruction of interlamellar elastin lamina is also associated with the loss of tropoelastin production, impairing rejuvenation [133]. The destruction of the vascular interlamellar elastin results in an eventual decrease in arterial elastic energy storage capability, compliance, and resilience [134]. In addition, short peptides, released during elastolysis, known as elastokines, actively participate in the onset and progression of arterial inflammation and calcification.
3.2.3. Calcification
Arterial calcification plays a crucial role in the development of arterial stiffening [135]. Increased calcium deposits, an element of calcification, are markedly increased in the arterial wall with aging [136, 137]. The morphology of older VSMCs appears osteoblasts-like, producing large amounts of bone-like substrates, such as collagen II [59]. The development of arterial calcification is dependent upon a balance of pro-/anti-calcification molecules. Overexpression of alkaline phosphatase, a pro-calcification molecule, increased arterial calcification and is one of the pro- calcification molecules that is found with greater frequency in old or senescent VSMCs . In addition, anti-calcification molecules, such as osteonectin, and osteopontin (OPN) are simultaneously reduced in old VSMCs [59]. Notably, age-associated increases in PDGF, a powerful cellular mitogen, also significantly accelerates the process of arterial calcification [138].
3.2.4. Amyloidosis
With advancing age, mis-folded aggregated amyloid proteins and fibrils are increased in arterial walls [62–65]. One of the main constituents of arterial amyloid fibrils is a 5.5 kDa fragment of MFG-E8, known as medin, which is markedly increased in the aged arterial wall [61, 62, 65]. Medin has a high capacity for binding to elastin fibers, potentially increasing stiffness and calcification [61–65]. Thus, medin amyloid is implicated as highly affecting the elasticity of aged arteries and needs further investigation.
3.2.5. Advanced glycoxidation end-products
Advanced glycoxidation end-products (AGEs) are increased in aged arterial wall. It is well known that AGEs accumulation contributes to multiple structural and functional alterations in the arterial system such as senescence, proinflammation, and stiffening [4, 52, 139–141]. AGEs are often generated by reactions between sugar chains and biologic amines of oxidized collagen. Older, cleaved/degraded, oxidized, collagen fibers are common molecular targets that are easily modified via a reaction between ROS and sugars in the arterial wall. Aging increases AGEs and promotes collagen production, through activation of its receptor, RAGE, in a feed-back manner.
3.3. Summary
Aging can be described as a form of sub-clinical pathological conditions. Proinflammatory molecular signaling acts on arterial cells, generating age-associated cellular and matrix phenotype changes as illustrated in Figure 1. These phenotypes are all observed in the aged arterial wall and have been reproduced in vivo and in vitro. These characteristic cellular and matrix alterations can also be induced in young animals under experimental proinflammatory stimulation. Proinflammatory structural phenotype clinical changes may be observed in aged population without evidence of cardiovascular disease. The signaling by these phenotypes is complex and it is unknown how multiple cellular and matrix events are controlled and how a sub-clinical symptom evolves into a clinical disease. For example, how does arterial stiffness syndrome evolve into a clinical pathological condition such as hypertension and atherosclerosis. It is important to decode the signaling network and find the key switch for the diagnosis, prevention, and treatment of adverse arterial remodeling with aging.
4. Arterial sub-clinical phenotypes with aging
Age-associated cellular and extracellular phenotypic shifts ultimately lead to “arterial proinflammatory stiffness syndrome”, including IMT, endothelial dysfunction, stiffening, and BP increase (Figure 2).
Figure 2. Diagram of proinflammatory arterial stiffness syndrome.
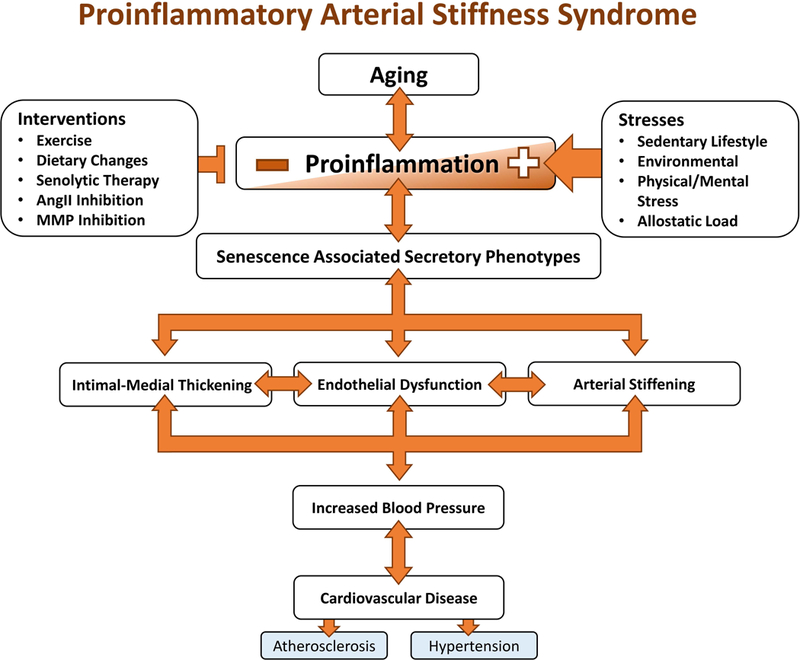
The phenotype changes made at the cellular and matrix level characterize the final stage of the vascular aging cascade that ultimately leads to proinflammatory structural phenotype changes and eventually cardiovascular disease.
4.1. Intimal-medial thickening
IMT can be accurately evaluated by B-mode ultrasound and other noninvasive imaging and is a hallmark of age-associated arterial remodeling [3, 8, 23, 116, 142–149]. Expansion of the intimal layer, rather than the media is mainly responsible for increased IMT [150], which is linked to increases in both vascular relaxation and stiffening [143, 144, 151, 152]. Notably, there is also an increase in VSMC progenitor cells promoting age-associated IMT [153].
4.2. Endothelial dysfunction
The arterial endothelial barrier becomes disrupted with advancing age. More senescent ECs, with reduced telomerase, contribute to endothelial-dependent dysfunction by increasing the number of defective sites along the lumen [85, 88, 99, 154, 155]. Circulating platelets adhere and infiltrate via the damaged sites into the arterial wall initiating inflammatory responses. Increased adhesive platelets on the inner surface of the arterial lumen not only inhibit the proliferation and migration of local endothelial cells but also exhausts endogenous repair by progenitor cells [99]. Therefore, increases in activated and aggregated platelets damages the integrity of the aged arterial endothelial barrier and also promotes endothelial dysfunction [145]. Furthermore, EC contractility is enhanced with aging, leading to increased endothelial permeability and intimal stiffening [25, 26, 38, 72, 83, 85, 87, 93, 148].
Aging also increases monocytosis and enhances macrophage trans-differentiation and accumulation within the aorta [145, 156]. The accumulation of macrophages within the arterial wall leads to metabolic impairment and subsequently accelerates arterial remodeling [145, 157]. Increased amounts of activated neutrophils and lymphocytes infiltrate the intramural space and interacts with ECs and VSMCs, facilitating ROS production, senescence, and endothelial function [68, 145, 158, 159]. In addition, older subjects with higher cardiovascular risk factors, have lower numbers of circulating endothelial progenitor cells, which is linked to endogenous regenerative potential, suggesting the reparative capacity of the endothelia is decreased [142].
4.3. Arterial Stiffening
Arterial stiffness, including intimal, medial and adventitial stiffness, is dependent on an intrinsic stress/strain relationships determined by both cell and matrix stiffness [77, 118, 120, 123, 125, 135, 139, 147, 148, 151, 154, 160–163]. Pulse wave velocity (PWV) has emerged as a gold-standard for non-invasive assessment of central arterial stiffness, a predictor of the incidence of hypertension and all-cause mortality, and increases with advancing age [144, 151, 162–164]. The Baltimore Longitudinal Study of Aging (BLSA) has demonstrated that increased PWV was associated with increased systolic blood pressure (SBP) and also a greater incidence of hypertension [165]. Further, the aortic-brachial PWV ratio has emerged as a novel index of BP- independent of vascular aging [144] and the carotid-radial/carotid-femoral PWV ratio is an accurate predictor of all-cause mortality [151].
4.4. Blood Pressure Increase
Increases in SBP and pulse pressure (PP) occurs after the sixth decade of life, becoming a hallmark of arterial aging [135, 143–145, 147, 160, 166]. Further, BP measurements are closely associated with PWV [143].
4.5. Summary
The accumulation of these molecular, cellular, and matrix phenotypes in the arterial wall with aging is manifested as arterial proinflammatory stiffness syndrome. The clinic arterial phenotype is illustrated in Figure 2, including intimal -medial thickening, endothelial dysfunction, stiffening, and blood pressure increase. These sub-clinical conditions are detected in the elderly without overt cardiovascular events, which we regard as arterial proinflammatory syndrome. Further studies are needed to understand the mechanism by which accelerated aging leads to clinical arterial phenotype and reduces the disease threshold which may ultimately lead to clinical cardiovascular disease.
5. Interventions to Counteract Arterial Aging
Since proinflammation is central to arterial aging, then efforts to reduce proinflammation could help reduce the clinical progression of arterial aging. A healthy life style and regular exercise can prevent age-associated senescence and secretion.
Pharmacological interventions may intervene to disrupt the progression of vascular aging by inhibiting the AAASP/SASP directly or through the removal of senescent vascular cells with senolytic drugs may modify arterial aging and diminish the proinflammatory cascades [2, 167–169] (Figure 3).
Figure 3. Age-associated proinflammatory arterial stiffness syndrome interventions.
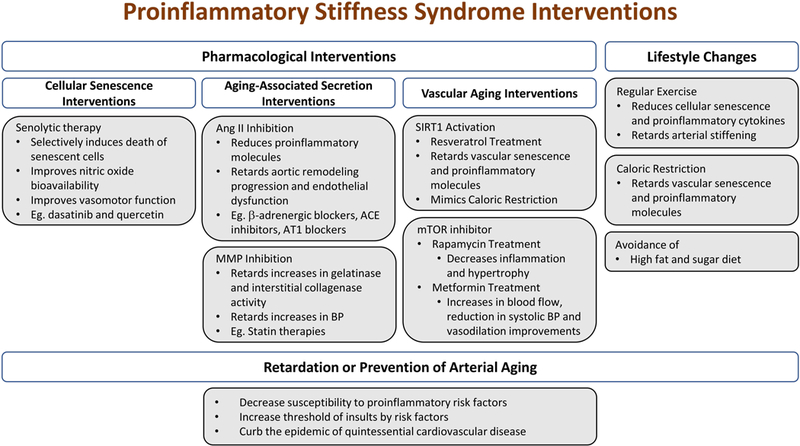
Vascular aging can be mitigated through various approaches that target the proinflammatory cascades. The eventual structural phenotype changes are not inevitable and cardiovascular disease may be prevented.
Abbreviations and acronyms: ACE: angiotensin converting enzyme; Ang II: angiotensin II; AT1: Ang II receptor; BP: blood pressure; MMPs: matrix metalloproteases; mTOR: mammalian target of rapamycin; Sirt1: silent information regulation 2 homolog 1
5.1. Diet
Long term caloric restriction retards both vascular senescence and proinflammatory molecules such as the decreases in MCP-1, ROS, and MMP activation and the improvements in the NO bioavailability and endothelial function, preventing arterial stiffening with aging [86, 170–172] . Not only are these molecules disrupted, but the clinical signs of aging, such as body weight, blood pressure, cholesterol levels, and arterial stiffness have improved [86, 170–172].
A high fat and sugar diet (HFS) drives arterial aging in nonhuman primates [170, 171]. Animals fed a HFS diet for 2-years showed increases not only in body weight and circulating cholesterol, but also exhibited signs of central arterial wall stiffening and inflammation [164]. Further study showed that the stiffening associated loss of endothelial cell integrity, lipid and macrophage infiltration, and calcification of the arterial wall were driven by genomic and proteomic disorders of oxidative stress and inflammation [164].
5.2. Exercise
Regular exercise substantially reduces ET-1 signaling, TGF-β1 activity, cellular senescence, proinflammation, and calcification in the aged arterial wall [76, 78, 86, 89, 141, 173]. Importantly, regular exercise effectively prevents age-associated SNA, BP increase, arterial stiffening, endothelial dysfunction [11, 16, 78, 89, 152, 174].
5.3. Senolysis
Clearance of senescent cells using transgenic and pharmacological approaches retards arterial aging. Senolytics, such as dasatinib and quercetin, have been utilized to retard vascular aging. Dasatinib primarily eliminates senescent progenitor cells while Quercetin is more effective against senescent endothelial cells [175]. The combined treatment of dasatinib and quercetin significantly reduces arterial senescent cell burden in the arterial wall, increases NO bioavailability and improves vasomotor dysfunction with aging [85]. Senolytic therapy can also reduce the progress of age-associated atherosclerosis [176, 177].
5.4. Inhibition of Ang II Signaling
The Ang II proinflammatory signaling cascade has been widely studied [20, 24–26, 44, 81, 178] . Chronic administration of young rats with Ang II not only increases the BP, but also enhances the activity of MMP-2, TGF-β1, calpain-1, MFG-E8, and collagen production within the arterial wall, similar to that of untreated old animals [27, 29, 59, 143, 179]. In contrast, chronic ACE inhibition of the Ang II receptor, an AT1 antagonist, significantly reduces the abundance of proinflammatory molecules and retards the progression of adverse aortic remodeling in experimental animals with aging [20, 24–26, 178].
5.5. Inhibition of MMPs
Activated MMPs are common elements of the SASP and AAASP. Chronic administration of PD166793, a broad spectrum MMP inhibitor, significantly retards age-associated increases in gelatinase and interstitial collagenase activity, ET-1 expression, elastic fiber fragmentation, and collagen production in the arterial wall of rats [39]. Interestingly, MMP inhibition also substantially retards increases in blood pressure with advancing age [39].
5.6. Activation of SIRT1
Resveratrol treatment, a caloric restriction mimicking small molecule and an agonist of SIRT1, effectively prevented the HFS-induced arterial wall inflammation and arterial stiffening in nonhuman primates [164]. These findings suggest that dietary resveratrol, like caloric restriction, holds a promise to ameliorate age-associated arterial inflammation, elastolysis, and stiffening [81, 92, 96, 180]
5.7. Inhibition of mTOR
Other treatments, like rapamycin and metformin, focus on decreasing inflammation and arterial stiffening and improving endothelial dysfunction through the inhibition of a mammalian target of rapamycin (mTOR) [181, 182].
5.8. Summary
Pharmacological and lifestyle interventions of age-associated proinflammatory stiffness syndrome are illustrated in Figure 3. These interventions are largely derived from studies of experimental animals and it is difficult to translate these approaches to the clinic. It is important to perform a large double blind, random clinical trial to find the most effective time, dose, and side effects for potential treatments. Since advanced aging is a form of sub-clinical disease, targeting proinflammation may be the best approach to mitigating cardiovascular disease, which evolves into clinical conditions through either reduced disease threshold or increased susceptibility and vulnerability.
6. Concluding Remarks
Age-associated arterial structural and functional remodeling are driven by chronic increases in proinflammatory signaling causing proinflammatory stiffness syndrome. At the cellular level, increases in senescent and senescence-associated molecular signaling have been observed both in vivo as well as in vitro. Microscopically, intimal-medial thickening, endothelial disruption, cellular senescence, senescence-associated cellular and matrix phenotypes are characteristics of the aged arterial wall. These adverse molecular, cellular, and matrix events, presenting as “old phenotypes”, are also observed in young animals experimentally infused with proinflammatory stimulants. Alternatively, in old animals, these adverse remodeling events are alleviated by inhibition of cellular senescence and senescence-associated phenotypes resulting in more “youthful phenotypes”. Arterial senescence and senescence-associated heterogeneous phenotypes cause proinflammatory stiffness syndrome present as sub-clinical conditions and may provide the fertile soil for the initiation and progression of hypertension and atherosclerosis at the molecular, cellular, and vascular levels. Thus, interventions that suppress or prevent proinflammatory stiffness syndrome at different levels, may hold great promise for treating and preventing the age- associated vascular diseases such as hypertension and atherosclerosis. Questions still remain regarding the mutability of the proinflammatory cascades and the triggers that control each level. Future studies are needed to decode the proinflammatory signaling network and understand how sub-clinical conditions evolve into ending cardiovascular disease.
Acknowledgements
I, Mingyi Wang, would like to thank, my advisor and mentor, Edward G. Lakatta, MD, the chief in the Laboratory of Cardiovascular Science, National Institution on Aging, National Institutes of Health, for introducing me to the wonders and frustrations of scientific research on cardiovascular aging.
Funding sources
This research was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Footnotes
Conflicts of interest
None
Contributor Information
Mingyi Wang, Laboratory of Cardiovascular Science, National Institute on Aging, National Institutes of Health, Biomedical Research Center (BRC), 251 Bayview Blvd, Baltimore, MD 21224..
Robert E Monticone, Laboratory of Cardiovascular Science, National Institute on Aging, National Institutes of Health, Biomedical Research Center (BRC), 251 Bayview Blvd, Baltimore, MD 21224..
Kimberly R McGraw, Laboratory of Cardiovascular Science, National Institute on Aging, National Institutes of Health, Biomedical Research Center (BRC), 251 Bayview Blvd, Baltimore, MD 21224..
References
- 1.Michaud M, et al. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc, 2013. 14(12): p. 877–82. [DOI] [PubMed] [Google Scholar]
- 2.Soto-Gamez A and Demaria M, Therapeutic interventions for aging: the case of cellular senescence. Drug Discov Today, 2017. 22(5): p. 786–795. [DOI] [PubMed] [Google Scholar]
- 3.Wang M, et al. Proinflammation: the key to arterial aging. Trends Endocrinol Metab, 2014. 25(2): p. 72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang M, Khazan B, and Lakatta EG, Central Arterial Aging and Angiotensin II Signaling. Curr Hypertens Rev, 2010. 6(4): p. 266–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang M, Monticone RE, and Lakatta EG, Arterial aging: a journey into subclinical arterial disease. Curr Opin Nephrol Hypertens, 2010. 19(2): p. 201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Go AS, et al. Heart disease and stroke statistics−-2014 update: a report from the American Heart Association. Circulation, 2014. 129(3): p. e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang M and Shah AM, Age-associated pro-inflammatory remodeling and functional phenotype in the heart and large arteries. J Mol Cell Cardiol, 2015. 83: p. 101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinenno FA, et al. Age-associated arterial wall thickening is related to elevations in sympathetic activity in healthy humans. Am J Physiol Heart Circ Physiol, 2000. 278(4): p. H1205–10. [DOI] [PubMed] [Google Scholar]
- 9.Gordon RD, et al. Role of the sympathetic nervous system in regulating renin and aldosterone production in man. J Clin Invest, 1967. 46(4): p. 599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplon RE, Walker AE, and Seals DR, Plasma norepinephrine is an independent predictor of vascular endothelial function with aging in healthy women. J Appl Physiol (1985), 2011. 111(5): p. 1416–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka H, Dinenno FA, and Seals DR, Reductions in central arterial compliance with age are related to sympathetic vasoconstrictor nerve activity in healthy men. Hypertens Res, 2017. [DOI] [PubMed]
- 12.Arnold AC, Gallagher PE, and Diz DI, Brain renin-angiotensin system in the nexus of hypertension and aging. Hypertens Res, 2013. 36(1): p. 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnes JN, et al. Aging enhances autonomic support of blood pressure in women. Hypertension, 2014. 63(2): p. 303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruno RM, et al. Sympathetic regulation of vascular function in health and disease. Front Physiol, 2012. 3: p. 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaye DM and Esler MD, Autonomic control of the aging heart. Neuromolecular Med, 2008. 10(3): p. 179–86. [DOI] [PubMed] [Google Scholar]
- 16.Laitinen T, et al. Age dependency of cardiovascular autonomic responses to head-up tilt in healthy subjects. J Appl Physiol (1985), 2004. 96(6): p. 2333–40. [DOI] [PubMed] [Google Scholar]
- 17.Barrett-O’Keefe Z, et al. Angiotensin II potentiates alpha-adrenergic vasoconstriction in the elderly. Clin Sci (Lond), 2013. 124(6): p. 413–22. [DOI] [PubMed] [Google Scholar]
- 18.Rudner XL, et al. Subtype specific regulation of human vascular alpha(1)-adrenergic receptors by vessel bed and age. Circulation, 1999. 100(23): p. 2336–43. [DOI] [PubMed] [Google Scholar]
- 19.Xu B and Li H, Brain mechanisms of sympathetic activation in heart failure: Roles of the reninangiotensin system, nitric oxide and proinflammatory cytokines (Review). Mol Med Rep, 2015. 12(6): p. 7823–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michel JB, et al. Effect of chronic ANG I-converting enzyme inhibition on aging processes. II. Large arteries. Am J Physiol, 1994. 267(1 Pt 2): p. R124–35. [DOI] [PubMed] [Google Scholar]
- 21.Wang M, et al. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension, 2007. 50(1): p. 219–27. [DOI] [PubMed] [Google Scholar]
- 22.Wang M, et al. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension, 2003. 41(6): p. 1308–16. [DOI] [PubMed] [Google Scholar]
- 23.Harvey A, Montezano AC, and Touyz RM, Vascular biology of ageing-Implications in hypertension. J Mol Cell Cardiol, 2015. 83: p. 112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basso N, et al. Protective effect of long-term angiotensin II inhibition. Am J Physiol Heart Circ Physiol, 2007. 293(3): p. H1351–8. [DOI] [PubMed] [Google Scholar]
- 25.Flavahan S, Chang F, and Flavahan NA, Local renin-angiotensin system mediates endothelial dilator dysfunction in aging arteries. Am J Physiol Heart Circ Physiol, 2016. 311(3): p. H849–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukai Y, et al. Inhibition of renin-angiotensin system ameliorates endothelial dysfunction associated with aging in rats. Arterioscler Thromb Vasc Biol, 2002. 22(9): p. 1445–50. [DOI] [PubMed] [Google Scholar]
- 27.Wang M, et al. Angiotensin II activates matrix metalloproteinase type II and mimics age- associated carotid arterial remodeling in young rats. Am J Pathol, 2005. 167(5): p. 1429–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu Z, et al. Milk fat globule protein epidermal growth factor-8: a pivotal relay element within the angiotensin II and monocyte chemoattractant protein-1 signaling cascade mediating vascular smooth muscle cells invasion. Circ Res, 2009. 104(12): p. 1337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang L, et al. Increased aortic calpain-1 activity mediates age-associated angiotensin II signaling of vascular smooth muscle cells. PLoS One, 2008. 3(5): p. e2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishimura A, et al. Purinergic P2Y6 receptors heterodimerize with angiotensin AT1 receptors to promote angiotensin II-induced hypertension. Sci Signal, 2016. 9(411): p. ra7. [DOI] [PubMed] [Google Scholar]
- 31.Nanba K, et al. Age-Related Autonomous Aldosteronism. Circulation, 2017. 136(4): p. 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seals DR and Esler MD, Human ageing and the sympathoadrenal system. J Physiol, 2000. 528(Pt 3): p. 407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krug AW, et al. Elevated mineralocorticoid receptor activity in aged rat vascular smooth muscle cells promotes a proinflammatory phenotype via extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase and epidermal growth factor receptor-dependent pathways. Hypertension, 2010. 55(6): p. 1476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DuPont JJ, et al. Vascular mineralocorticoid receptor regulates microRNA-155 to promote vasoconstriction and rising blood pressure with aging. JCI Insight, 2016. 1(14): p. e88942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCurley A, et al. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med, 2012. 18(9): p. 1429–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galmiche G, et al. Smooth muscle cell mineralocorticoid receptors are mandatory for aldosterone-salt to induce vascular stiffness. Hypertension, 2014. 63(3): p. 520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koenig JB and Jaffe IZ, Direct role for smooth muscle cell mineralocorticoid receptors in vascular remodeling: novel mechanisms and clinical implications. Curr Hypertens Rep, 2014. 16(5): p. 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goel A, et al. Increased endothelial exocytosis and generation of endothelin-1 contributes to constriction of aged arteries. Circ Res, 2010. 107(2): p. 242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang M, et al. Chronic matrix metalloproteinase inhibition retards age-associated arterial proinflammation and increase in blood pressure. Hypertension, 2012. 60(2): p. 459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdalvand A, et al. Matrix metalloproteinase enhances big-endothelin-1 constriction in mesenteric vessels of pregnant rats with reduced uterine blood flow. Hypertension, 2013. 61(2): p. 488–93. [DOI] [PubMed] [Google Scholar]
- 41.Lekontseva ON, et al. Ovariectomy in aged versus young rats augments matrix metalloproteinase-mediated vasoconstriction in mesenteric arteries. Menopause, 2010. 17(3): p. 516–23. [DOI] [PubMed] [Google Scholar]
- 42.Simeone SM, et al. Vascular gene expression in mice overexpressing human endothelin-1 targeted to the endothelium. Physiol Genomics, 2011. 43(3): p. 148–60. [DOI] [PubMed] [Google Scholar]
- 43.Tomobe Y, et al. Mechanisms of altered sensitivity to endothelin-1 between aortic smooth muscles of spontaneously hypertensive and Wistar-Kyoto rats. J Pharmacol Exp Ther, 1991. 257(2): p. 555–61. [PubMed] [Google Scholar]
- 44.Hao Q, et al. Curcumin Attenuates Angiotensin II-Induced Abdominal Aortic Aneurysm by Inhibition of Inflammatory Response and ERK Signaling Pathways. Evid Based Complement Alternat Med, 2014 2014: p. 270930. [DOI] [PMC free article] [PubMed]
- 45.Lu XT, et al. Chronic psychological stress induces vascular inflammation in rabbits. Stress, 201316(1): p. 87–98. [DOI] [PubMed] [Google Scholar]
- 46.Spinetti G, et al. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol, 2004. 24(8): p. 1397–402. [DOI] [PubMed] [Google Scholar]
- 47.Ferlosio A, et al. Age-related increase of stem marker expression influences vascular smooth muscle cell properties. Atherosclerosis, 2012. 224(1): p. 51–7. [DOI] [PubMed] [Google Scholar]
- 48.Lu P, et al. Role of TGF-beta1/Smad3 signaling pathway in secretion of type I and III collagen by vascular smooth muscle cells of rats undergoing balloon injury. J Biomed Biotechnol, 2012 2012: p. 965953. [DOI] [PMC free article] [PubMed]
- 49.Wang M, et al. Matrix metalloproteinase 2 activation of transforming growth factor-beta1 (TGF- beta1) and TGF-beta1-type II receptor signaling within the aged arterial wall. Arterioscler Thromb Vasc Biol, 2006. 26(7): p. 1503–9. [DOI] [PubMed] [Google Scholar]
- 50.Randell A and Daneshtalab N, Elastin microfibril interface-located protein 1, transforming growth factor beta, and implications on cardiovascular complications. J Am Soc Hypertens, 2017. 11(7): p. 437–448. [DOI] [PubMed] [Google Scholar]
- 51.Tang Y, et al. Mechanisms of TGF-beta-induced differentiation in human vascular smooth muscle cells. J Vasc Res, 2011. 48(6): p. 485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lozhkin A, et al. NADPH oxidase 4 regulates vascular inflammation in aging and atherosclerosis. J Mol Cell Cardiol, 2017. 102: p. 10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiasson VL, et al. Endothelial cell transforming growth factor-beta receptor activation causes tacrolimus-induced renal arteriolar hyalinosis. Kidney Int, 2012. 82(8): p. 857–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bai H, et al. Suppression of Transforming Growth Factor-beta Signaling Delays Cellular Senescence and Preserves the Function of Endothelial Cells Derived from Human Pluripotent Stem Cells. Stem Cells Transl Med, 2017. 6(2): p. 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang M, et al. A local proinflammatory signalling loop facilitates adverse age-associated arterial remodeling. PLoS One, 2011. 6(2): p. e16653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Z, et al. Increased expression of matrix metalloproteinase-2 in the thickened intima of aged rats. Hypertension, 1999. 33(1): p. 116–23. [DOI] [PubMed] [Google Scholar]
- 57.Spiers JP, et al. Alterations in vascular matrix metalloproteinase due to ageing and chronic hypertension: effects of endothelin receptor blockade. J Hypertens, 2005. 23(9): p. 1717–24. [DOI] [PubMed] [Google Scholar]
- 58.Wang M and Lakatta EG, Altered regulation of matrix metalloproteinase-2 in aortic remodeling during aging. Hypertension, 2002. 39(4): p. 865–73. [DOI] [PubMed] [Google Scholar]
- 59.Jiang L, et al. Calpain-1 regulation of matrix metalloproteinase 2 activity in vascular smooth muscle cells facilitates age-associated aortic wall calcification and fibrosis. Hypertension, 2012. 60(5): p. 1192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nabeshima Y, et al. Calpain 1 inhibitor BDA-410 ameliorates alpha-klotho-deficiency phenotypes resembling human aging-related syndromes. Sci Rep, 2014. 4: p. 5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davies HA, et al. Oxidative Stress Alters the Morphology and Toxicity of Aortic Medial Amyloid. Biophys J, 2015. 109(11): p. 2363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haggqvist B, et al. Medin: an integral fragment of aortic smooth muscle cell-produced lactadherin forms the most common human amyloid. Proc Natl Acad Sci U S A, 1999. 96(15): p. 8669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peng S, et al. Medin and medin-amyloid in ageing inflamed and non-inflamed temporal arteries. J Pathol, 2002. 196(1): p. 91–6. [DOI] [PubMed] [Google Scholar]
- 64.Peng S, Glennert J, and Westermark P, Medin-amyloid: a recently characterized age-associated arterial amyloid form affects mainly arteries in the upper part of the body. Amyloid, 2005. 12(2): p. 96–102. [DOI] [PubMed] [Google Scholar]
- 65.Larsson A, et al. Lactadherin binds to elastin--a starting point for medin amyloid formation? Amyloid, 2006. 13(2): p. 78–85. [DOI] [PubMed] [Google Scholar]
- 66.Brissette MJ, et al. MFG-E8 released by apoptotic endothelial cells triggers anti-inflammatory macrophage reprogramming. PLoS One, 2012. 7(4): p. e36368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thorp E and Tabas I, Mechanisms and consequences of efferocytosis in advanced atherosclerosis. J Leukoc Biol, 2009. 86(5): p. 1089–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ait-Oufella H, et al. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation, 2007. 115(16): p. 2168–77. [DOI] [PubMed] [Google Scholar]
- 69.Fleenor BS, et al. Curcumin ameliorates arterial dysfunction and oxidative stress with aging. Exp Gerontol, 2013. 48(2): p. 269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gong X, et al. Long-term atorvastatin improves age-related endothelial dysfunction by ameliorating oxidative stress and normalizing eNOS/iNOS imbalance in rat aorta. Exp Gerontol, 2014. 52C: p. 9–17. [DOI] [PubMed] [Google Scholar]
- 71.Roos CM, et al. Transcriptional and phenotypic changes in aorta and aortic valve with aging and MnSOD deficiency in mice. Am J Physiol Heart Circ Physiol, 2013. 305(10): p. H1428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lund DD, et al. Protective effect of extracellular superoxide dismutase on endothelial function during aging. Am J Physiol Heart Circ Physiol, 2009. 296(6): p. H1920–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rice KM, et al. Chronic paracetamol treatment influences indices of reactive oxygen species accumulation in the aging Fischer 344 X Brown Norway rat aorta. Ann Clin Lab Sci, 2012. 42(2): p. 152–61. [PubMed] [Google Scholar]
- 74.van der Loo B, et al. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med, 2000. 192(12): p. 1731–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang M, et al. Involvement of NADPH oxidase in age-associated cardiac remodeling. J Mol Cell Cardiol, 2010. 48(4): p. 765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barrett-O’Keefe Z, et al. Endothelin-A-mediated vasoconstriction during exercise with advancing age. J Gerontol A Biol Sci Med Sci, 2015. 70(5): p. 554–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wirth A, et al. Age-dependent blood pressure elevation is due to increased vascular smooth muscle tone mediated by G-protein signalling. Cardiovasc Res, 2016. 109(1): p. 131–40. [DOI] [PubMed] [Google Scholar]
- 78.Fleenor BS, et al. Arterial stiffening with ageing is associated with transforming growth factor- beta1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol, 2010. 588(Pt 20): p. 3971–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vendrov AE, et al. NOX4 NADPH Oxidase-Dependent Mitochondrial Oxidative Stress in Aging- Associated Cardiovascular Disease. Antioxid Redox Signal, 2015. 23(18): p. 1389–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mikhed Y, Daiber A, and Steven S, Mitochondrial Oxidative Stress, Mitochondrial DNA Damage and Their Role in Age-Related Vascular Dysfunction. Int J Mol Sci, 2015. 16(7): p. 15918–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim MY, et al. The PPARdelta-mediated inhibition of angiotensin II-induced premature senescence in human endothelial cells is SIRT1-dependent. Biochem Pharmacol, 2012. 84(12): p. 1627–34. [DOI] [PubMed] [Google Scholar]
- 82.Craige SE and Keaney JF Jr., Polyploidy and dysregulated ROS signaling: the school of hard Nox? Cell Cycle, 2009. 8(6): p. 797. [DOI] [PubMed] [Google Scholar]
- 83.Zarzuelo MJ, et al. SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: implications for vascular aging. Biochem Pharmacol, 2013. 85(9): p. 1288–96. [DOI] [PubMed] [Google Scholar]
- 84.Santhanam L, et al. Decreased S-nitrosylation of tissue transglutaminase contributes to age- related increases in vascular stiffness. Circ Res, 2010. 107(1): p. 117–25. [DOI] [PubMed] [Google Scholar]
- 85.Roos CM, et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell, 2016. 15(5): p. 973–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Donato AJ, et al. Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell, 2013. 12(5): p. 772–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Donato AJ, et al. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J Physiol, 2011. 589(Pt 18): p. 4545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Novella S, et al. Aging-related endothelial dysfunction in the aorta from female senescence- accelerated mice is associated with decreased nitric oxide synthase expression. Exp Gerontol, 2013. 48(11): p. 1329–37. [DOI] [PubMed] [Google Scholar]
- 89.Steppan J, et al. Exercise, vascular stiffness, and tissue transglutaminase. J Am Heart Assoc, 2014. 3(2): p. e000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hezel M, et al. Dietary nitrate improves age-related hypertension and metabolic abnormalities in rats via modulation of angiotensin II receptor signaling and inhibition of superoxide generation. Free Radic Biol Med, 2016. 99: p. 87–98. [DOI] [PubMed] [Google Scholar]
- 91.Alghasham A, et al. Impact of anti-peroxynitrite-damaged-thymidine-monophosphate antibodies on disease activity in patients with systemic lupus erythematosus. Nucleosides Nucleotides Nucleic Acids, 2015. 34(1): p. 56–68. [DOI] [PubMed] [Google Scholar]
- 92.Csiszar A, et al. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci, 2012. 67(8): p. 811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Donato AJ, et al. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res, 2007. 100(11): p. 1659–66. [DOI] [PubMed] [Google Scholar]
- 94.Donato AJ, et al. Aging is associated with greater nuclear NF kappa B, reduced I kappa B alpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell, 2008. 7(6): p. 805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ungvari Z, et al. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-{kappa}B activation in the nonhuman primate Macaca mulatta. J Gerontol A Biol Sci Med Sci, 2011. 66(8): p. 866–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tang Y, et al. Resveratrol reduces vascular cell senescence through attenuation of oxidative stress by SIRT1/NADPH oxidase-dependent mechanisms. J Nutr Biochem, 2012. 23(11): p. 1410–6. [DOI] [PubMed] [Google Scholar]
- 97.Thompson AM, Wagner R, and Rzucidlo EM, Age-related loss of SirT1 expression results in dysregulated human vascular smooth muscle cell function. Am J Physiol Heart Circ Physiol, 2014. 307(4): p. H533–41. [DOI] [PubMed] [Google Scholar]
- 98.Badi I, et al. MicroRNA-34a Induces Vascular Smooth Muscle Cells Senescence by SIRT1 Downregulation and Promotes the Expression of Age-Associated Pro-inflammatory Secretory Factors. J Gerontol A Biol Sci Med Sci, 2015. 70(11): p. 1304–11. [DOI] [PubMed] [Google Scholar]
- 99.Bochenek ML, Schutz E, and Schafer K, Endothelial cell senescence and thrombosis: Ageing clots. Thromb Res, 2016. 147: p. 36–45. [DOI] [PubMed] [Google Scholar]
- 100.Herbert KE, et al. Angiotensin II-mediated oxidative DNA damage accelerates cellular senescence in cultured human vascular smooth muscle cells via telomere-dependent and independent pathways. Circ Res, 2008. 102(2): p. 201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shan H, et al. From autophagy to senescence and apoptosis in Angiotensin II-treated vascular endothelial cells. APMIS, 2014. 122(10): p. 985–92. [DOI] [PubMed] [Google Scholar]
- 102.Shan H, Bai X, and Chen X, Angiotensin II induces endothelial cell senescence via the activation of mitogen-activated protein kinases. Cell Biochem Funct, 2008. 26(4): p. 459–66. [DOI] [PubMed] [Google Scholar]
- 103.Tanaka Y, Moritoh Y, and Miwa N, Age-dependent telomere-shortening is repressed by phosphorylated alpha-tocopherol together with cellular longevity and intracellular oxidative- stress reduction in human brain microvascular endotheliocytes. J Cell Biochem, 2007. 102(3): p. 689–703. [DOI] [PubMed] [Google Scholar]
- 104.Chang E and Harley CB, Telomere length and replicative aging in human vascular tissues. Proc Natl Acad Sci U S A, 1995. 92(24): p. 11190–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Beyer AM, et al. Critical Role for Telomerase in the Mechanism of Flow-Mediated Dilation in the Human Microcirculation. Circ Res, 2016. 118(5): p. 856–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gardner SE, et al. Senescent Vascular Smooth Muscle Cells Drive Inflammation Through an Interleukin-1alpha-Dependent Senescence-Associated Secretory Phenotype. Arterioscler Thromb Vasc Biol, 2015. 35(9): p. 1963–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kinoshita D, et al. Progerin impairs vascular smooth muscle cell growth via the DNA damage response pathway. Oncotarget, 2017. 8(21): p. 34045–34056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Uryga AK and Bennett MR, Ageing induced vascular smooth muscle cell senescence in atherosclerosis. J Physiol, 2016. 594(8): p. 2115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang J, et al. Vascular Smooth Muscle Cell Senescence Promotes Atherosclerosis and Features of Plaque Vulnerability. Circulation, 2015. 132(20): p. 1909–19. [DOI] [PubMed] [Google Scholar]
- 110.Watson A, et al. Nicotinamide Phosphoribosyltransferase in Smooth Muscle Cells Maintains Genome Integrity, Resists Aortic Medial Degeneration, and Is Suppressed in Human Thoracic Aortic Aneurysm Disease. Circ Res, 2017. 120(12): p. 1889–1902. [DOI] [PubMed] [Google Scholar]
- 111.McCrann DJ, et al. Upregulation of Nox4 in the aging vasculature and its association with smooth muscle cell polyploidy. Cell Cycle, 2009. 8(6): p. 902–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mistry Y, et al. A role for mitochondrial oxidants in stress-induced premature senescence of human vascular smooth muscle cells. Redox Biol, 2013. 1(1): p. 411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Porter LJ, et al. Prelamin A Accumulation Attenuates Rac1 Activity and Increases the Intrinsic Migrational Persistence of Aged Vascular Smooth Muscle Cells. Cells, 2016. 5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bennett MR, Sinha S, and Owens GK, Vascular Smooth Muscle Cells in Atherosclerosis. Circ Res, 2016. 118(4): p. 692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang M, et al. MFG-E8 activates proliferation of vascular smooth muscle cells via integrin signaling. Aging Cell, 2012. 11(3): p. 500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lakatta EG, Wang M, and Najjar SS, Arterial aging and subclinical arterial disease are fundamentally intertwined at macroscopic and molecular levels. Med Clin North Am, 2009. 93(3): p. 583–604, Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Humphrey JD, Dufresne ER, and Schwartz MA, Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol, 2014. 15(12): p. 802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sehgel NL, Vatner SF, and Meininger GA, “Smooth Muscle Cell Stiffness Syndrome”-Revisiting the Structural Basis of Arterial Stiffness. Front Physiol, 2015. 6: p. 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Qiu H, et al. Short communication: vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ Res, 2010. 107(5): p. 615–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Langlois B, et al. Vimentin knockout results in increased expression of sub-endothelial basement membrane components and carotid stiffness in mice. Sci Rep, 2017. 7(1): p. 11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Galmiche G, et al. Inactivation of serum response factor contributes to decrease vascular muscular tone and arterial stiffness in mice. Circ Res, 2013. 112(7): p. 1035–45. [DOI] [PubMed] [Google Scholar]
- 122.Zhu W, et al. A new twist to the primitive mechanical fate identifies an aged-associated TGFβ1 nexus in vascular smooth muscle cells. The FASEB Journal, 2016. 30(1 Supplement): p. 1300.3–1300.3.26631482 [Google Scholar]
- 123.Steppan J, et al. Tissue Transglutaminase Modulates Vascular Stiffness and Function Through Crosslinking-Dependent and Crosslinking-Independent Functions. J Am Heart Assoc, 2017. 6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dinardo CL, et al. Variation of mechanical properties and quantitative proteomics of VSMC along the arterial tree. Am J Physiol Heart Circ Physiol, 2014. 306(4): p. H505–16. [DOI] [PubMed] [Google Scholar]
- 125.Sehgel NL, et al. Augmented vascular smooth muscle cell stiffness and adhesion when hypertension is superimposed on aging. Hypertension, 2015. 65(2): p. 370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chatterjee S, et al. Mechanosignaling in the vasculature: emerging concepts in sensing, transduction and physiological responses. Am J Physiol Heart Circ Physiol, 2015. 308(12): p. H1451–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Burton DG, Matsubara H, and Ikeda K, Pathophysiology of vascular calcification: Pivotal role of cellular senescence in vascular smooth muscle cells. Exp Gerontol, 2010. 45(11): p. 819–24. [DOI] [PubMed] [Google Scholar]
- 128.Cattell MA, Anderson JC, and Hasleton PS, Age-related changes in amounts and concentrations of collagen and elastin in normotensive human thoracic aorta. Clin Chim Acta, 1996. 245(1): p. 73–84. [DOI] [PubMed] [Google Scholar]
- 129.Taghizadeh H and Tafazzoli-Shadpour M, Characterization of mechanical properties of lamellar structure of the aortic wall: Effect of aging. J Mech Behav Biomed Mater, 2016. 65: p. 20–28. [DOI] [PubMed] [Google Scholar]
- 130.Antonicelli F, et al. Elastin-elastases and inflamm-aging. Curr Top Dev Biol, 2007. 79: p. 99–155. [DOI] [PubMed] [Google Scholar]
- 131.Maurice P, et al. Elastin fragmentation and atherosclerosis progression: the elastokine concept. Trends Cardiovasc Med, 2013. 23(6): p. 211–21. [DOI] [PubMed] [Google Scholar]
- 132.Duca L, et al. Matrix ageing and vascular impacts: focus on elastin fragmentation. Cardiovasc Res, 2016. 110(3): p. 298–308. [DOI] [PubMed] [Google Scholar]
- 133.Fritze O, et al. Age-related changes in the elastic tissue of the human aorta. J Vasc Res, 2012. 49(1): p. 77–86. [DOI] [PubMed] [Google Scholar]
- 134.Ferruzzi J, et al. Loss of Elastic Fiber Integrity Compromises Common Carotid Artery Function: Implications for Vascular Aging. Artery Res, 2016. 14: p. 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Joly L, et al. Influence of Thoracic Aortic Inflammation and Calcifications on Arterial Stiffness and Cardiac Function in Older Subjects. J Nutr Health Aging, 2016. 20(3): p. 347–54. [DOI] [PubMed] [Google Scholar]
- 136.Derlin T, et al. Age-related differences in the activity of arterial mineral deposition and regional bone metabolism: a 18F-sodium fluoride positron emission tomography study. Osteoporos Int, 2015. 26(1): p. 199–207. [DOI] [PubMed] [Google Scholar]
- 137.Lee SH, et al. Coronary Calcification Is Reversely Related with Bone and Hair Calcium: The Relationship among Different Calcium Pools in Body. J Bone Metab, 2016. 23(4): p. 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ouyang L, et al. Roles of platelet-derived growth factor in vascular calcification. J Cell Physiol, 2017. [DOI] [PubMed]
- 139.Semba RD, et al. Serum carboxymethyl-lysine, an advanced glycation end product, is associated with arterial stiffness in older adults. J Hypertens, 2015. 33(4): p. 797–803; discussion 803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang Y, et al. Effect of glucose on the biomechanical function of arterial elastin. J Mech Behav Biomed Mater, 2015. 49: p. 244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lesniewski LA, et al. Aerobic exercise reverses arterial inflammation with aging in mice. Am J Physiol Heart Circ Physiol, 2011. 301(3): p. H1025–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Al Mheid I, et al. Age and Human Regenerative Capacity Impact of Cardiovascular Risk Factors. Circ Res, 2016. 119(7): p. 801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.AlGhatrif M, et al. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension, 2013. 62(5): p. 934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fortier C, et al. Aortic-Brachial Pulse Wave Velocity Ratio: A Blood Pressure-Independent Index of Vascular Aging. Hypertension, 2017. 69(1): p. 96–101. [DOI] [PubMed] [Google Scholar]
- 145.Furman D, et al. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat Med, 2017. 23(2): p. 174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Humphrey JD and Milewicz DM, Aging, Smooth Muscle Vitality, and Aortic Integrity. Circ Res, 2017. 120(12): p. 1849–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mitchell GF, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility--Reykjavik study. Brain, 2011. 134(Pt 11): p. 3398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Paneni F, et al. The Aging Cardiovascular System: Understanding It at the Cellular and Clinical Levels. J Am Coll Cardiol, 2017. 69(15): p. 1952–1967. [DOI] [PubMed] [Google Scholar]
- 149.Whitlock MC and Hundley WG, Noninvasive Imaging of Flow and Vascular Function in Disease of the Aorta. JACC Cardiovasc Imaging, 2015. 8(9): p. 1094–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Virmani R, et al. Effect of aging on aortic morphology in populations with high and low prevalence of hypertension and atherosclerosis. Comparison between occidental and Chinese communities. Am J Pathol, 1991. 139(5): p. 1119–29. [PMC free article] [PubMed] [Google Scholar]
- 151.Niiranen TJ, et al. Aortic-Brachial Arterial Stiffness Gradient and Cardiovascular Risk in the Community: The Framingham Heart Study. Hypertension, 2017. 69(6): p. 1022–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Raghu R, et al. Comparative study of viscoelastic arterial wall models in nonlinear one- dimensional finite element simulations of blood flow. J Biomech Eng, 2011. 133(8): p. 081003. [DOI] [PubMed] [Google Scholar]
- 153.Majesky MW, et al. Vascular smooth muscle progenitor cells: building and repairing blood vessels. Circ Res, 2011. 108(3): p. 365–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yin H and Pickering JG, Cellular Senescence and Vascular Disease: Novel Routes to Better Understanding and Therapy. Can J Cardiol, 2016. 32(5): p. 612–23. [DOI] [PubMed] [Google Scholar]
- 155.Yeh HI, et al. Age-related alteration of gap junction distribution and connexin expression in rat aortic endothelium. J Histochem Cytochem, 2000. 48(10): p. 1377–89. [DOI] [PubMed] [Google Scholar]
- 156.Du W, et al. Age-associated vascular inflammation promotes monocytosis during atherogenesis. Aging Cell, 2016. 15(4): p. 766–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Guzik TJ and Cosentino F, Epigenetics and Immunometabolism in Diabetes and Aging. Antioxid Redox Signal, 2017. [DOI] [PMC free article] [PubMed]
- 158.Nogueira-Neto J, et al. Basal neutrophil function in human aging: Implications in endothelial cell adhesion. Cell Biol Int, 2016. 40(7): p. 796–802. [DOI] [PubMed] [Google Scholar]
- 159.Joffre J, et al. Genetic and Pharmacological Inhibition of TREM-1 Limits the Development of Experimental Atherosclerosis. J Am Coll Cardiol, 2016. 68(25): p. 2776–2793. [DOI] [PubMed] [Google Scholar]
- 160.Niiranen TJ, et al. Prevalence, Correlates, and Prognosis of Healthy Vascular Aging in a Western Community-Dwelling Cohort: The Framingham Heart Study. Hypertension, 2017. 70(2): p. 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sazonova OV, et al. Extracellular matrix presentation modulates vascular smooth muscle cell mechanotransduction. Matrix Biol, 2015. 41: p. 36–43. [DOI] [PubMed] [Google Scholar]
- 162.Tan I, et al. Effect of Heart Rate on Arterial Stiffness as Assessed by Pulse Wave Velocity. Curr Hypertens Rev, 2017. [DOI] [PubMed]
- 163.Xiao H, et al. Arterial viscoelasticity: role in the dependency of pulse wave velocity on heart rate in conduit arteries. Am J Physiol Heart Circ Physiol, 2017. 312(6): p. H1185–H1194. [DOI] [PubMed] [Google Scholar]
- 164.Mattison JA, et al. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab, 2014. 20(1): p. 183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Najjar SS, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol, 2008. 51(14): p. 1377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Franklin SS, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation, 1997. 96(1): p. 308–15. [DOI] [PubMed] [Google Scholar]
- 167.Malavolta M, et al. Pleiotropic Effects of Tocotrienols and Quercetin on Cellular Senescence: Introducing the Perspective of Senolytic Effects of Phytochemicals. Curr Drug Targets, 2016. 17(4): p. 447–59. [DOI] [PubMed] [Google Scholar]
- 168.Maria J and Ingrid Z, Effects of bioactive compounds on senescence and components of senescence associated secretory phenotypes in vitro. Food Funct, 2017. 8(7): p. 2394–2418. [DOI] [PubMed] [Google Scholar]
- 169.Schmitt R, Senotherapy: growing old and staying young? Pflugers Arch, 2017. [DOI] [PubMed]
- 170.Mattison JA, et al. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun, 2017. 8: p. 14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mattison JA, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature, 2012. 489(7415): p. 318–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Weiss EP and Fontana L, Caloric restriction: powerful protection for the aging heart and vasculature. Am J Physiol Heart Circ Physiol, 2011. 301(4): p. H1205–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rossman MJ, et al. Endothelial Cell Senescence with Aging in Healthy Humans: Prevention by Habitual Exercise and Relation to Vascular Endothelial Function. Am J Physiol Heart Circ Physiol, 2017: p. ajpheart 00416 2017. [DOI] [PMC free article] [PubMed]
- 174.Gauche R, et al. Blood pressure reactivity to mental stress is attenuated following resistance exercise in older hypertensive women. Clin Interv Aging, 2017. 12: p. 793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhu Y, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell, 2015. 14(4): p. 644–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Childs BG, et al. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science, 2016. 354(6311): p. 472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jeon OH, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med, 2017. 23(6): p. 775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Huang W, Alhenc Gelas F, and Osborne-Pellegrin MJ, Protection of the arterial internal elastic lamina by inhibition of the renin-angiotensin system in the rat. Circ Res, 1998. 82(8): p. 879–90. [DOI] [PubMed] [Google Scholar]
- 179.Wang M, et al. Method for the diagnosis of age-associated vascular disorders 2010, Google Patents. [Google Scholar]
- 180.Baur JA, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature, 2006. 444(7117): p. 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lesniewski LA, et al. Dietary rapamycin supplementation reverses age-related vascular dysfunction and oxidative stress, while modulating nutrient-sensing, cell cycle, and senescence pathways. Aging Cell, 2017. 16(1): p. 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Xu X, et al. Metformin protects against systolic overload-induced heart failure independent of AMP-activated protein kinase alpha2. Hypertension, 2014. 63(4): p. 723–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


