Abstract
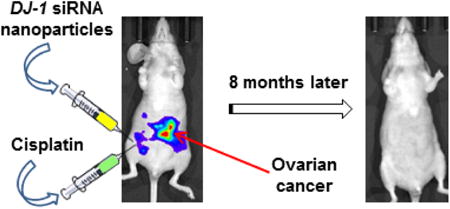
Herein, we report an efficient combinatorial therapy for metastatic ovarian cancer based on siRNA-mediated suppression of DJ-1 protein combined with a low dose of cisplatin. DJ-1 protein modulates, either directly or indirectly, different oncogenic pathways that support and promote survival, growth, and invasion of ovarian cancer cells. To evaluate the potential of this novel therapy, we have engineered a cancer-targeted nanoplatform and validated that DJ-1 siRNA delivered by this nanoplatform after intraperitoneal injection efficiently downregulates the DJ-1 protein in metastatic ovarian cancer tumors and ascites. In vivo experiments revealed that DJ-1 siRNA monotherapy outperformed cisplatin alone by inhibiting tumor growth and increasing survival of mice with metastatic ovarian cancer. Finally, three cycles of siRNA-mediated DJ-1 therapy in combination with a low dose of cisplatin completely eradicated ovarian cancer tumors from the mice, and there was no cancer recurrence detected for the duration of the study, which lasted 35 weeks.
Keywords: ovarian cancer, DJ-1, cisplatin, combinatorial therapy, siRNA
Graphical abstract
We have developed and validated combinatorial treatment for metastatic ovarian cancer which consists of two monotherapies administered sequentially via intraperitoneal route for three cycles: (1) siRNA-loaded nanoparticles aimed to suppress DJ-1 protein, and (2) the chemotherapy agent, cisplatin. DJ-1 suppression by siRNA can simultaneously interfere with the expression of multiple oncogenic proteins responsible for ovarian cancer cells survival, migration, proliferation and resistance to platinum-based drugs. The developed therapy eradicated metastatic ovarian cancer tumors from the mice, and there was no cancer recurrence detected during the duration of the study.
Introduction
Ovarian cancer is the deadliest gynecologic malignancy.1 The high mortality of ovarian cancer stems from its unique metastatic behavior not shared with other cancers. Following exfoliation at primary tumor sites, ovarian cancer cells are disseminated within the abdominal cavity via a peritoneal fluid.2, 3 As a result, malignant cells encase the reproductive organs, bladder, sigmoid colon, and omentum, leading to the accumulation of abdominal ascites and severe pain.3 The standard therapy for patients with metastatic ovarian cancer consists of primary debulking surgery followed by platinum-taxane based chemotherapy.1, 4 Due to the diffuse nature of ovarian cancer, surgery rarely enables the complete removal of all metastases, and the incorporation of chemotherapy is of utmost importance to eliminate any residual tumors.4, 5 The standard chemotherapy regimen for ovarian cancer is a combination of two or more platinum and taxane drugs administered intravenously (IV) or intraperitoneally (IP) for three to six cycles.6 Application of multiple chemotherapeutic agents allows the oncologists to target a variety of oncogenic pathways that promote growth and dissemination of primary tumor in the form of metastases. Clinical studies revealed that IP chemotherapy is more efficient than IV chemotherapy, but it also causes more deleterious side effects making some patients discontinue their treatment before its completion.7 Enhanced therapeutic efficacy and the severe side effects that encompass IP chemotherapy are due to the high concentrations of the chemotherapeutic agents that can be achieved within the peritoneal cavity.7 Therefore, there is an urgent need to develop novel IP therapies that allow for a reduction in the number of cycles and doses of chemotherapeutic drugs used in the treatment of ovarian cancer, while improving treatment outcomes and efficacy. Identification and suppression of a single multifunctional protein, responsible for malignant proliferation, invasion, chemoresistance, and overall cellular survival, is a promising therapeutic avenue that would satisfy all the aforementioned needs.
DJ-1 is a multifunctional protein that has recently been implicated as a key oncogenic driver and biomarker for various cancers, including ovarian cancer.8, 9 Previous studies revealed that DJ-1 is expressed in more than 80% of cases of advanced ovarian carcinoma at all anatomical sites.10 It was also documented that increased DJ-1 expression positively correlates with both lower survival in ovarian cancer patients and enhanced the resistance of ovarian cancer cells to platinum-based drugs.11-13 DJ-1 has been shown to modulate (either directly or indirectly) various cellular pathways that support and promote survival, growth, and invasion of cancer cells.12,13 It is known that DJ-1 binds to and downregulates the activity of phosphatase and tensin homolog (PTEN), a protein that functions as a biological brake for Akt pathways, thus increasing the growth, proliferation, and migration of tumor cells.8, 14, 15 Furthermore, DJ-1 directly inhibits tumor suppressor protein 53 (p53), a cell cycle regulation protein responsible for cell cycle arrest.16 DJ-1 enhances the activity of NRF2 - an antioxidant protein that, when translocated to the nucleus, upregulates a cohort of factors responsible for modulating the cellular redox homeostasis in response to elevated levels of intracellular reactive oxygen species (ROS).17-19 Finally, DJ-1 upregulates glutamate cysteine ligase - the rate-limiting enzyme in glutathione synthesis further increasing cellular antioxidant defenses.20
We have recently demonstrated in vitro that DJ-1 is highly overexpressed in ovarian cancer cells and its siRNA-mediated suppression substantially decreases cell proliferation, viability, and migration.11 Moreover, we discovered that DJ-1 suppression in combination with a low dose of cisplatin provides a superior therapeutic response in the studied ovarian cancer cell lines.11 Therefore, we hypothesized that novel IP therapy based on siRNA-mediated DJ-1 suppression combined with low doses of cisplatin could provide a promising treatment modality for metastatic ovarian cancer. Herein, we report the first use of DJ-1 suppression as a therapeutic approach for the treatment of metastatic ovarian cancer.
Methods
Development of a murine model for metastatic human ovarian cancer
Animal studies were performed according to the Humane Care and Use of Laboratory Animals Policy and were approved by Institutional Animal Care and Use Committee of Oregon Health and Science University. Experiments were carried out on female Nu/Nu Nude mice bearing intraperitoneal xenograft of luciferase-expressing ES-2 (ES-2-luc) human ovarian cancer cells (Supplementary materials).
Synthesis and characterization of a nanoplatform for siRNA delivery
The LHRH-targeted nanoplatform for DJ-1 siRNA delivery was prepared and characterized according to our developed procedures (Supplementary Materials).11, 19, 21
Evaluation of the nanoplatform efficiency to suppress the targeted protein in vivo
Three weeks following ES-2-luc inoculation, five mice were IP injected twice per week (Tuesday and Friday) with 0.5 mL of nanoparticles loaded with DJ-1 siRNA at a 50 μM concentration. In the control group, five mice were injected with saline. 24 h after the second injection, mice were euthanized, and both solid tumors and ascites fluid were collected. A portion of the solid tumors was digested using a tissue homogenizer in 250 μL of RIPA buffer. The ascites cells were centrifuged at 3,500 rpm for 3 min, and the cell pellet resuspended in 250 μL of RIPA buffer. The immunoblots were performed according to our previously published protocol.11, 19
Animals dosing regimen
The control and cisplatin monotherapy groups were IP dosed once a week at the beginning of each week (Monday). The control group was given a normal saline injection at a volume of 1 mL. The cisplatin monotherapy group was given an IP injection of cisplatin at a concentration of 0.05 mg (1.85 mg/kg), in a volume of 1 mL. Finally, the DJ-1 monotherapy group was IP dosed twice per week (Tuesday and Friday) with nanoparticles at a 50 μM siRNA concentration in a volume of 0.5 mL. For the combinatorial treatment group, the cisplatin and DJ-1 monotherapy groups dosing regimens were combined. All treatment groups were given their respective therapies for a total of 3 weeks.
Statistical Analysis
The data were analyzed using descriptive statistics and presented as mean values ± standard deviation (SD) from 3-6 independent measurements. The comparison among groups was performed by the independent sample Student's t-test. The difference between variants was considered significant at p < 0.05. Kaplan-Meier estimator curves and median survival times were performed using “survfit” in the “survival” R package. Log-Rank-Test for differences between groups was performed using “survdiff” in “survival.” Hazard ratios for treatments and overall Likelihood Ratio Test were calculated using “coxph” in “survival.” The proportional hazards assumptions were confirmed using the Z:ph test “cox.zph” in “survival.”
Results
Development and characterization of a murine model for metastatic human ovarian cancer
To validate the therapeutic efficacy of the novel IP therapy, we established a murine model of metastatic ovarian cancer by inoculating ES-2 human ovarian clear cell carcinoma cells, into the peritoneal cavity of nude mice. Cancer progression in the mouse's body was monitored by recording the bioluminescence signal generated by ES-2 cells that are stably transfected with a luciferase reporter gene (Figure 1 A-D). ES-2 cells were selected based on five key intrinsic features required to rigorously evaluate the efficacy of the proposed treatment on an aggressive ovarian metastatic cancer model.
Figure 1.
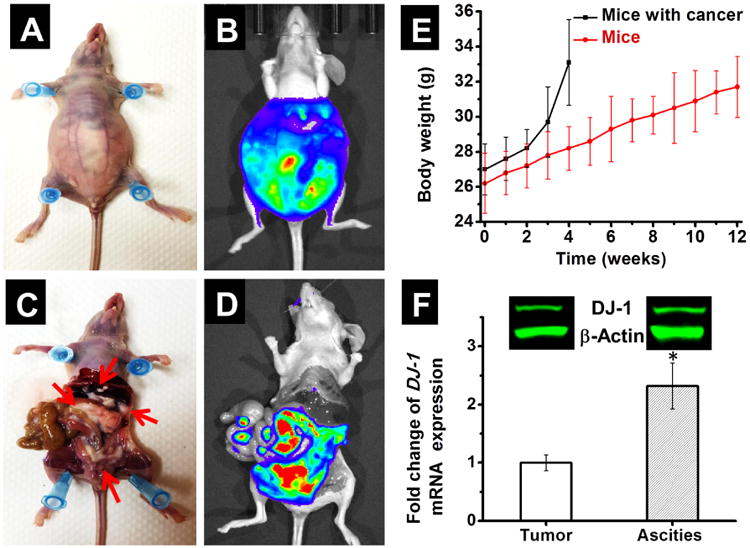
Murine model of metastatic ovarian cancer. Representative photographs (A and C) and bioluminescence images (B and D) of a mouse 4 weeks after IP injection with ES-2-luc ovarian cancer cells. (C) Arrows indicate cancer tissues. (E) Changes in body weight of mice injected with ES-2-luc cells (black line) when compared to mice without cancer (red line). (F) Basal levels of DJ-1 mRNA in solid ovarian cancer tumors and ascites obtained from mice 4 weeks post ES-2-luc cells inoculation. The intracellular level of DJ-1 mRNA in solid tumors was set to 1. *p < 0.05 when compared with solid tumors. Inset: Representative western blot images of DJ-1 protein and β-actin expression in solid cancer tumors and ascites.
First, ES-2 cells are classified as an ovarian clear cell carcinoma, which is an aggressive subtype of epithelial ovarian cancer (EOC) characterized by poor response to platinum-based drugs, high recurrence, and lower survival rates when compared to other types of EOC.22, 23 Therefore, novel treatment modalities such as the developed therapy are highly warranted to increase the survival rate of patients with advanced ovarian clear cell carcinoma.23
Second, ES-2 cells have a rapid growth rate, with a doubling time of ∼19 hours.11, 24 Consequently, they could be employed to develop an animal model of aggressive ovarian cancer that mimics the abdominal metastasis and ascites formation observed in patients with stage III and IV ovarian cancer. Our studies validated the aggressive nature of ES-2 cells inoculated into the peritoneal cavity of mice. The cancer's dissemination within the peritoneal cavity was rapid and mice had to be euthanized at the end of week 4 following ES-2 cell injection due to lethargy and mobility issues caused by a dramatic accumulation of ascites fluid leading to a marked increase in body weight and visible external tumors protruding from the lower abdomen (Figure 1A-E). Peritoneal lavage was performed in euthanized mice to evaluate the dissemination of the solid tumors. By using bioluminescence imaging and visual techniques, we confirmed that tumor nodules were disseminated widely through the peritoneal cavity and attached to various organs including liver, colon and gastrointestinal tract (Figure 1 C and D). Of note, the average volume of ascites fluid was about 5 mL per animal.
Third, we have previously demonstrated that ES-2 cells overexpress the DJ-1 protein, a main target of the proposed therapy.11 In the current study, DJ-1 overexpression has also been confirmed in both solid tumors and the cells collected from the ascites fluid by qPCR and immunoblot analyses (Figure 1F). In addition, the basal level of DJ-1 mRNA was 2.3 times higher in ascites relative to solid tumors. To the best of our knowledge, this is the first report demonstrating differential expression of DJ-1 in solid tumors and malignant ascites. This correlation could be related to the fact that malignant ascites-derived cells exhibit enhanced resistance to chemotherapies, and increased metastatic potential.25 Thus, differential expression of key oncogenic drivers such as DJ-1 is expected. Similarly, Mo et al. demonstrated that expression of the ABC transporters (MDR1, MRP1, and BCRP) associated with chemoresistance was significantly higher in ascites-derived human ovarian cancer cells when compared to cells derived from primary tumor.26
Fourth, ES-2 cells exhibit moderate resistance to platinum chemotherapeutic agents.11, 24 Platinum resistance is of an immense clinical importance as it has been reported that a majority of patients with cancer relapse show either a decreased sensitivity or complete resistance to platinum chemotherapy.27
Fifth, ES-2 cells have been shown to overexpress the luteinizing hormone releasing hormone receptor (LHRHR) used for growth and proliferation.28 Therefore, our nanoparticles, equipped with the targeting LHRH peptide, can be used to efficiently deliver DJ-1 siRNA to ES-2 tumors after IP administration.11 LHRHR are expressed in about 80% of endometrial and ovarian cancers, as well as other cancers.29-33 Shah et al. demonstrated that metastatic and primary tumors collected from advanced ovarian carcinoma patients as well as cancer cells isolated from malignant ascites overexpressed LHRHR, while expression of this receptor in other organs was minimal or undetectable.34 We anticipate the LHRH-targeted nanoplatform prepared and evaluated in the present study can be potentially extended to a variety of ovarian tumors.
Synthesis and characterization of a nanoplatform for siRNA delivery
The instability in blood and low accumulation in the targeted tissue after systemic administration and limited internalization into cellular cytoplasm are the major issues preventing the application of siRNA for the developed therapeutic approach.35 Therefore, we have engineered a delivery system for the transportation of the DJ-1 siRNA to the ovarian cancer tumors after IP administration (Figure 2). The core of the system is based on the nanoparticles formed as a result of the spontaneous electrostatic complexation between the DJ-1 siRNA and generation 4 PPI dendrimer (PPI G4).36 The positively charged primary amines of the PPI G4 interact with the negatively charged phosphate groups of the siRNA's backbone (Figure 2A). An excess of the dendrimer provides encapsulation of siRNA inside of nanoparticles, which in turn protects the siRNA against Ribonuclease-mediated degradation in the serum and facilitates cellular uptake.21 The gel retardation assay validated the complete complexation of the siRNA by PPI G4 at nitrogen to phosphate (N/P) ratio of 2 (Figure S1), indicating that the resulting nanoparticles contain 0.38 nanomoles of siRNA per 1 nanomole of PPI G4. According to DLS analysis, the formed nanoparticles featured an average hydrodynamic diameter of 100.8 ± 15.5 nm (Figure S2) and a narrow size distribution (polydispersity index (PDI) = 0.13 ± 0.07). The protonated amines in the dendrimer structure, which are important for siRNA complexation, introduce positive charge onto the surface of nanoparticles (+28.0 ± 4.1 mV). A positively charged surface could promote nanoparticle aggregation in the blood, due to electrostatic association with negatively charged serum proteins.37, 38 To avoid this, we have modified the surface of nanoparticles with polyethylene glycol (PEG), which contains a thiol-reactive maleimide and amine-reactive NHS ester on the opposite ends.21 The modification has been carried out by coupling NHS groups of PEG to amino groups on the nanoparticle surface via amide bonds (Figure 2B). Finally, the LHRH peptide was conjugated to the distal end of the PEG moiety by coupling its MAL group to a thiol group (SH) presented in the peptide structure (Figure 2C).21 The presence of LHRH peptide on the nanoparticles surface was confirmed by Bicinchoninic acid protein assay according to the manufacturer's protocol and our published procedure.21 The obtained results revealed that the nanoparticles loaded with 50 nanomoles of siRNA are modified with 1.6 μmoles of the LHRH peptide. The resulting nanoparticles have a hydrodynamic diameter of 145.2 ± 9.1 nm with a PDI of 0.18 ± 0.02 (Figure S2), and slightly positive surface charge (+7.7 ± 1.6 mV).
Figure 2.

Preparation of siRNA-loaded nanoplatform. (A) Complexation of negatively charged siRNA by positively charged PPI G4 dendrimers into nanoparticles. (B) Modification of the siRNA-loaded nanoparticles by conjugation of NHS-activated PEG to dendrimer amino groups on the nanoparticle surface via an amide bond. (C) Conjugation of LHRH peptide to the distal end of PEG layer through the maleimide groups on PEG and the thiol groups in LHRH.
Since premature release of siRNA molecules from the delivery system following IP administration could compromise their therapeutic efficacy, we evaluated the stability of nanoparticles in terms of siRNA release in ascitic fluid. The obtained data demonstrates that less than 10% of encapsulated siRNA molecules were released within 48 h (Figure S3).
We evaluated the tumor targeting capability of both non- and LHRH-modified nanoparticles following their IP administration into mice with intraperitoneal xenografts of ovarian cancer (Figure 3A-D). The IVIS imaging system (PerkinElmer, Waltham, MA, USA) was used to monitor bioluminescence representing dissemination of luciferase-expressing cancer cells as well as NIR fluorescence generated by the Cy5.5-labeled siRNA. The recorded fluorescence images revealed that siRNA molecules delivered by both non- and LHRH-targeted nanoparticles were distributed in the abdominal cavities 24 h after IP injection (Figure 3B and D). Furthermore, comparison of bioluminescence and fluorescence images indicates a strong overlap between bioluminescence and fluorescence signals, generated by cancer cells and Cy5.5-labeled siRNA delivered with LHRH-targeted nanoparticles, respectively (Figure 3C and D). Quantitative analysis confirmed that fluorescence intensity of Cy5.5-labeled siRNA overlapped with bioluminescence was 2.3 times higher in case of LHRH-targeted nanoparticles when compared to non-targeted (PEG-modified) ones, suggesting that LHRH peptide molecules enhance accumulation of the nanoparticles in the cancer tumors. The obtained in vivo data is in good agreement with our published in vitro results that demonstrated the ability of LHRH peptide to improve internalization of the nanoparticles into the ovarian cancer cells.11
Figure 3.
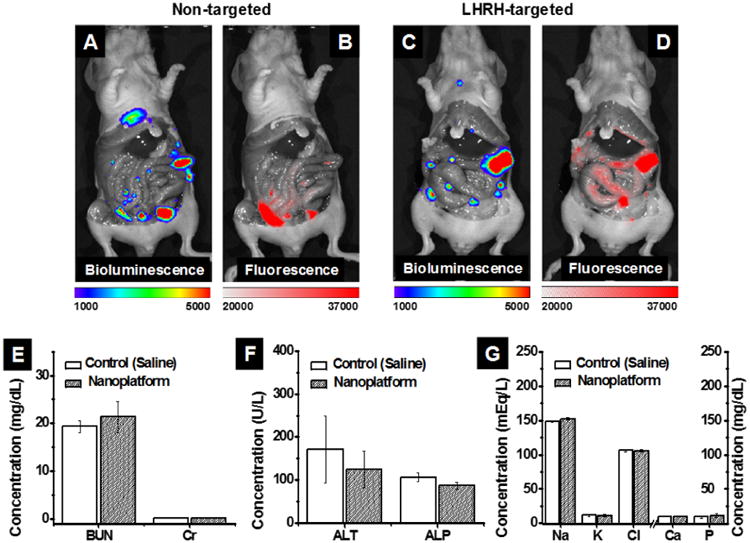
Distribution of bioluminescence (A and C) and Cy5.5-siRNA-generated fluorescence (B and D) 24 h after IP injection of non-targeted (A and B) and LHRH-targeted (C and D) nanoparticles into mice with intraperitoneal xenograft of ES2 ovarian cancer cells. Note: only cancer tissues generate bioluminescence signal in D-luciferin-injected animals. The blood levels of BUN and creatinine (E), ALT and ALP (F), and (G) electrolytes (sodium (Na), potassium (K), chloride (Cl), calcium (Ca), phosphorus (P)) in mice injected IP with saline (control) and DJ-1 siRNA-loaded nanoparticles (nanoplatform, a 50 μM siRNA concentration in a volume of 0.5 mL) during four weeks (total eight injections, three days apart).
To assess the potential acute toxicity of the LHRH-targeting nanoplatform, healthy mice were treated with the DJ-1 siRNA-loaded nanoparticles during four weeks (eight IP injections, three days apart) and the concentrations of surrogate markers in blood for kidney function (blood urea nitrogen (BUN) and creatinine (Cr)) and liver function (alanine transaminase (ALT) and alkaline phosphatase (ALP)) were measured at the end of the treatment (Figure 3E and F).39 In addition, the serum levels of blood electrolytes were evaluated as an indicator of major organ toxicity (Figure 3G).40 The concentrations of surrogate markers and blood electrolytes in mice injected with saline (control) or the nanoparticles were similar (Figure 3E and G) and within the normal ranges,41 indicating that the developed nanoparticles do not exhibit any acute toxicity.
The DJ-1 siRNA's ability to suppress the targeted protein in both solid tumors and ascites was evaluated in mice inoculated with ES-2 cells 3 weeks before nanoparticle administration. Ascites and solid tumors were collected after one round of siRNA therapy (two IP injections, 3 days apart) and DJ-1 protein levels were analyzed by immunoblotting and compared to the cancer samples obtained from animals injected with saline. The results revealed that siRNA delivered by our nanoparticles significantly decreases DJ-1 basal levels in both ascites and solid tumors (Figure 4 and Figure S4). Of note, DJ-1 suppression was more efficient in ascites as compared to solid tumors. The observed difference is likely due to the fact that ascites acts as a physiological barrier between the injected therapy and the solid tumors. Consequently, the cells within the ascites fluid absorb a substantial portion of the injected nanoparticles before they can reach solid tumors. These results reinforce the importance of both the timing of treatment, and the removal of abdominal ascites before the commencement of therapy.
Figure 4.

Representative western blot images of DJ-1, p-Akt, NRF2, p53 and CASP-3 expression in both ascites and solid tumors collected from the mice after treatment with saline (control) and nanoparticles loaded with DJ-1 siRNA. β-Actin was used as the loading control.
We also evaluated changes in the expression of critical oncogenic proteins modulated by DJ-1 such as p-Akt, NRF2, and p53, following IP administration of the nanoparticles. Previous studies demonstrated that DJ-1 protein enhances Akt phosphorylation by binding to PTEN and thereby promoting proliferation and migration of cancer cells.8, 14, 15 Overexpression of DJ-1 also results in elevated levels of NRF2 that induces expression of various antioxidant factors, thereby protecting cancer cells from ROS-mediated cell death.11, 20, 42 Indeed, the immunoblot analyses confirmed that basal levels of p-Akt and NRF2 were downregulated in both solid tumors and cells isolated from ascites fluid after siRNA-mediated DJ-1 suppression (Figure 4 and Figure S4). DJ-1 has also been reported to promote cancer cells growth and resistance to chemotherapeutic drugs by sequestering tumor suppressor protein p53 and consequently inhibiting the apoptotic p53-Bax-Caspase pathway.8, 11, 16 It was consistently observed that the levels of p53 and pro-apoptotic cleaved caspase 3 (CASP-3) were substantially elevated in both ascites and solid tumors following the siRNA-mediated attenuation of DJ-1 (Figure 4 and Figure S4). Moreover, changes in the expression of the above-mentioned proteins in solid tumors were confirmed by immunohistochemistry (Figure S5).
Design and evaluation of the combinatorial therapy for metastatic ovarian cancer
The developed combinatorial treatment consists of two monotherapies administered sequentially via IP route for three cycles: (1) the chemotherapy agent, cisplatin, and (2) siRNA-loaded nanoparticles aimed to suppress DJ-1 protein (Figure 5).
Figure 5.

The schematic shows treatment schedules for the combinatorial therapy and corresponding monotherapies.
For the treatment of Stage III and IV ovarian cancer in a clinical setting, IP administration of cisplatin (75 mg/m2) is recommended to be given on day one of each cycle and must be incorporated with the use of IV paclitaxel or cyclophosphamide for a treatment duration of 6 cycles.43 Our strategy for developing the new IP treatment modality has been to use a single chemotherapeutic agent below its recommended therapeutic dose and decrease the number of treatment cycles from six to three in order to minimize severe side effects of conventional IP chemotherapy. Based on the recommended 75 mg/m2 dose, an average woman with a weight of 60 kg and body surface area (BSA) of 1.62 m2 would require an IP injection of ∼120 mg (2 mg/kg) cisplatin.44 By assuming an equivalent surface area dosage conversion factor of 12.3,44 the IP does of ∼ 0.5 mg (∼25 mg/kg) cisplatin would be expected for a mouse with a BSA of approximately 0.007 m2. We reduced the last mentioned IP dose of cisplatin for our therapy by a factor of 10 resulting in 0.05 mg (1.85 mg/kg), which is reported to be safe in nude mice.45. In considering the accepted dosing regimen, scaled for species' size variability, the combinatorial treatment included an IP injection of cisplatin dosed at 0.05 mg (1.85 mg/kg) once a week for 3 weeks (three cycles) on day one of each cycle (Monday, Figure 5).
Our in vitro studies demonstrated that siRNA therapy must be initiated prior to cisplatin administration in order to achieve the superior therapeutic outcome (Figure S6). Such regimen is due to the long half-life (∼24-30 h) of DJ-1;17, 46 thus siRNA-mediated silencing of DJ-1 transcript is needed before chemotherapy to substantially deplete its basal levels in cancer cells. To achieve and maintain steady-state suppression of DJ-1 protein, siRNA therapy was initiated one week prior to, and continued during cisplatin-based chemotherapy via IP injections of siRNA-loaded nanoparticles on Tuesday and Friday of each week during 3 weeks (total of 6 injections, Figure 5). Our in vivo data provided in Figure 4 validates that two IP injections of siRNA-loaded nanoparticles administered 3 days apart provided a substantial decrease of DJ-1 protein levels in both solid tumors and ascites. The siRNA dosing regimen was based on our previous in vitro data, which demonstrated that following one siRNA transfection, DJ-1 protein suppression lasts for more than 3 days in cultured ovarian cancer cells.11
The efficacy of our combinatorial therapy was assessed by long-term survival studies and compared to the corresponding monotherapies and non-treated animals. The following control groups were tested (Figure 5): (1) no treatment: one IP injection of saline on Monday of each treatment week (total of 3 injections); (2) cisplatin monotherapy: one IP injection of cisplatin (Cis) on Monday of each treatment week (total of 3 injections); and (3) siRNA monotherapy: two IP injections of siRNA-loaded nanoparticles on Tuesday and Friday of each treatment week (total of 6 injections). All treatments were initiated one week after inoculation of ES-2 ovarian cancer cells into mice and animals were monitored for fitness and survival during the study. Additonally, both body weight and bioluminescence images of mice were recorded weekly. Three weeks following treatment initiation, mice injected with saline displayed signs of severe ascites characterized by obvious abdominal distension, the rapid increase in body weight (Figure S7), lethargy and impaired mobility. Also, the tumors were widely disseminated accross the abdominal cavity as indicated by bioluminescence imaging (Figure 6). Consequently, animals had to be euthanized according to the approved protocol. The cisplatin monotherapy prolonged the median survival time of mice to 6 weeks whereas that of the saline-treated group was 3 weeks after the start of treatment (Table 1, Figures 6 and 7). Interestingly, the siRNA monotherapy further extended the survival time of mice by 2 weeks over that of cisplatin alone. Finally, ovarian cancer tumors were completely eradicated from the mice treated with a combinatorial therapy, and there was no cancer recurrence detected during the duration of experiment (Figure 6). The animals from this group were observed for 35 weeks and euthanized. Because a single non-cancer-related death occurred in this group, median survival time could not be calculated but would be greater than 35 weeks (Figure 7 and Table 1).
Figure 6.
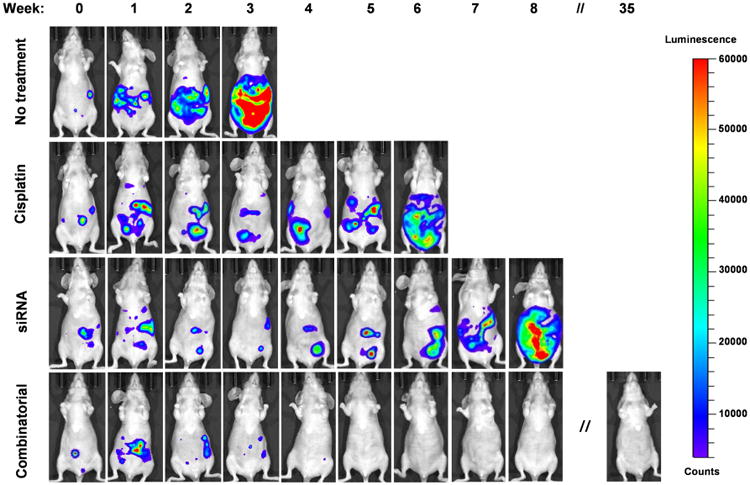
Representative whole-body bioluminescence images of ES-2-luc tumor-bearing nude mice at various time points after treatment with saline (no treatment), cisplatin, DJ-1 siRNA-loaded nanoparticles (siRNA) and combinatorial therapy. Note: only cancer tissues and ascites generate bioluminescence signal in D-luciferin-injected animals.
Table 1. Parameters from survival studies.
| Therapy | Median survival time (weeks) | Hazard ratio (95% CI)* | Reduction in the hazard# |
|---|---|---|---|
| Saline | 3 | ||
| Cisplatin | 6 | 0.10306 | 89.7% |
| DJ-1 siRNA | 8 | 0.00893 | 99.1% |
| Combinatorial | >35 | 0.00092 | 99.9% |
Compared to saline-treated group
Reduction in the probability of death at a given time point compared to saline-treated animals
Figure 7.

Kaplan-Meier survival curves of ES-2-luc tumor-bearing nude mice after treatment with saline (no treatment), cisplatin, DJ-1 siRNA-loaded nanoparticles (siRNA) and combinatorial therapy.
Hazard Ratios, a measure of the effect of treatment on an outcome of interest over time, were calculated for each group. Cisplatin monotherapy displayed a Hazard Ratio of 0.10306 (95% CI: 0.02151-0.49365), a reduction in the hazard (death probability at a given time point) by 89.7% compared to saline-treated animals (Table 1). Cisplatin-treated mice at any given time point during the study were 89.7% less likely to die at the next time point compared with mice in the saline treated group. The siRNA monotherapy and combinatorial treatment displayed Hazard Ratios of 0.00893 (95% CI: 0.00072-0.11120) and 0.00092 (95% CI: 0.00003-0.03296), showing a reduction in the hazard by 99.1% and 99.9%, respectively (Table 1). Mice treated with siRNA-loaded nanoparticles and combinatorial treatment at any given time point during the study were 99.1% and 99.9% less likely to die at the following time point compared to mice in the saline treated group, respectively. The calculated hazard ratios show that the combinatorial therapy had the greatest reduction in hazard of all the treatment groups, meaning that cisplatin combined with DJ-1 siRNA has the most pronounced effect on ovarian cancer cell death leading to increased survival within the combinatorial group. An overall difference between the four treatment groups was found to be significant (LRT: χ2=25, df=3, p=1.57e-05). Within the Cox-Proportional Hazards Model, both treatments were considered significant (Cisplatin monotherapy: Z=-2.84, p=0.00447; siRNA monotherapy: Z=-3.67, p=0.00025), with the overall model significance (LRT=29.3, df=2, p=4.37e-07).
Discussion
An efficient approach for ovarian cancer treatment can be developed by combining two or more therapeutic agents at their non-toxic doses in a manner wherein cytotoxic mechanisms of action synergistically complement each other ideally affecting multiple pathways that lead to the cancer growth and survival. Here, we have combined the first line chemotherapy agent cisplatin and a novel siRNA-based gene therapy focused on the suppression of the multifunctional DJ-1 protein. Their cytotoxic mechanisms of action target multiple distinctly different intracellular pathways and thus make it extremely difficult for the cancer cells to compensate and upregulate anti-apoptotic pathways in order to survive. Cisplatin primarily stops the proliferation of cancer cells by crosslinking DNA, usually at guanine residues, thereby disrupting DNA repair mechanisms, causing DNA damage, and subsequently inducing apoptosis when repair proves impossible.47 Several studies also reported that cisplatin can induce an intracellular level of toxic ROS via the reduction of the mitochondrial membrane potential.11, 48 In contrast to cisplatin, DJ-1 suppression by siRNA can simultaneously interfere with the expression of multiple oncogenic proteins responsible for ovarian cancer cells survival, migration, proliferation, and resistance to platinum-based drugs.8 Our data indicate that DJ-1 silencing correlates with a significant upregulation of p53 in tumors and ascites, a protein that negatively affects proliferation and viability of malignant cells.8, 16 We also validated that siRNA-mediated DJ-1 suppression is accompanied by downregulation of p-Akt levels. Akt phosphorylation promotes survival and migration of cancer cells while reducing their apoptosis. 8, 14, 15 Finally, we revealed that DJ-1 suppression leads to a substantial reduction in the intracellular level of NRF2. It is known that enhanced levels of NRF2 protect cancer cells from ROS-mediated death.20, 42
Our present in vivo data suggests that siRNA-mediated suppression of DJ-1 can be a potent strategy to enhance the anticancer effects of conventional chemotherapy while reducing severe side effects. Taking advantage of the prepared nanoplatform, we demonstrated for the first time that DJ-1 siRNA downregulates the targeting protein in metastatic ovarian cancer tumors and ascites following IP injection. DJ-1 siRNA monotherapy outperformed cisplatin-based treatment by inhibiting tumor growth and increasing survival of mice by two weeks. The superior therapeutic effect could be attributed to the fact that siRNA-mediated DJ-1 suppression disrupts more oncogenic pathways in ovarian cancer cells when compared to cisplatin alone.11 Our in vivo results are in good agreement with previously published in vitro data indicating that DJ-1 siRNA monotherapy provides higher efficacy than cisplatin regarding decreasing cancer cells viability, proliferation, and migration while enhancing intracellular ROS production and cell cycle arrest.11 Although treatment of mice with metastatic ovarian cancer by DJ-1 siRNA monotherapy significantly reduced the growth rate of tumors in comparison to cisplatin treatment, complete remission of cancer was not attained and mice had to be euthanized at the end of week 8. The observed therapeutic outcome could be explained by the fact that siRNA treatment was completed at the end of week 3 and due to only temporary effects of siRNAs on target genes (several days).11, 12 As a result, DJ-1 protein suppression by three cycles of siRNA therapy may not last long enough to kill all the cancer cells. In contrast, combinatorial siRNA-mediated DJ-1 therapy with a low dose of cisplatin completely eradicated ovarian cancer tumors from the mice, and no cancer recurrence was detected during the 35-weeks trial. These results strongly suggest that the suppression of DJ-1 protein may dramatically enhance the therapeutic activity of cisplatin and could be explored as a therapeutic target in combination with other chemotherapy agents.
In conclusion, this work validates therapeutic efficacy of a novel combinatorial treatment for metastatic ovarian cancer and highlights the importance of selecting therapeutic agents whose cytotoxic mechanisms of action complement one another in the context of a treatment regimen. Our animal studies establish a solid foundation for the potential application of DJ-1 siRNA therapy as an adjuvant therapeutic option to reinforce the existing conventional chemotherapeutic regimens in order to attain decreased systemic side effects and increase the efficacy of traditional anti-cancer interventions.
Supplementary Material
Acknowledgments
This research was supported by the College of Pharmacy at Oregon State University (OSU), and the National Institutes of Health (NIH/NBIB 1R15EB020351-01A1 and 5R01 GM108975). The funding sources had no involvement in the collection, analysis and interpretation of the data; and in the decision to submit the article for publication.
Abbreviations
- IV chemotherapy
intravenous chemotherapy
- IP chemotherapy
intraperitoneal chemotherapy
- PTEN
phosphatase and tensin homolog
- p53
tumor suppressor protein 53
- NRF2
nuclear factor (erythroid-derived 2)-like 2
- ROS
reactive oxygen species
- PPIG4
Generation 4 poly(propylene imine) dendrimer
- MAL-PEG-NHS
α-Maleimide-ω-N-hydroxysuccinimide ester poly(ethylene glycol)
- LHRH
Luteinizing Hormone-Releasing Hormone
- qPCR
real-time quantitative polymerase chain reaction
- ES-2-luc
luciferase-expressing ES-2 human ovarian cancer cells
- NRS
normal rabbit serum
- PBS
phosphate buffered saline
- PEG
polyethylene glycol
- p-Akt
phosphorylated Akt
- LRT
Likelihood Ratio Test
- CI
confidence interval
- SD
standard deviation
Footnotes
The authors declare no conflicts of interest.
The authors declare no commercial associations, current and within the past five years, that might pose a potential, perceived or real conflict of interest.
No competing interests are present.
The authors declare no prior or upcoming presentations of abstracts at meetings regarding the research on the title page.
Appendix A. Supplementary Materials: Supplementary materials to this article can be found online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cancer facts and figures, American Cancer Society. 2016 http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/
- 2.Romero I, Bast RC., Jr Minireview: human ovarian cancer: biology, current management, and paths to personalizing therapy. Endocrinology. 2012;153:1593–602. doi: 10.1210/en.2011-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bast RC, Jr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9:415–28. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGuire WP, 3rd, Markman M. Primary ovarian cancer chemotherapy: current standards of care. Br J Cancer. 2003;89 Suppl 3:S3–8. doi: 10.1038/sj.bjc.6601494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaaback K, Johnson N, Lawrie TA. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev. 2016:CD005340. doi: 10.1002/14651858.CD005340.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mueller JJ, Kelly A, Zhou Q, Iasonos A, Long Roche K, Sonoda Y, et al. Intraperitoneal chemotherapy after interval debulking surgery for advanced-stage ovarian cancer: Feasibility and outcomes at a comprehensive cancer center. Gynecol Oncol. 2016;143:496–503. doi: 10.1016/j.ygyno.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu Z, Wang J, Wientjes MG, Au JL. Intraperitoneal therapy for peritoneal cancer. Future Oncol. 2010;6:1625–41. doi: 10.2217/fon.10.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao J, Lou S, Ying M, Yang B. DJ-1 as a human oncogene and potential therapeutic target. Biochem Pharmacol. 2015;93:241–50. doi: 10.1016/j.bcp.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Kang M, Lu W, Guo Q, Zhang B, Xie Q, et al. DJ-1, a novel biomarker and a selected target gene for overcoming chemoresistance in pancreatic cancer. J Cancer Res Clin Oncol. 2012;138:1463–74. doi: 10.1007/s00432-012-1205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson B, Hadar R, Schlossberg A, Sternlicht T, Slipicevic A, Skrede M, et al. Expression and clinical role of DJ-1, a negative regulator of PTEN, in ovarian carcinoma. Hum Pathol. 2008;39:87–95. doi: 10.1016/j.humpath.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Schumann C, Chan S, Khalimonchuk O, Khal S, Moskal V, Shah V, et al. Mechanistic nanotherapeutic approach based on siRNA-mediated DJ-1 protein suppression for platinum-resistant ovarian cancer. Mol Pharm. 2016;13:2070–83. doi: 10.1021/acs.molpharmaceut.6b00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang AZ. Nanoparticle Formulations of siRNA: The next generation of targeted therapy for lymphomas and leukemias? EBioMedicine. 2014;1:101–2. doi: 10.1016/j.ebiom.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng HZ, Qu YQ, Zhang WJ, Xiu B, Deng AM, Liang AB. Proteomic analysis identified DJ-1 as a cisplatin resistant marker in non-small cell lung cancer. Int J Mol Sci. 2011;12:3489–99. doi: 10.3390/ijms12063489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hlobilkova A, Knillova J, Bartek J, Lukas J, Kolar Z. The mechanism of action of the tumour suppressor gene PTEN. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2003;147:19–25. [PubMed] [Google Scholar]
- 15.Kim RH, Peters M, Jang Y, Shi W, Pintilie M, Fletcher GC, et al. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell. 2005;7:263–73. doi: 10.1016/j.ccr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Fan J, Ren H, Jia N, Fei E, Zhou T, Jiang P, et al. DJ-1 decreases Bax expression through repressing p53 transcriptional activity. J Biol Chem. 2008;283:4022–30. doi: 10.1074/jbc.M707176200. [DOI] [PubMed] [Google Scholar]
- 17.Gan L, Johnson DA, Johnson JA. Keap1-Nrf2 activation in the presence and absence of DJ-1. Eur J Neurosci. 2010;31:967–77. doi: 10.1111/j.1460-9568.2010.07138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP. DJ-1, a cancer- and Parkinson's disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci USA. 2006;103:15091–6. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schumann C, Taratula O, Khalimonchuk O, Palmer AL, Cronk LM, Jones CV, et al. ROS-induced nanotherapeutic approach for ovarian cancer treatment based on the combinatorial effect of photodynamic therapy and DJ-1 gene suppression. Nanomedicine. 2015;11:1961–70. doi: 10.1016/j.nano.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Zhou W, Freed CR. DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T alpha-synuclein toxicity. J Biol Chem. 2005;280:43150–8. doi: 10.1074/jbc.M507124200. [DOI] [PubMed] [Google Scholar]
- 21.Taratula O, Garbuzenko OB, Kirkpatrick P, Pandya I, Savla R, Pozharov VP, et al. Surface-engineered targeted PPI dendrimer for efficient intracellular and intratumoral siRNA delivery. J Control Release. 2009;140:284–93. doi: 10.1016/j.jconrel.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pectasides D, Pectasides E, Psyrri A, Economopoulos T. Treatment issues in clear cell carcinoma of the ovary: a different entity? Oncologist. 2006;11:1089–94. doi: 10.1634/theoncologist.11-10-1089. [DOI] [PubMed] [Google Scholar]
- 23.Mabuchi S, Sugiyama T, Kimura T. Clear cell carcinoma of the ovary: molecular insights and future therapeutic perspectives. J Gynecol Oncol. 2016;27:e31. doi: 10.3802/jgo.2016.27.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beaufort CM, Helmijr JC, Piskorz AM, Hoogstraat M, Ruigrok-Ritstier K, Besselink N, et al. Ovarian cancer cell line panel (OCCP): clinical importance of in vitro morphological subtypes. PLoS One. 2014;9:e103988. doi: 10.1371/journal.pone.0103988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmed N, Stenvers KL. Getting to know ovarian cancer ascites: opportunities for targeted therapy-based translational research. Front Oncol. 2013;3:256. doi: 10.3389/fonc.2013.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mo L, Pospichalova V, Huang Z, Murphy SK, Payne S, Wang F, et al. Ascites increases expression/function of multidrug resistance proteins in ovarian cancer cells. PLoS One. 2015;10:e0131579. doi: 10.1371/journal.pone.0131579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luvero D, Milani A, Ledermann JA. Treatment options in recurrent ovarian cancer: latest evidence and clinical potential. Ther Adv Med Oncol. 2014;6:229–39. doi: 10.1177/1758834014544121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsui KH, Lee WL, Seow KM, Yang LW, Wang SY, Wang PH, et al. Effect of gonadotropin-releasing hormone agonist on ES-2 ovarian cancer cells. Taiwan J Obstet Gynecol. 2014;53:35–42. doi: 10.1016/j.tjog.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Dharap SS, Wang Y, Chandna P, Khandare JJ, Qiu B, Gunaseelan S, et al. Tumor-specific targeting of an anticancer drug delivery system by LHRH peptide. Proc Natl Acad Sci USA. 2005;102:12962–67. doi: 10.1073/pnas.0504274102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grundker C, Volker P, Griesinger F, Ramaswamy A, Nagy A, Schally AV, et al. Antitumor effects of the cytotoxic luteinizing hormone-releasing hormone analog AN-152 on human endometrial and ovarian cancers xenografted into nude mice. Am J Obstetr Gynecol. 2002;187:528–37. doi: 10.1067/mob.2002.124278. [DOI] [PubMed] [Google Scholar]
- 31.Shah V, Taratula O, Garbuzenko OB, Taratula OR, Rodriguez-Rodriguez L, M T. Targeted nanomedicine for suppression of CD44 and simultaneous cell death induction in ovarian cancer: an optimal delivery of siRNA and anticancer drug. Clin Cancer Res. 2013;19:6193–204. doi: 10.1158/1078-0432.CCR-13-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engel J, Emons G, Pinski J, Schally AV. AEZS-108: a targeted cytotoxic analog of LHRH for the treatment of cancers positive for LHRH receptors. Expert Opin Investig Drugs. 2012;21:891–99. doi: 10.1517/13543784.2012.685128. [DOI] [PubMed] [Google Scholar]
- 33.Straub B, Muller M, Krause H, Schrader M, Goessl C, Heicappell R, et al. Increased incidence of luteinizing hormone-releasing hormone receptor gene messenger RNA expression in hormone-refractory human prostate cancers. Clin Cancer Res. 2001;7:2340–43. [PubMed] [Google Scholar]
- 34.Shah V, Taratula O, Garbuzenko OB, Taratula OR, Rodriguez-Rodriguez L, Minko T. Targeted nanomedicine for suppression of CD44 and simultaneous cell death induction in ovarian cancer: an optimal delivery of siRNA and anticancer drug. Clin Cancer Res. 2013;19:6193–204. doi: 10.1158/1078-0432.CCR-13-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seth S, Johns R, Templin MV. Delivery and biodistribution of siRNA for cancer therapy: challenges and future prospects. Ther Deliv. 2012;3:245–61. doi: 10.4155/tde.11.155. [DOI] [PubMed] [Google Scholar]
- 36.Taratula O, Savla R, He H, Minko T. Poly (propyleneimine) dendrimers as potential siRNA delivery nanocarrier: from structure to function. Int J Nanotechnol. 2010;8:36–52. [Google Scholar]
- 37.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5:505–15. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanoparticle PEGylation for imaging and therapy. Nanomedicine. 2011;6:715–28. doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campion S, Aubrecht J, Boekelheide K, Brewster DW, Vaidya VS, Anderson L, et al. The current status of biomarkers for predicting toxicity. Expert Opin Drug Metab Toxicol. 2013;9:1391–408. doi: 10.1517/17425255.2013.827170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah VM, Nguyen DX, Alfatease A, Bracha S, Alani AW. Characterization of pegylated and non-pegylated liposomal formulation for the delivery of hypoxia activated vinblastine-N-oxide for the treatment of solid tumors. J Control Release. 2017;253:37–45. doi: 10.1016/j.jconrel.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 41.Schnell MA, Hardy C, Hawley M, Propert KJ, Wilson JM. Effect of blood collection technique in mice on clinical pathology parameters. Hum Gene Ther. 2002;13:155–61. doi: 10.1089/10430340152712700. [DOI] [PubMed] [Google Scholar]
- 42.Liu C, Chen Y, Kochevar IE, Jurkunas UV. Decreased DJ-1 leads to impaired Nrf2-regulated antioxidant defense and increased UV-A-induced apoptosis in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2014;55:5551–60. doi: 10.1167/iovs.14-14580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vermorken JB. Intraperitoneal chemotherapy in advanced ovarian cancer: recognition at last. Ann Oncol. 2006;17 Suppl 10:x241–6. doi: 10.1093/annonc/mdl267. [DOI] [PubMed] [Google Scholar]
- 44.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jandial DD, Messer K, Farshchi-Heydari S, Pu M, Howell SB. Tumor platinum concentration following intraperitoneal administration of cisplatin versus carboplatin in an ovarian cancer model. Gynecol Oncol. 2009;115:362–6. doi: 10.1016/j.ygyno.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Honbou K, Suzuki NN, Horiuchi M, Niki T, Taira T, Ariga H, et al. The crystal structure of DJ-1, a protein related to male fertility and Parkinson's disease. J Biol Chem. 2003;278:31380–4. doi: 10.1074/jbc.M305878200. [DOI] [PubMed] [Google Scholar]
- 47.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–78. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi YM, Kim HK, Shim W, Anwar MA, Kwon JW, Kwon HK, et al. Mechanism of Cisplatin-Induced Cytotoxicity Is Correlated to Impaired Metabolism Due to Mitochondrial ROS Generation. PLoS One. 2015;10:e0135083. doi: 10.1371/journal.pone.0135083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


