Abstract
Genistein is a phytoestrogen found in soy. We previously found that adult exposure to dietary levels of genistein affected gestation time, parturition time, litter size, pup weight, and pup mortality in CD-1 mice. The present study investigated the effects of adult genistein exposure on follicle number and gene expression in the ovaries of CD-1 mice. We found that exposure to genistein had no effect on follicle number, but it did affect the expression of apoptotic regulatory genes (Bax, Bcl-2, Bid, and Dffa) in the ovary.
1. Introduction
Genistein is an isoflavone found in many plants, but it is particularly abundant in soy. Genistein is an estrogenic isoflavone and has a twenty-fold selectivity for estrogen receptor (ER) β over ERα [1]. Human exposure to genistein can occur either through the diet in the form of soy products like tofu, tempeh, and soy milk, or through dietary supplements.
Women are exposed to genistein primarily through soy-based foods and dietary supplements. Asian populations typically show high serum levels of genistein due to the prevalence of soy in the diet, whereas European and American populations have lower and highly variable serum levels [2]. Dietary supplements containing phytoestrogens are often promoted as safe alternatives to synthetic estrogens and are thus a major route of genistein exposure [2].
Genistein has been implicated as a potential preventative agent against various diseases including cancer, postmenopausal bone loss, and cardiovascular disease [3, 4]. However, the broader health effects of genistein are relatively underexplored. Genistein has been shown to affect biological systems via several diverse mechanisms, including inhibition of protein tyrosine kinases, inhibition of topoisomerase II, as well as actions at estrogen receptors [1, 3]. In particular, the ability of genistein to bind to and signal through estrogen receptors is of particular concern to female reproductive health because female reproductive tissues contain many estrogen receptors. Further, previous work has shown that xenoestrogens, including bisphenol A (BPA), the synthetic estrogen diethylstilbestrol (DES), and phytoestrogens can adversely affect measures of female reproductive health [5-8].
Estrogens are known to play important roles in regulating ovarian follicle development and function [9]. Estrogen deficient mice have decreased numbers of primordial and primary follicles as well as impaired antral follicle development [10]. Like estrogen, genistein is known to have activity through the estrogen receptors. Genistein has been shown to stimulate estrogen-responsive pS2 mRNA expression in the estrogen receptor positive human breast cancer cell line MCF-7 in vitro [11]. These effects were blocked by the addition of tamoxifen, suggesting that genistein was indeed acting on MCF-7 cells by interacting with estrogen receptors. Interestingly, genistein was found to have proliferative effects at low doses, but anti-proliferative effects at high doses, indicating that like many xenoestrogens, genistein can have non-monotonic effects in biological systems [11]. Genistein has also produced estrogen receptor-mediated changes in the thymus of mice [12]. Previous work has also shown that genistein can affect cognitive changes similar to those of estradiol in ovariectomized rats [13]. The relative binding affinities of genistein for ERα and ERβ are 0.7 and 13, compared to an affinity of 100 for both receptors with estradiol [1]. Thus, given the activity of genistein at the estrogen receptors, and the role of estrogens in regulating follicular growth and development, it is important to investigate the potential effects of genistein on ovarian follicles.
Our previous work showed that genistein exposure inhibited mouse antral follicle growth, altered steroid hormone levels, and changed gene expression in vitro [14]. Specifically, genistein exposure increased gene expression of the cell cycle regulators cyclin dependent kinase inhibitor 1A (Cdkn1a), cyclin B1 (Ccnb1), and cyclin E1 (Ccne1). Genistein exposure also increased the expression of the steroidogenic enzymes steroidogenic acute regulatory protein (Star) and cytochrome P450 family 11 subfamily A member 1 (Cyp11a1), while decreasing the expression of cytochrome P450 family 17 subfamily A member 1 (Cyp17a1) and 3beta-hydroxysteroid dehydrogenase/delta(5)-delta(4)isomerase type I (Hsd3b1). These results suggest that genistein can affect ovarian function in vitro. Thus, the proposed study was designed to investigate whether genistein exposure also affects ovarian function in vivo. To closely model human exposure to genistein, we used oral consumption of genistein that resulted in serum genistein levels in mice similar to those observed in humans after exposure to genistein through the diet. We then investigated ovarian follicle number in response to treatment with genistein. We also investigated the expression of genes involved in apoptosis, cell cycle regulation, ovarian steroidogenesis, as well as the expression of estrogen receptor genes.
2. Methods
2.1. Chemicals and diet formulation
Genistein (98% purified determined via high-pressure liquid chromatography, Botanical Research Center, University of Illinois) was sent to Envigo-Teklad (Madison, WI) to formulate custom diets. The AIN-93G, soy and phytoestrogen free diet was used as the control as well as the base diet. Genistein was added to the base diet at a concentration of 0, 300, 500, or 1000 parts per million (ppm). Previous work has shown that mice consuming these diets have serum genistein levels of 1.0, 1.8, and 2.6 μM respectively [15]. These serum levels are within the range of those observed after human soy supplementation [16].
2.2. Animals
Female CD-1 (30 days old) mice were purchased from Charles River Laboratories (Wilmington, MA) and were allowed to acclimate to the facility prior to adjustment to diet. The mice were housed four per cage at the University of Illinois at Urbana-Champaign Veterinary Medicine Animal Facility and were provided food and water ad libitum. Temperature was maintained at 22 ± 1 °C and animals were exposed to 12-h light and dark cycles.
2.3. Study design
This study was conducted as described in Patel et al. [7]. Briefly, beginning on post-natal day (PND) 35, female CD-1 mice were fed diets containing 0, 300, 500, or 1000 ppm genistein ad libitum for 30, 60, 150, or 240 days. All mice and food were weighed and recorded twice a week to ensure all the animals were eating similar amounts of food and that they did not experience abnormal weight changes. After each dosing period, three to six mice from each treatment group were euthanized in diestrus. After euthanasia, blood and tissues were collected for analyses as described below.
2.4. Assessment of ovarian follicle numbers
Follicle numbers were quantified as previously described [17]. Ovaries were fixed with paraformaldehyde and transferred into 70% ethanol before being embedded in paraffin wax and sectioned (8 μm) using a microtome. Sections were mounted onto glass slides and then stained with hematoxylin and eosin. Follicle numbers were counted in every 10th section. Follicles were classified as primordial if they contained an oocyte surrounded by a single layer of squamous granulosa cells, primary if they contained an oocyte surrounded by a single layer of cuboidal granulosa cells, preantral if they contained an oocyte surrounded by at least two layers of cuboidal granulosa cells and theca cells, and antral if they contained an oocyte with a fluid-filled antral space surrounded by multiple layers of cuboidal granulosa cells and theca cells. Preantral and antral follicles were only counted if the oocyte contained nuclear material to avoid double counting the larger follicle types that can span multiple sections. Primordial and primary follicles were counted in each section regardless of the presence of nuclear material in the oocyte. Transitioning follicles were classified as the more immature state.
2.5. Gene expression analysis
Gene expression was analyzed as described in Berger et al. [18]. RNA from snap frozen ovaries was extracted using a miRNeasy Micro Kit (Qiagen, Inc., Valencia, CA) according to the manufacturer’s protocol. The samples were treated with DNase (Qiagen, Inc., Valencia, CA) during the process. RNA (100 ng) was reversed transcribed to cDNA and subjected to quantitative real time PCR (qPCR) using the CFX96 Real-Time PCR Detection System (Bio-Rad Inc.) and accompanying software (CFX Manager Software). The initial incubation temperature was 95 °C for 5 min. This was followed by 36 cycles at 95 °C for 10 s, at 60 °C for 10 s, and at 72 °C for 10 s. Melting from 65 °C to 95 °C was followed by final extension at 72 °C for 2 min. Standard curves, melting temperature graphs, and threshold cycle (Ct) values were generated for each run. Primer sequences for each gene are listed in Table 1. All samples were run in duplicate. The expression data from each sample were normalized to the corresponding values of beta-actin (Actb). Individual relative fold changes were calculated by the Pfaffl method [19], then average fold changes for each group were represented as a ratio to the average fold change of the control group.
Table 1.
| Gene | Species | Forward sequence | Reverse sequence |
|---|---|---|---|
| Actb | mouse | AGC ACA GCT TCT TTG CAG CTC CTT | CAG CGC AGC GAT ATC GTC ATC CAT |
| Aifm1 | mouse | AGG ACG GTG AGC AAC ATG AA | GTT CTA TCC ACC CAT CCC GC |
| Bax | mouse | TGA AGA CAG GGG CCT TTT TG | AAT TCG CCG GAG ACA CTC G |
| Bcl-2 | mouse | ATG CCT TTG TGG AAC TAT ATG GC | GGT ATG CAC CCA GAG TGA TGC |
| Bcl2l10 | mouse | CGC TAC ACA CAC TGA CTG GA | CTT TAG GAT CCC CTG CCC TG |
| Bid | mouse | AGC AAA TGT TCC CTC CGC TTC TGT | GTA GGC TGT GGC GGC TCG TG |
| Bok | mouse | CTG CCC CTG GAG GAC GCT TG | CCG TCA CCA CAG GCT CCG AC |
| Ccna2 | mouse | GCT CTA CTG CCC GGA GGC TGA | TGG CCT ACA TGT CCT CTG GGG AA |
| Ccnb1 | mouse | TGC ATT CTC TCA GTG CCC TCC ACA | AGA CAG GAG TGG CGC CTT GGT |
| Ccnd2 | mouse | CCT TTG ACG CAG GCT CCC TTC T | ACC CTG GTG CAC GCA TGC AAA |
| Ccnel | mouse | GGT GTC CTC GCT GCT TCT GCT T | CCG GAT AAC CAT GGC GAA CGG A |
| Cdk4 | mouse | AGA AAC CCT CGC TGA AGC GGC A | TGG GGG TGA ACC TCG TAA GGA GA |
| Cdkn1a | mouse | TTA GGC AGC TCC AGT GGC AAC C | ACC CCC ACC ACC ACA CAC CAT A |
| Cyp1a1 | mouse | TGT CAG ATG ATA AGG TCA TCA CG | TCT CCA GAA TGA AGG CCT CCA G |
| Cyp1b1 | mouse | GCG ACG ATT CCT CCG GGC TG | TGC ACG CGG GCC TGA ACA TC |
| Cyp11a1 | mouse | AGA TCC CTT CCC CTG GTG ACA ATG | CGC ATG AGA AGA GTA TCG ACG CAT C |
| Cyp17a1 | mouse | CCA GGA CCC AAG TGT GTT CT | CCT GAT ACG AAG CAC TTC TCG |
| Cyp19a1 | mouse | CAT GGT CCC GGA AAC TGT GA | GTA GTA GTT GCA GGC ACT TC |
| Dffa | mouse | GCC AGA TCC TTA CCA CAC TGA | TTA TGT CCC AGC TCA GAG CGA |
| Esr1 | mouse | CCG TGT GCA ATG ACT ATG CC | GTG CTT CAA CAT TCT CCC TCC TC |
| Esr2 | mouse | GGA ATC TCT TCC CAG CAG CA | GGG ACC ACA TTT TTG CAC TT |
| Hsd17b1 | mouse | AAG CGG TTC GTG GAG AAG TAG | ACT GTG CCA GCA AGT TTG CG |
| Hsd3b2 | mouse | CAG GAG AAA GAA CTG CAG GAG GTC | GCA CAC TTG CTT GAA CAC AGG C |
| Star | mouse | CAG GGA GAG GTG GCT ATG CA | CCG TGT CTT TTC CAA TCC TCT G |
2.6. Statistical analysis
If data met assumptions of normal distribution and homogeneity of variance, data were analyzed by one-way analysis of variance (ANOVA) followed by Dunnett (2-sided) post-hoc comparisons. If data were not normally distributed, and/or did not meet homogeneity of variance assumptions, the independent sample Kruskal-Wallis H followed by Mann-Whitney U nonparametric tests were performed. Statistical significance was assigned at P ≤ 0.05.
3. Results
3.1. The effects of genistein on ovarian follicle number
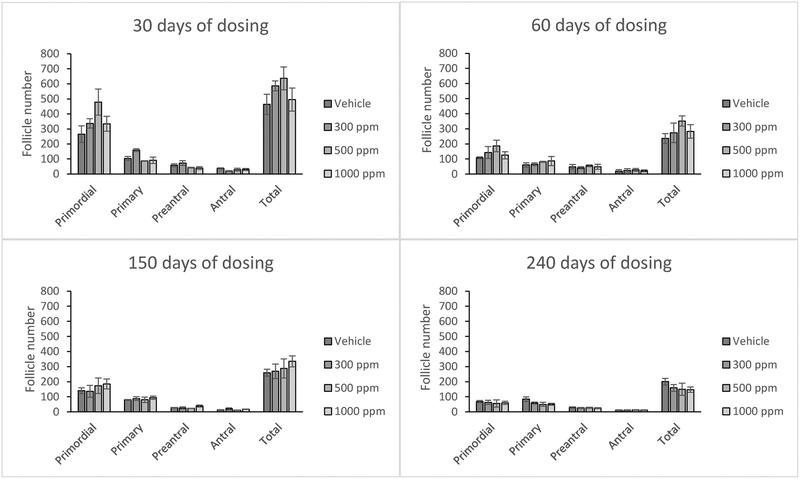
Genistein exposure did not significantly affect the number of primordial, preantral, antral, or total follicles at any of the selected doses (Figure 1). Further, genistein exposure did not significantly affect follicle numbers at any time point (Figure 1).
Figure 1:
Effects of genistein exposure on follicle numbers in mice treated with 0, 300, 500, or 1000 ppm genistein. The numbers of primordial, primary, preantral, antral, and total ovarian follicles were counted at 30, 60, 150, and 240 days of age. Graphs represent means ± SEM from 4-6 females per treatment group in the F1 generation.
3.2. The effects of genistein on gene expression in the ovary
3.2.1. Apoptosis genes
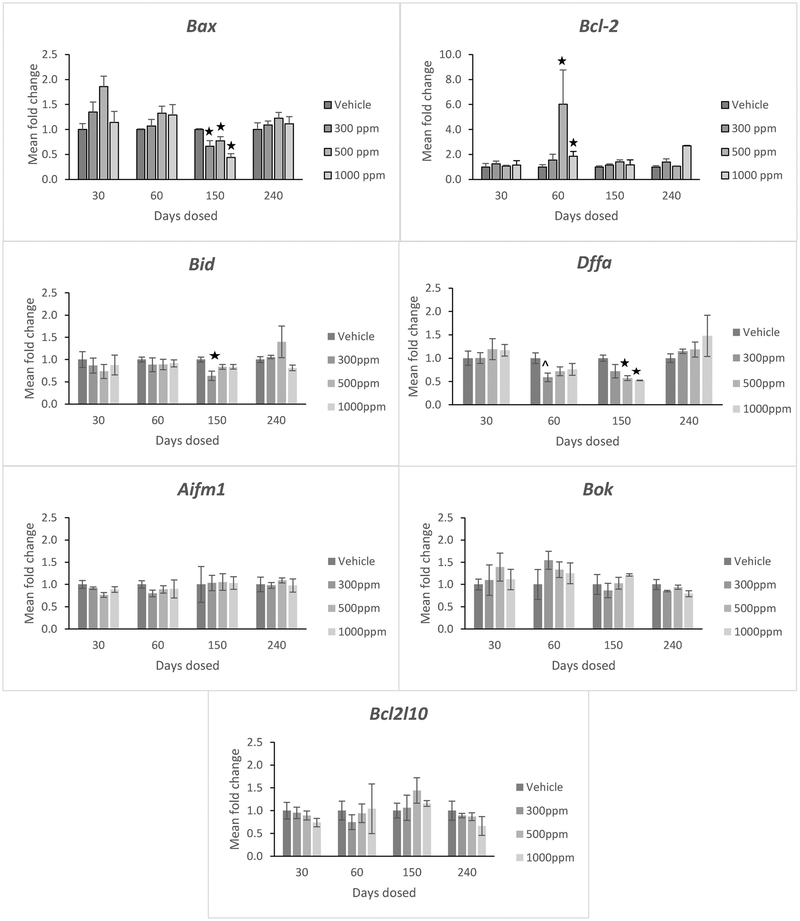
After 60 days of exposure, genistein exposure at 500 and 1000 ppm significantly increased the expression of the anti-apoptotic factor known as B-cell lymphoma 2 (Bcl-2) (Figure 2, p <0.05). However, genistein exposure did not significantly affect Bcl-2 expression in the ovary at any other time point. It also did not affect the expression of the anti-apoptotic factor known as Bcl-2-like protein 10 (Bcl2l10) at any time point (Figure 2).
Figure 2:
Effects of genistein exposure on gene expression of Bax, Bcl-2, Bid, Dffa, Aifm1, Bok, and Bcl2l10 in mice treated with 0, 300, 500, or 1000 ppm genistein. Gene expression was measured after 30, 60, 150, and 240 days of exposure to genistein. RNA was extracted from snap-frozen ovaries, reversed transcribed to cDNA, and subjected to quantitative real time PCR. Graphs represent means ± SEM from 3 females per treatment group. Asterisks indicate significant differences compared to the control (p ≤ 0.05). ^indicates p = 0.064.
After 150 days of exposure, genistein treatment at 300, 500, and 1000 ppm significantly reduced expression of the pro-apoptotic factor Bcl-2 associated X, apoptosis regulator (Bax) compared to control (Figure 2, p <0.05), but it did not significantly affect Bax expression in the ovary at any other time point. Genistein exposure also did not affect expression of the pro-apoptotic factor known as apoptosis-inducing factor mitochondrial 1 (Aifm1) or the pro-apoptotic factor known as Bcl-2 related ovarian killer protein (Bok) in the ovary at any time point (Figure 2).
Interestingly, genistein exposure reduced the expression of other pro-apoptotic factors known as BH3-interacting domain against death (Bid) and DNA fragmentation alpha (Dffa) in the ovary (Figure 2, p<0.05). Specifically, at 150 days, genistein exposure at 300 ppm reduced Bid expression and genistein exposure at 500 ppm and 1000 ppm reduced Dffa expression in the ovary compared to control (Figure 2, p<0.05).
3.2.2. Cell cycle regulator genes
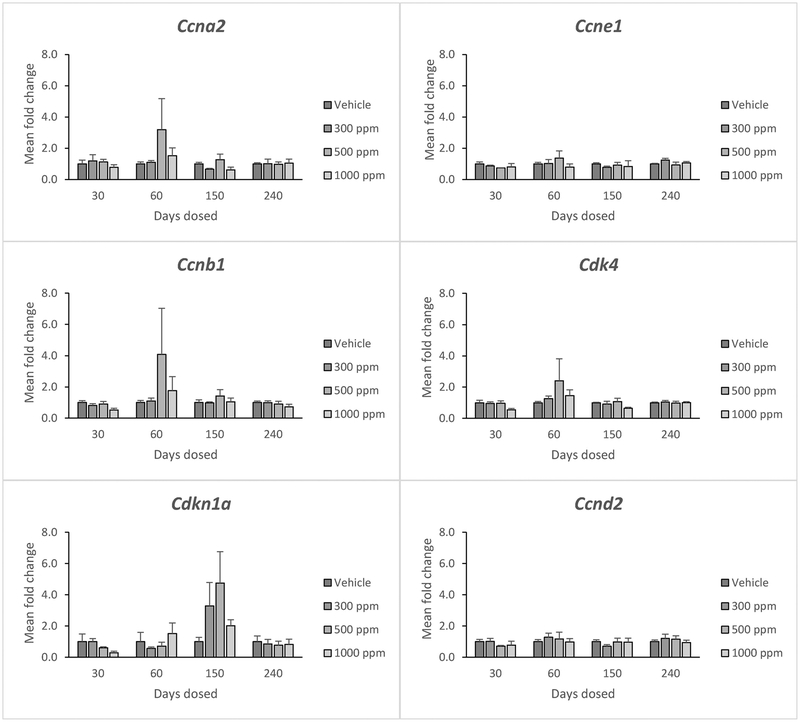
Genistein did not significantly affect the expression of cyclin A2 (Ccna2), Ccne1, Ccnb1, cyclin dependent kinase 4 (Cdk4), Cdkn1a, or cyclin D2 (Ccnd2) at any dose (Figure 3). Further, it did not affect the selected cell cycle regulators at any time point (Figure 3).
Figure 3:
Effects of genistein exposure on gene expression of Ccna2, Ccne1, Ccnb1, Cdk4, Cdkn1a, and Ccnd2 in mice treated with 0, 300, 500, or 1000 ppm genistein. Gene expression was measured after 30, 60, 150, and 240 days of exposure to genistein. RNA was extracted from snap-frozen ovaries, reversed transcribed to cDNA, and subjected to quantitative real time PCR. Graphs represent means ± SEM from 3 females per treatment group.
3.2.3. Cytochrome P450 genes
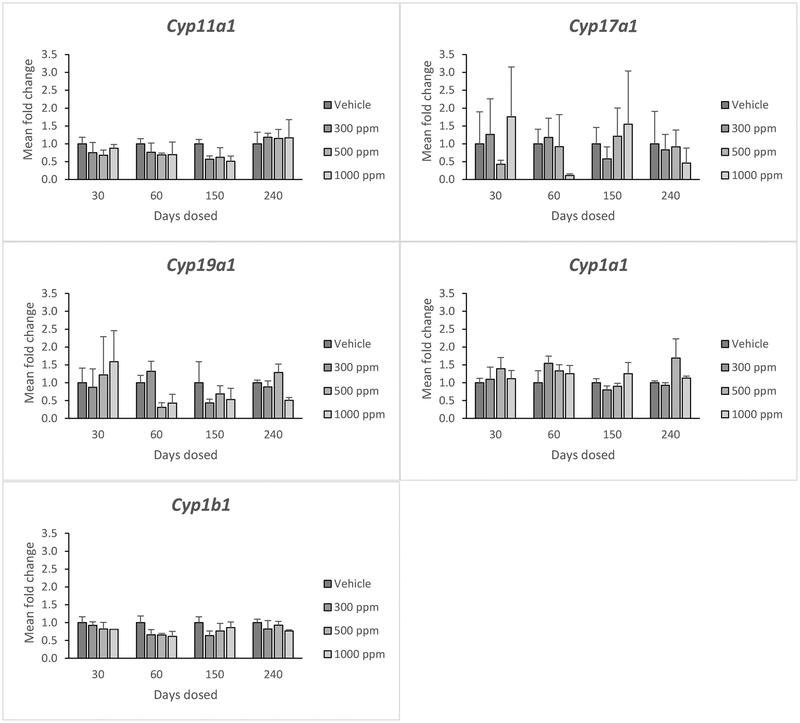
Genistein did not significantly affect the expression of Cyp11a1, Cyp17a1, cytochrome P450 family 19 subfamily A member 1 (Cyp19a1), cytochrome P450, family 1, subfamily A, polypeptide 1 (Cyp1a1), or cytochrome P450 family 1 subfamily B member 1 (Cyp1b1) at any dose (Figure 4). Further, it did not affect expression of these cytochrome P450 genes at any time point (Figure 4).
Figure 4:
Effects of genistein exposure on gene expression of Cyp11a1, Cyp17a1, Cyp19a1, Cyp1a1, and Cyp1b1 in mice treated with 0, 300, 500, or 1000 ppm genistein. Gene expression was measured after 30, 60, 150, and 240 days of exposure to genistein. RNA was extracted from snap-frozen ovaries, reversed transcribed to cDNA, and subjected to quantitative real time PCR. Graphs represent means ± SEM from 3 females per treatment group.
3.2.4. Steroidogenesis genes
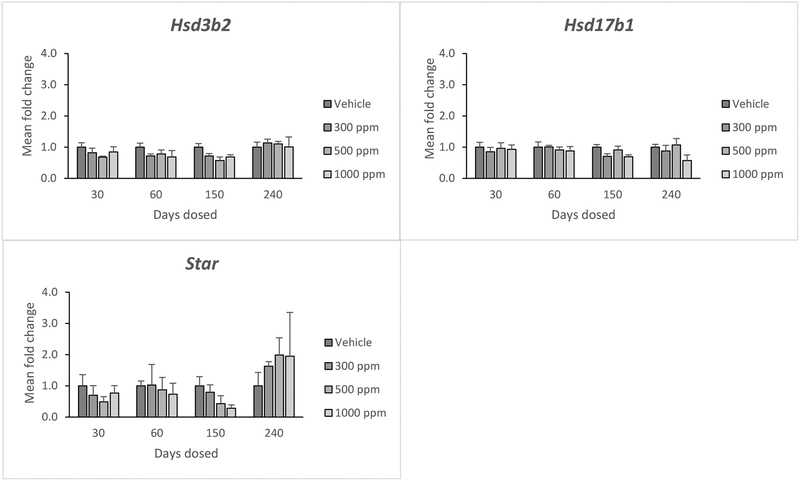
Genistein did not significantly affect the expression of hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 2 (Hsd3b2), hydroxysteroid 17-beta dehydrogenase 1 (Hsd17b1) or Star at any dose (Figure 5). Further, it did not affect expression of these steroidogenesis genes at any time point (Figure 5).
Figure 5:
Effects of genistein exposure on gene expression of Hsd3b2, Hsd17b, and Star in mice treated with 0, 300, 500, or 1000 ppm genistein. Gene expression was measured after 30, 60, 150, and 240 days of exposure to genistein. RNA was extracted from snap frozen ovaries, reversed transcribed to cDNA, and subjected to quantitative real time PCR. Graphs represent means ± SEM from 3 females per treatment group.
3.2.5. Estrogen receptor genes
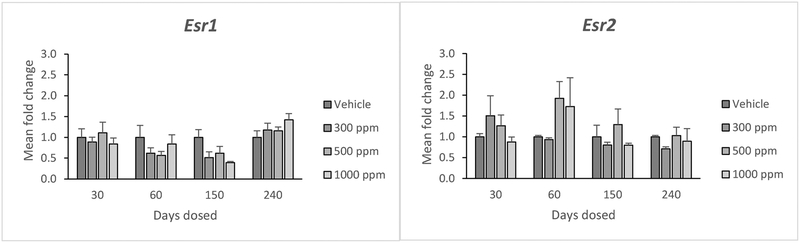
Genistein did not significantly affect the expression of estrogen receptor 1 (Esr1) or estrogen receptor 2 (Esr2) at any dose (Figure 6). Further, it did not affect expression of these genes at any time point (Figure 6).
Figure 6.
Effects of genistein exposure on gene expression of Esr1 and Esr2 in mice treated with 0, 300, 500, or 1000 ppm genistein. Gene expression was measured after 30, 60, 150, and 240 days of exposure to genistein. RNA was extracted from snap-frozen ovaries, reversed transcribed to cDNA, and subjected to quantitative real time PCR. Graphs represent means ± SEM from 3 females per treatment group.
Discussion
This study investigated the effects of chronic oral exposure to dietary levels of genistein on ovarian follicle numbers and gene expression in the ovary. The results show that exposure to 300, 500, or 1000 ppm genistein did not affect follicle numbers at any time-point in mice. The results also show that exposure to 300, 500 or 1000 ppm genistein did not affect the expression of several cell cycle regulatory genes (Ccna2, Ccne1, Ccnb1, Cdk4, Cdkn1a, Ccnd2), members of the cytochrome P450 superfamily of genes (Cyp11a1, Cyp17a1, Cyp19a1, Cyp1a1, Cyp1b1), key genes involved in steroidogenesis (Hsd3b2, Hsd17b1, Star), or estrogen receptor genes (Esr1, Esr2) in the ovary. However, genistein exposure did increase ovarian expression of the anti-apoptotic factor Bcl-2 (500 and 1000 ppm) and decrease ovarian expression of the pro-apoptotic factors known as Bax (300, 500, and 1000 ppm), Bid (500 ppm), and Dffa (500 and 1000 ppm).
We previously found that exposure to genistein in the same paradigm as the current study resulted in the disruption of several indices of fertility. Mice treated with 500 or 1000 ppm genistein for 30 days had significantly decreased gestation times compared to control mice. Mice treated with 500 ppm genistein for 60 days had decreased litter size and increased average pup weight compared to control mice. Additionally, mice treated with 300 ppm genistein for 150 days had an increased risk of prolonged parturition compared to control mice [7]. Interestingly, Patel et al. [7] showed that genistein exposure also had a non-linear effect on fertility in mice, with the 300 ppm genistein group showing reduced fertility at 240 days compared to control mice, but the 500 and 1000 ppm genistein groups showing higher fertility at 240 days compared to control mice. Genistein exposure did not affect levels of estradiol or progesterone in Patel et al. [7]. Taken together with the results of the current study, these data suggest that chronic oral exposure to environmentally relevant levels of genistein can affect female reproductive health, but that these effects are likely not mediated by changes in follicle number, changes in estradiol or progesterone biosynthesis, or changes in the expression of the cell cycle regulatory genes Ccna2, Ccne1, Ccnb1, Cdk4, Cdkn1a, or Ccnd2 in the ovary. They are also not likely due to changes in ovarian steroidogenesis or in the number of estrogen receptors in the ovary, as shown by the lack of changes in gene expression of key members of the cytochrome P450 superfamily (Cyp11a1, Cyp17a1, Cyp19a1, Cyp1a1, and Cyp1b1) as well as the lack of changes in gene expression of Esr1 and Esr2.
Given the changes in Bcl-2, Bax, Bid, and Dffa with exposure to genistein that were found in the present study, it is possible that some of the effects of genistein in the ovary are mediated by changes in apoptotic regulation. Bcl-2 is generally considered to be anti-apoptotic [20,22], whereas Bax, Bid, and Dffa are generally considered to be pro-apoptotic [21, 22]. Thus, the pattern of gene expression found in the present study suggests that chronic exposure to genistein might act in an anti-apoptotic manner in the ovary, providing a possible mechanism for the increased fertility found in aged mice given high levels of genistein in Patel et al. [7]. However, it is important to note that this study did not find any effect of genistein exposure on the expression other selected regulators of the apoptotic pathway (Bok, Aifm1, and Bcl2l10). It is possible that the current study missed changes in expression of these genes because potential changes occurred earlier or later than the times examined in this study. It is also possible that genistein exposure alters the protein levels, but not the transcript levels of apoptotic regulators. Thus, future studies should examine the effects of genistein exposure on protein levels of regulators of apoptosis.
The results from the present study are in contrast with our previous work in which genistein, while not altering levels of Bax or Bcl-2, did alter the expression of the cell cycle regulatory genes Cdkn1a, Ccnb1, and Ccne1 as well as the steroidogenic enzymes Star, Cyp11a1, Hsd3b1 and Cyp17a1 at the highest dose (36μM) [14]. However, the Patel et al. study [14] was conducted in vitro and the doses used in that study were higher than the doses found in vivo in rodents after dietary exposure to genistein. Additionally, the in vitro paradigm exposed the follicles to genistein directly, which differs from genistein exposure to the ovaries in vivo after oral dosing due to hepatic metabolism. Additionally, it should be noted that in the present study, due to small sample sizes, variability in gene expression was high, thus potentially obscuring small changes in gene expression between exposure groups.
Other studies have also shown effects of genistein on reproductive health in female rodents. Genistein exposure early in life was shown to cause hyperplasia of the mammary glands, abnormal cellular maturation in the vagina as well as abnormal ovarian antral follicles in female rats [23]. It was also shown to alter the numbers of corpora lutea and ovulated oocytes in mice [24]. Neonatal exposure to environmentally relevant doses of genistein was shown to increase estrous cycle length and decrease the percentage of live pup births in exposed females compared to control female mice [24]. Further, it was shown to inhibit oocyte nest breakdown in mice [25, 26] and increase the number of multi-oocyte follicles in rats and mice [27, 28].
Previous research also suggests that soy isoflavone exposure, especially early in life, can affect reproductive health in human populations [29]. Infants fed soy formula have exhibited premature development of breast buds as well as the vaginal epithelium when compared to their cow milk or breast milk fed counterparts [30, 31]. Additionally, one study has found that women who were fed soy formula as infants report slightly longer menstrual bleeding duration as well as more menstrual discomfort than those fed milk-based formulas [32]. A recent large study of adult women found that women who were fed soy-based formulas as infants were more likely to develop smooth muscle tumors in the uterus than women fed milk-based formulas [33]. Infants fed soy-based formulas have serum genistein levels of 1.4–4.5 μM [34], which are comparable to the serum genistein levels (1, 1.8, and 2.6 μM) in the present study. Thus, the results of the present study may be applicable to this population. However, it should be noted that exposure in the present study started when mice were 35 days old, not at an age comparable to human infancy.
Collectively, genistein exposure has been shown to affect reproductive outcomes in rodents as well as in humans. Women continue to be exposed to genistein through the consumption of soy-based food products as well as through dietary supplements, yet much remains unknown about this potent phytoestrogen. Future studies should further investigate the mechanisms by which genistein could be affecting female reproductive health. This study and others like it are critical for understanding the broader physiological effects of genistein.
Highlights:
Genistein exposure does not affect ovarian follicle number
Genistein exposure does not affect expression of cell cycle regulators
Genistein exposure does not affect expression of steroidogenic enzymes
2Genistein exposure alters expression of apoptotic regulator genes
Acknowledgements
This work was supported by NIH R03ES023972 (JAF), NIH R03ES023972S1, the Botanical Estrogen Research Center at the University of Illinois (WGH), a Billie A. Field Fellowship (SP), and a Toxicology Scholar Award (PK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–63. [DOI] [PubMed] [Google Scholar]
- [2].Jefferson WN, Patisaul HB, Williams CJ. Reproductive consequences of developmental phytoestrogen exposure. Reproduction. 2012;143:247–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Polkowski K, Mazurek AP. Biological properties of genistein. A review of in vitro and in vivo data. Acta Pol Pharm 2000;57:135–55. [PubMed] [Google Scholar]
- [4].Russo M, Russo GL, Daglia M, Kasi PD, Ravi S, Nabavi SF, et al. Understanding genistein in cancer: The "good" and the "bad" effects: A review. Food Chem 2016;196:589–600. [DOI] [PubMed] [Google Scholar]
- [5].Maffini MV, Rubin BS, Sonnenschein C, Soto AM. Endocrine disruptors and reproductive health: the case of bisphenol-A. Mol Cell Endocrinol 2006;254-255:179–86. [DOI] [PubMed] [Google Scholar]
- [6].Palter SF, Tavares AB, Hourvitz A, Veldhuis JD, Adashi EY. Are estrogens of import to primate/human ovarian folliculogenesis? Endocr Rev 2001;22:389–424. [DOI] [PubMed] [Google Scholar]
- [7].Patel S, Hartman JA, Helferich WG, Flaws JA. Preconception exposure to dietary levels of genistein affects female reproductive outcomes. Reprod Toxicol 2017;74:174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Patel S, Brehm E, Gao L, Rattan S, Ziv-Gal A, Flaws JA. Bisphenol A Exposure, Ovarian Follicle Numbers, and Female Sex Steroid Hormone Levels: Results From a CLARITY-BPA Study. Endocrinology. 2017;158:1727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Britt KL, Findlay JK. Estrogen actions in the ovary revisited. J Endocrinol 2002;175:269–76. [DOI] [PubMed] [Google Scholar]
- [10].Britt KL, Saunders PK, McPherson SJ, Misso ML, Simpson ER, Findlay JK. Estrogen actions on follicle formation and early follicle development. Biol Reprod 2004;71:1712–23. [DOI] [PubMed] [Google Scholar]
- [11].Wang TT, Sathyamoorthy N, Phang JM. Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis. 1996;17:271–5. [DOI] [PubMed] [Google Scholar]
- [12].Cooke PS, Selvaraj V, Yellayi S. Genistein, estrogen receptors, and the acquired immune response. J Nutr 2006;136:704–8. [DOI] [PubMed] [Google Scholar]
- [13].Pisani SL, Neese SL, Doerge DR, Helferich WG, Schantz SL, Korol DL. Acute genistein treatment mimics the effects of estradiol by enhancing place learning and impairing response learning in young adult female rats. Horm Behav 2012;62:491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Patel S, Peretz J, Pan YX, Helferich WG, Flaws JA. Genistein exposure inhibits growth and alters steroidogenesis in adult mouse antral follicles. Toxicol Appl Pharmacol 2016;293:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Naaz A, Yellayi S, Zakroczymski MA, Bunick D, Doerge DR, Lubahn DB, et al. The soy isoflavone genistein decreases adipose deposition in mice. Endocrinology. 2003;144:3315–20. [DOI] [PubMed] [Google Scholar]
- [16].Albertazzi P, Pansini F, Bottazzi M, Bonaccorsi G, De Aloysio D, Morton MS. Dietary soy supplementation and phytoestrogen levels. Obstet Gynecol 1999;94:229–31. [DOI] [PubMed] [Google Scholar]
- [17].Hannon PR, Niermann S, Flaws JA. Acute exposure to di(2-ethylhexyl) phthalate in adulthood causes adverse reproductive outcomes later in life and accelerates reproductive aging in female mice. Toxicol Sci 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Berger A, Ziv-Gal A, Cudiamat J, Wang W, Zhou C, Flaws JA. The effects of in utero bisphenol A exposure on the ovaries in multiple generations of mice. Reprod Toxicol 2015;60:39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001;29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Belka C, Budach W. Anti-apoptotic Bcl-2 proteins: structure, function and relevance for radiation biology. Int J Radiat Biol 2002;78:643–58. [DOI] [PubMed] [Google Scholar]
- [21].Pawlowski J, Kraft AS. Bax-induced apoptotic cell death. Proc Natl Acad Sci U S A. 2000;97:529–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Matsuda F, Inoue N, Manabe N, Ohkura S. Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. J Reprod Dev 2012;58:44–50. [DOI] [PubMed] [Google Scholar]
- [23].Delclos KB, Bucci TJ, Lomax LG, Latendresse JR, Warbritton A, Weis CC, et al. Effects of dietary genistein exposure during development on male and female CD (Sprague-Dawley) rats. Reprod Toxicol 2001;15:647–63. [DOI] [PubMed] [Google Scholar]
- [24].Jefferson WN, Padilla-Banks E, Newbold RR. Adverse effects on female development and reproduction in CD-1 mice following neonatal exposure to the phytoestrogen genistein at environmentally relevant doses. Biol Reprod 2005;73:798–806. [DOI] [PubMed] [Google Scholar]
- [25].Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology. 2007;148:3580–90. [DOI] [PubMed] [Google Scholar]
- [26].Jefferson W, Newbold R, Padilla-Banks E, Pepling M. Neonatal genistein treatment alters ovarian differentiation in the mouse: inhibition of oocyte nest breakdown and increased oocyte survival. Biol Reprod 2006;74:161–8. [DOI] [PubMed] [Google Scholar]
- [27].Cimafranca MA, Davila J, Ekman GC, Andrews RN, Neese SL, Peretz J, et al. Acute and chronic effects of oral genistein administration in neonatal mice. Biol Reprod 2010;83:114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Losa SM, Todd KL, Sullivan AW, Cao J, Mickens JA, Patisaul HB. Neonatal exposure to genistein adversely impacts the ontogeny of hypothalamic kisspeptin signaling pathways and ovarian development in the peripubertal female rat. Reprod Toxicol 2011;31:280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hooper L, Madhavan G, Tice JA, Leinster SJ, Cassidy A. Effects of isoflavones on breast density in pre- and post-menopausal women: a systematic review and meta-analysis of randomized controlled trials. Hum Reprod Update. 2010;16:745–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bernbaum JC, Umbach DM, Ragan NB, Ballard JL, Archer JI, Schmidt-Davis H, et al. Pilot studies of estrogen-related physical findings in infants. Environ Health Perspect 2008;116:416–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zung A, Glaser T, Kerem Z, Zadik Z. Breast development in the first 2 years of life: an association with soy-based infant formulas. J Pediatr Gastroenterol Nutr 2008;46:191–5. [DOI] [PubMed] [Google Scholar]
- [32].Strom BL, Schinnar R, Ziegler EE, Barnhart KT, Sammel MD, Macones GA, et al. Exposure to soy-based formula in infancy and endocrinological and reproductive outcomes in young adulthood. Jama. 2001;286:807–14. [DOI] [PubMed] [Google Scholar]
- [33].D'Aloisio AA, Baird DD, DeRoo LA, Sandler DP. Association of intrauterine and early-life exposures with diagnosis of uterine leiomyomata by 35 years of age in the Sister Study. Environ Health Perspect. 2010;118:375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet. 1997;350:23–7. [DOI] [PubMed] [Google Scholar]