What have we learned about gastric cancer in the 75 years since the first issue of Gastroenterology? The earliest, to our knowledge, comprehensive review in Gastroenterology on this topic was by Russell Boles, a gastroenterologist from the University of Pennsylvania. He posed 7 questions that he identified as the most important unanswered issues facing the field1:
The environmental, genetic, and injurious factors that give rise to gastric cancer are uncertain.
In certain countries, like Japan, gastric cancer is for some reason far more frequent than in the US and in Northern and Western Europe.
Certain histological and physiological changes, like atrophic gastritis and pernicious anemia, increase risk for progression, yet “little can be offered in explaining their relationship”. Interestingly, Boles pointed out that Sir Arthur Hurst preceded Boles by 30 years in discussing the importance of understanding “certain conditions that were regarded as precursors of the disease.” Unfortunately, Boles noted: “Conceptions in these respects have changed little since Hurst’s time.”
Boles was excited about new work that showed that achlorhydria, decreased pepsin secretion, and pernicious anemia (ie chronically decreased gastric intrinsic factor secretion) all correlated positively with cancer (see, e.g., October, 1955 issue of Gastroenterology2 as well as another important paper from Sara Jordan and coworkers in October, 19523).
Boles commented on the uncertain relationship of gastric peptic ulcers to cancer. It wasn’t clear if ulcers that harbored cancers were simply cancers that caused ulcers or if benign ulcers increased the risk for progression to cancer.
Boles analyzed total funding from the U.S. Public Health Service (largely via the newly established National Cancer Institute) for gastric cancer research relative to funding for other cancers, and as a function of deaths caused by cancer. He found that not much more than 2% of total cancer funding in the decade preceding his article went to gastric cancer research, yet cancer of the stomach accounted for nearly 12% of cancer death. Thus, he said: “One of the purposes of this paper…was to focus on the relative indifference being shown in the field of gastric cancer research.” He claimed: “One can only speculate on the reasons for this….”
Boles also cited his Gastroenterology paper from 1955 (March issue4) showing data noting the beginning of a trend that would continue for much of the 20th century, which is that rates of gastric cancer in the United States were suddenly (within the span of a decade) beginning to dramatically decrease, in particular among white men.
Here, we discuss the progress made since 1958 on these 7 issues, focusing on key articles in Gastroenterology that highlight where the field has evolved over the past 60 years. We will commence with the areas where the field, arguably, has not progressed substantially.
Inadequate Funding for Gastric Cancer Research
Relative to morbidity/mortality and to nearly every other cancer, gastric cancer is still strikingly neglected in terms of research investment. As of 2013, one study showed that gastric cancer was last or nearly last in funding in the U.S., normalized in several ways5. A subsequent study showed that stomach cancer funding was last among all cancer types examined in both the U.K. and U.S., normalized to years of life lost6. And it was among the least funded in a follow-up study on U.K. funding7. Gastric cancer continues to be a significant health burden, estimated to be the cause of 10,800 deaths in the U.S. this year (NCI). Thus, to paraphrase Boles, we still can only speculate about why gastric cancer research is poorly funded.
Basis for Differences in Gastric Cancer Incidence
With regard to issue #7, the decline in gastric cancer in white American men, which Boles was among the first to recognize by the mid-20th century4, persisted to nearly the end of the century. Since 1992, in people younger than 65, the decreasing incidence has essentially stopped8 and might even be reversing if not rising again in recent years among younger (<50) males8. Other than the fact that human colonization by one of the key etiological agents, Helicobacter pylori (see below), has also declined over the last century and a half, the reasons for the decrease in rates of gastric cancer are still not apparent. Similarly, with regard to Boles’s issue #2, rates of gastric cancer and death from gastric cancer are still much higher in Japan, perhaps due in part to higher rates of infection with H. pylori or at least with more oncogenic strains thereof, along with poorly understood environmental factors (discussed below and reviewed in ref.9). Notably, rates in Japan have also been decreasing, as have rates in other countries with traditionally higher incidence9.
Etiology of Gastric Cancer: H. pylori, and Genetic and Environmental Factors
With regard to Boles’s issue #1 (“environmental, genetic, and injurious factors,”), there has been a considerable increase in our understanding of “injurious factors” to the stomach (discussed below), and regarding the environmental and genetic factors, the field has made important progress as well. The game-changing environmental agent H. pylori, first discovered by Marshall and Warren in 2005 (see Gastroenterology, December, 200510, was shown to be almost required for nearly 90% of global gastric cancer11. H. pylori has been estimated to be the single most common cancer-causing infectious agent in the world, responsible for over one-third of cancers caused by infections11. Statistics like those, as well as studies showing the decrease in gastric cancer caused by population eradication efforts (see, e.g., May, 2016 Gastroenterology12), lends support to the Japanese effort to eradicate H. pylori in the population at large (see, e.g., the Japanese approach discussed in Gastroenterology from January, 201813). However, the vast majority of people colonized by H. pylori do not get gastric cancer. A recent meta-analysis in the August, 2017 Gastroenterology showed that almost 4 and a half billion people are infected worldwide14, yet far less than 1% will develop gastric cancer. What is different about cases where gastric cancer occurs? In part, it may be the strain of H. pylori, with those expressing the oncogenic toxin CagA much more tumorigenic than CagA- strains. There are even variants of CagA in East Asian strains that may further increase risk15 (see also Gastroenterology, July, 200816). A recent article (February, 2018) in Gastroenterology highlighted how the rapidly mutating H. pylori genetic and epigenetic landscape might complicate population-based studies of how different strains might increase cancer risk17.
Host genetic and epigenetic variation may also affect whether H pylori causes gastric cancer. Multiple articles published over the past two decades in Gastroenterology chart our growing understanding of the influence of host genetics on disease progression; for example, in reverse chronological order just from the past decade: 1) FOXD3 was shown to be epigenetically dysregulated in gastric carcinogenesis (January, 2013)18, 2) variants in the autophagy pathway-associated gene ATG16L1 were shown to increase risk to H. pylori infection (May, 2012)19, 3) TFF2 methylation was shown to increase in H. pylori infection and gastric tumorigenesis (December, 2010)20, 4) the RNA/DNA editing enzyme activation-induced cytidine deaminase (AID) shows aberrant expression in H. pylori infected patients, which may cause mutation of the tumor suppressor CDKN2b-CDKN2a locus (December, 2010)21, 5) methylation of O6-methylguanine DNA methyltransferase (MGMT) is increased in patients infected with CagA+ H. pylori, suggesting disrupted DNA repair (May, 2010)22, 6) polymorphism in the TLR4 gene increases risk of gastric cancer (March, 2007)23. One of the first host polymorphisms to be described that increases risk for gastric cancer precursor lesions was in the gene IL1B, reported in the July, 2002 issue24, an article cited over 270 times.
In addition to numerous gene polymorphisms that increase risk -- especially in the context of H. pylori infection – discussed above, the field has progressed tremendously in the understanding of the genetic determinants for diffuse gastric cancers of the signet ring variety, which are rarer and less associated with H pylori infection. Mutations in the CDH1 (E-cadherin) gene have been shown to be responsible for a large fraction of hereditary diffuse gastric cancer cases25–28. The association between CHD1 mutations and breast and gastric cancer was studied in a large cohort and published in the December, 2001 Gastroenterology29.
Other environmental factors interact with H. pylori to help promote gastric cancer. For example, lower host cholesterol levels can block H. pylori pathogenesis (reviewed in9), with an article currently in press in Gastroenterology indicating that the bacteria may scavenge cholesterol to help mute the inflammatory response30. Iron deficiency seems to accelerate gastric carcinogenesis via interaction with H. pylori (reviewed in9 and see especially ref31); a relationship between H. pylori and iron was noted as early as the August, 1998 issue of Gastroenterology32. Other environmental factors that may influence risk for gastric cancer include the level of salt (high salt increases risk) and fresh fruits and vegetables (high and diverse intake decrease risk). Thus, many environmental factors, most centered around H. pylori infection, have been discovered to increase gastric cancer risk since the Boles review in 1958, and much of the key work has been published in Gastroenterology.
Advances in understanding mechanisms of H. pylori function as a carcinogen along with other environmental and host factors has emerged from research on rodent models, such as a seminal article in January, 2000 on how mice with genetically-induced hypergastrinemia model gastric tumorigenesis33 (a paper cited over 425 times) with a follow up in June, 2003 (cited over 141 times), and the introduction of a Mongolian gerbil model in the September, 1998 issue34 (cited over 844 times), that helped fulfill Koch’s postulates as to the bacterium as a carcinogen35.
Relationship of Gastric Ulcers with Gastric Cancer
Progress has been made on Boles’s question #5 regarding the relationship between gastric ulcers and gastric cancer. Whether gastric cancers tended to arise in ulcers, or whether gastric cancers developed first and then frequently ulcerated has been an issue that has perplexed gastroenterologists since the time of Ménétrier36, who himself cited references up to a half century earlier than when he wrote on this topic in 1900. In fact, the issue of ulcers and cancer was in part the subject of Sara Jordan’s and Frank Lahey’s article on pages 1–12 of the first issue of Gastroenterology in 194337, the anniversary of which we are commemorating with this series of reviews! Our understanding since the era of Boles and his predecessors has increased substantially as we now know that H. pylori is also the cause of nearly all duodenal and the majority of gastric peptic ulcers. An early article on this link appeared in the February, 1989 Gastroenterology38 (cited over 650 times), which described how Helicobacter (then called Campylobacter) pylori gastritis in the antrum led to chronic increase in acid production, which was the underlying cause of peptic ulcers. In a Reply to the 1989 article (November, 1990), a link was also noted between H. pylori and atrophic gastritis39, which, as we discuss below, is a lesion that indicates increased risk for gastric cancer.
Atrophic gastritis occurs when, in some patients, H. pylori spreads proximally from its original and more common antral niche into the body and fundus of the stomach. The associated inflammatory reaction correlates with loss of parietal cells and decreased acid production. Thus, the consensus in the field is that peptic ulcers and gastric cancer are related in having H. pylori as an important etiological agent but largely reflect two different courses (or stages) of disease caused by the bacterium: one characterized by increased acid production and ulceration, and the other by decreased acid production and potential progression to cancer. Some authors, including the one who penned the 1989 article, argued that ulcers (especially those in the body/fundus) may also be earlier manifestations with cancer being a later manifestation of the disease40. In either case, the consensus is that the vast majority of ulcers in which cancer is found are due to the growth of the cancer in an ulcerated morphology and not due to cancer arising within an earlier, active, benign peptic ulcer. Thus, while a diagnostic challenge for the endoscopist as to whether an ulcer is benign and peptic or an ulcerated malignant cancer, the etiology of the two entities has been reasonably well established since the Boles review.
Cellular Remodeling, Gastric Metaplasia and Cancer Progression
Finally, now that we, as a field, have resolved how H. pylori plays a dual role in ulcers, and that benign peptic ulcers are not usually direct precursor lesions for gastric cancer, we can turn to Boles’s issues #3 (the role of precursor lesions like atrophic gastritis in tumorigenesis) and #4 (the link between achlorhydria and progression to cancer). We can also touch on the final aspect of issue #1 (injurious agents and cancer). The idea that gastric atrophy, involving loss of the normal mature cells of the body/fundus, was a precursor state for gastric cancer had already been postulated by Ménétrier, who noted by 1900 that cancers seem to arise in regions where the rich cellular architecture of gastric glands had atrophied to relatively simple glands filled with mucus-stuffed cells36. The pathologist George Adami in the same year had also remarked how, as glandular tissue aged, cancers seemed to arise after they’d atrophied41. Both had speculated that infectious agents or other irritants caused these changes. As discussed, we now know that H. pylori is the infectious agent at the root of most gastric cancer. In recent years, we have learned, with many of the most important papers published in Gastroenterology (e.g., April 200842 cited over 269 times), that, when H. pylori spreads beyond the antrum to cause gastritis of the whole stomach (“pangastritis”), it slowly causes the pattern of inflammation and cellular changes known as chronic atrophic gastritis40, 43. The cells that are atrophied are mature digestive-enzyme-secreting chief and acid-secreting parietal cells (see, e.g., February, 2011 issue44). Just as Ménétrier described, the metaplastic cells that replace the atrophied parietal and chief cells are stuffed with mucins (like MUC6), and with other proteins (like TFF2), whose expression is normally in a much more limited cohort of progenitor cells in the isthmus or neck of the gland (see, e.g., Gastroenterology45, 46. Thus, atrophic gastritis is invariably associated with metaplasia, which has been called pyloric or pseudopyloric (because the pattern of mucinous cells from top to bottom of the gastric unit resembles the antrum/pyloric region) or Spasmolytic Polypeptide Expressing Metaplasia (SPEM), as TFF2’s erstwhile name was Spasmolytic Polypeptide47. Additionally, it is the view of the authors that this pattern of metaplasia may resemble the juvenile or embryonic stomach most of all, and may represent a conserved repair process when deep glandular cells like parietal cells are injured48.
The link between SPEM/atrophy and risk for gastric cancer is well established, and, thus, the reason why achlorhydria indicates increased risk for gastric cancer is straightforward: the less acid, the less parietal cell mass, the more atrophy. Interestingly, another metaplasia has drawn a great deal more attention during the second half of the 20th Century: intestinal metaplasia. A relatively early mention of the potential role for “intestinalization” of the stomach as a gastric cancer precursor was in the March, 1964 Issue49. Given that eventual gastric cancers that arise have, to varying degrees from region to region and from person to person, intestinal morphological characteristics, has suggested that tumors arise from intestinal metaplasia. It seems clear that SPEM or pseudopyloric metaplasia arises first, and some have argued that intestinal metaplasia may arise from SPEM, making SPEM the root lesion, even if intestinal metaplasia or certain types of intestinal metaplasia may be the more proximate route to gastric cancer (see, e.g., June, 2010 issue50).
Current Questions
Why SPEM increases the risk for cancer is open to debate; however, the plasticity that must occur for the stomach to retool the types of cells that are produced may carry inherent risk43. Some have proposed that the change in cell fate may occur in the actively proliferating isthmal progenitor cells (e.g., June, 2017 issue51). In contrast, others have made the case in multiple issues of Gastroenterology52–55 that it seems more likely that mature chief cells scale down their secretory architecture through a conserved set of cellular and molecular events (recently dubbed “paligenosis”56) to become proliferative SPEM cells.
Currently, the field is in an active state of debate about the mechanisms that determine which cells fuel metaplasia (see, e.g., the summary on the Freston metaplasia meeting in the July, 2017 issue57). Time will tell whether a stem-cell based or mature-cell based plasticity event (or both) is the one that occurs in human patients. In multiple other organs, mature secretory cells, like chief cells, can undergo cellular reprogramming (paligenosis) to become proliferative56. There would be risks associated with a long-lived cell, such as a chief cell, that could warehouse mutations for months or years, recalled into a state wherein oncogenes and epigenetic changes favoring the progenitor or embryonic state could be unmasked. This would especially be true over cycles of recruitment as progenitor cells (via paligenosis) and redifferentiation43, 56 where mutations can accumulate over time.
More research – in our underfunded field! -- will eventually help reveal how atrophic gastritis and metaplasias contribute to gastric cancer, with many more exciting studies sure to come. The bulk of the most important work will likely continue to be published in Gastroenterology. Certainly, one can hope that we will be much farther into understanding the key questions on gastric cancer posed by Boles well before we reach the second diamond or sesquicentennial anniversary of the journal!
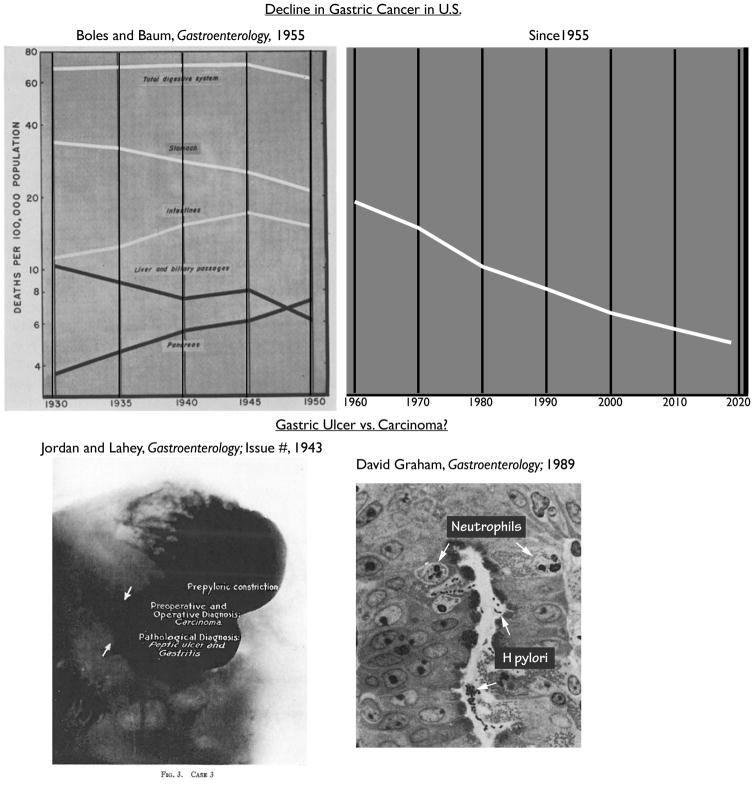
Figure. Gastric Cancer Then and Now.
In some cases, we have learned a lot since 1958 when Boles wrote a review in Gastroenterology on gastric cancers. Top-Boles had identified a surprising decline in deaths due to gastric cancer in his 1955 article4 on the subject in Gastroenterology (graphed are white male deaths). That trend continued through the end of the 20th Century and then has since leveled off (note log axis). Data in graph on right estimated from multiple sources but primarily based on the American Cancer Society online tool (https://cancerstatisticscenter.cancer.org). The reasons for the decline are not entirely clear but are discussed in the text. Bottom- Gastroenterologists have struggled with distinguishing benign gastric ulcers from cancer since the 18th Century and the relationship between the two had been unclear until the role of H pylori in ulcers and cancer was discovered. Depicted is a roentgenogram with contrast (Fig. 3 from the first article in the first issue of Gastroenterology) from a 58 year-old male who had black stools and 6 weeks of epigastric distress relieved somewhat by food; there was no weight loss. Achlorhydria and slight anemia with constricted antrum led to concern about gastric cancer and exploratory laparotomy with pathology showing a punched-out 1 cm ulcer and ultimate diagnosis of chronic peptic ulcer. At right, a portion of a photomicrograph from one of the early Gastroenterology papers discussing pathology caused by H pylori, the bacteria that were, at the time, being revealed to be the primary cause of both peptic ulcers and gastric cancer.
Acknowledgments
Funding: JCM is supported by grants NIDDK R01s (DK094989, DK105129, DK110406), Alvin J. Siteman Cancer Center/Barnes Jewish Hospital Foundation Cancer Frontier Fund, NIH NCI P30 CA091842, The Barnard Trust, and DeNardo Education & Research Foundation grants to J.C.M., L.C.S. has research support through NIH P01-DK062041, NIH 5R01-DK096972, NCI P50-CA130810 and the AGA Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Author names in bold designate shared co-first authorship
- 1.Boles RS. Cancer of the stomach. Gastroenterology. 1958;34:847–58. [PubMed] [Google Scholar]
- 2.Segal HL, Miller LL. Present status and possibilities on ion exchange compounds as tubeless agents for determining gastric acidity. Gastroenterology. 1955;29:633–9. discussion, 639–40. [PubMed] [Google Scholar]
- 3.Ross JR, Griffin BG, Jordan SM. “Gastritis” and its sequelae. Gastroenterology. 1952;22:205–13. [PubMed] [Google Scholar]
- 4.Boles RS, Baum WS. An apparent change of incidence in cancer of the stomach. Gastroenterology. 1955;28:367–77. [PubMed] [Google Scholar]
- 5.Carter AJ, Nguyen CN. A comparison of cancer burden and research spending reveals discrepancies in the distribution of research funding. BMC Public Health. 2012;12:526. doi: 10.1186/1471-2458-12-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter AJ, Delarosa B, Hur H. An analysis of discrepancies between United Kingdom cancer research funding and societal burden and a comparison to previous and United States values. Health Res Policy Syst. 2015;13:62. doi: 10.1186/s12961-015-0050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maruthappu M, Head MG, Zhou CD, et al. Investments in cancer research awarded to UK institutions and the global burden of cancer 2000–2013: a systematic analysis. BMJ Open. 2017;7:e013936. doi: 10.1136/bmjopen-2016-013936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merchant SJ, Kim J, Choi AH, et al. A rising trend in the incidence of advanced gastric cancer in young Hispanic men. Gastric Cancer. 2017;20:226–234. doi: 10.1007/s10120-016-0603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park JY, Forman D, Waskito LA, et al. Epidemiology of Helicobacter pylori and CagA-Positive Infections and Global Variations in Gastric Cancer. Toxins (Basel) 2018:10. doi: 10.3390/toxins10040163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang L. Barry Marshall 2005 Nobel laureate in medicine and physiology. Gastroenterology. 2005;129:1813–4. doi: 10.1053/j.gastro.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 11.Plummer M, de Martel C, Vignat J, et al. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4:e609–16. doi: 10.1016/S2214-109X(16)30143-7. [DOI] [PubMed] [Google Scholar]
- 12.Lee YC, Chiang TH, Chou CK, et al. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology. 2016;150:1113–1124. e5. doi: 10.1053/j.gastro.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 13.Sugano K, Hiroi S, Yamaoka Y. Prevalence of Helicobacter pylori Infection in Asia: Remembrance of Things Past? Gastroenterology. 2018;154:257–258. doi: 10.1053/j.gastro.2017.08.074. [DOI] [PubMed] [Google Scholar]
- 14.Hooi JKY, Lai WY, Ng WK, Suen MMY, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Higashi H, Tsutsumi R, Fujita A, et al. Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc Natl Acad Sci U S A. 2002;99:14428–33. doi: 10.1073/pnas.222375399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basso D, Zambon CF, Letley DP, et al. Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphisms. Gastroenterology. 2008;135:91–9. doi: 10.1053/j.gastro.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 17.Nell S, Estibariz I, Krebes J, et al. Genome and Methylome Variation in Helicobacter pylori With a cag Pathogenicity Island During Early Stages of Human Infection. Gastroenterology. 2018;154:612–623. e7. doi: 10.1053/j.gastro.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Cheng AS, Li MS, Kang W, et al. Helicobacter pylori causes epigenetic dysregulation of FOXD3 to promote gastric carcinogenesis. Gastroenterology. 2013;144:122–133. e9. doi: 10.1053/j.gastro.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Raju D, Hussey S, Ang M, et al. Vacuolating cytotoxin and variants in Atg16L1 that disrupt autophagy promote Helicobacter pylori infection in humans. Gastroenterology. 2012;142:1160–71. doi: 10.1053/j.gastro.2012.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson AJ, Menheniott TR, O’Connor L, et al. Helicobacter pylori infection promotes methylation and silencing of trefoil factor 2, leading to gastric tumor development in mice and humans. Gastroenterology. 2010;139:2005–17. doi: 10.1053/j.gastro.2010.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto Y, Marusawa H, Kinoshita K, et al. Up-regulation of activation-induced cytidine deaminase causes genetic aberrations at the CDKN2b-CDKN2a in gastric cancer. Gastroenterology. 2010;139:1984–94. doi: 10.1053/j.gastro.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Sepulveda AR, Yao Y, Yan W, et al. CpG methylation and reduced expression of O6-methylguanine DNA methyltransferase is associated with Helicobacter pylori infection. Gastroenterology. 2010;138:1836–44. doi: 10.1053/j.gastro.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 23.Hold GL, Rabkin CS, Chow WH, et al. A functional polymorphism of toll-like receptor 4 gene increases risk of gastric carcinoma and its precursors. Gastroenterology. 2007;132:905–12. doi: 10.1053/j.gastro.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 24.Furuta T, El-Omar EM, Xiao F, et al. Interleukin 1beta polymorphisms increase risk of hypochlorhydria and atrophic gastritis and reduce risk of duodenal ulcer recurrence in Japan. Gastroenterology. 2002;123:92–105. doi: 10.1053/gast.2002.34156. [DOI] [PubMed] [Google Scholar]
- 25.Grady WM, Willis J, Guilford PJ, et al. Methylation of the CDH1 promoter as the second genetic hit in hereditary diffuse gastric cancer. Nat Genet. 2000;26:16–7. doi: 10.1038/79120. [DOI] [PubMed] [Google Scholar]
- 26.Huntsman DG, Carneiro F, Lewis FR, et al. Early gastric cancer in young, asymptomatic carriers of germ-line E-cadherin mutations. N Engl J Med. 2001;344:1904–9. doi: 10.1056/NEJM200106213442504. [DOI] [PubMed] [Google Scholar]
- 27.Keller G, Vogelsang H, Becker I, et al. Diffuse type gastric and lobular breast carcinoma in a familial gastric cancer patient with an E-cadherin germline mutation. Am J Pathol. 1999;155:337–42. doi: 10.1016/S0002-9440(10)65129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richards FM, McKee SA, Rajpar MH, et al. Germline E-cadherin gene (CDH1) mutations predispose to familial gastric cancer and colorectal cancer. Hum Mol Genet. 1999;8:607–10. doi: 10.1093/hmg/8.4.607. [DOI] [PubMed] [Google Scholar]
- 29.Pharoah PD, Guilford P, Caldas C, et al. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology. 2001;121:1348–53. doi: 10.1053/gast.2001.29611. [DOI] [PubMed] [Google Scholar]
- 30.Morey P, Pfannkuch L, Pang E, et al. Helicobacter pylori Depletes Cholesterol in Gastric Glands to Prevent Interferon Gamma Signaling and Escape the Inflammatory Response. Gastroenterology. 2018;154:1391–1404. e9. doi: 10.1053/j.gastro.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Noto JM, Gaddy JA, Lee JY, et al. Iron deficiency accelerates Helicobacter pylori-induced carcinogenesis in rodents and humans. J Clin Invest. 2013;123:479–92. doi: 10.1172/JCI64373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milman N, Rosenstock S, Andersen L, et al. Serum ferritin, hemoglobin, and Helicobacter pylori infection: a seroepidemiologic survey comprising 2794 Danish adults. Gastroenterology. 1998;115:268–74. doi: 10.1016/s0016-5085(98)70192-1. [DOI] [PubMed] [Google Scholar]
- 33.Wang TC, Dangler CA, Chen D, et al. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118:36–47. doi: 10.1016/s0016-5085(00)70412-4. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe T, Tada M, Nagai H, et al. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology. 1998;115:642–8. doi: 10.1016/s0016-5085(98)70143-x. [DOI] [PubMed] [Google Scholar]
- 35.Wang TC, Fox JG. Helicobacter pylori and gastric cancer: Koch’s postulates fulfilled? Gastroenterology. 1998;115:780–3. doi: 10.1016/s0016-5085(98)70159-3. [DOI] [PubMed] [Google Scholar]
- 36.Ménétrier PE. ÉTIOLOGIE GÉNÉRALE DES TUMEURS. In: Bouchard CJ, editor. TRAITE DE PATHOLOGIE GÉNÉRALE. Vol. 3. Paris: G. Masson; 1900. pp. 762–796. [Google Scholar]
- 37.Jordan SM, FHL Prepyloric lesions of the stomach: medical and surgical aspects. Gastroenterology. 1943;1:1–12. [Google Scholar]
- 38.Graham DY. Campylobacter pylori and peptic ulcer disease. Gastroenterology. 1989;96:615–25. doi: 10.1016/s0016-5085(89)80057-5. [DOI] [PubMed] [Google Scholar]
- 39.Faisal MA, Russell RM, Samloff IM, et al. Helicobacter pylori infection and atrophic gastritis in the elderly. Gastroenterology. 1990;99:1543–4. doi: 10.1016/0016-5085(90)91213-p. [DOI] [PubMed] [Google Scholar]
- 40.Graham DY. History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J Gastroenterol. 2014;20:5191–204. doi: 10.3748/wjg.v20.i18.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adami JG. “Festschrift” in Honor of Abraham Jacobi, M.D., L.L.D.: To Commemorate the Seventieth Anniversary of His Birth. May Sixth, 1900. Knickerbocker Press; 1900. On Growth and Overgrowth; pp. 422–432. [Google Scholar]
- 42.de Vries AC, van Grieken NC, Looman CW, et al. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945–52. doi: 10.1053/j.gastro.2008.01.071. [DOI] [PubMed] [Google Scholar]
- 43.Saenz JB, Mills JC. Acid and the basis for cellular plasticity and reprogramming in gastric repair and cancer. Nat Rev Gastroenterol Hepatol. 2018;15:257–273. doi: 10.1038/nrgastro.2018.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mills JC, Shivdasani RA. Gastric epithelial stem cells. Gastroenterology. 2011;140:412–24. doi: 10.1053/j.gastro.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nomura S, Baxter T, Yamaguchi H, et al. Spasmolytic polypeptide expressing metaplasia to preneoplasia in H. felis-infected mice. Gastroenterology. 2004;127:582–94. doi: 10.1053/j.gastro.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 46.Nozaki K, Ogawa M, Williams JA, et al. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology. 2008;134:511–22. doi: 10.1053/j.gastro.2007.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt PH, Lee JR, Joshi V, et al. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest. 1999;79:639–46. [PubMed] [Google Scholar]
- 48.Keeley TM, Samuelson LC. Cytodifferentiation of the postnatal mouse stomach in normal and Huntingtin-interacting protein 1-related-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1241–51. doi: 10.1152/ajpgi.00239.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monroe LS, Boughton GA, Sommers SC. The Association of Gastric Epithelial Hyperplasia and Cancer. Gastroenterology. 1964;46:267–72. [PubMed] [Google Scholar]
- 50.Goldenring JR, Nam KT, Wang TC, et al. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology. 2010;138:2207–10. 2210 e1. doi: 10.1053/j.gastro.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayakawa Y, Wang TC. Isthmus Progenitors, Not Chief Cells, Are the Likely Origin of Metaplasia in eR1-CreERT; LSL-Kras(G12D) Mice. Gastroenterology. 2017;152:2078–2079. doi: 10.1053/j.gastro.2017.02.043. [DOI] [PubMed] [Google Scholar]
- 52.Goldenring JR, Mills JC. Isthmus Time Is Here: Runx1 Identifies Mucosal Stem Cells in the Gastric Corpus. Gastroenterology. 2017;152:16–19. doi: 10.1053/j.gastro.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 53.Hibdon ES, Samuelson LC. Cellular Plasticity in the Stomach: Insights Into the Cellular Origin of Gastric Metaplasia. Gastroenterology. 2018;154:801–803. doi: 10.1053/j.gastro.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsuo J, Kimura S, Yamamura A, et al. Identification of Stem Cells in the Epithelium of the Stomach Corpus and Antrum of Mice. Gastroenterology. 2017;152:218–231. e14. doi: 10.1053/j.gastro.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 55.Radyk MD, Burclaff J, Willet SG, et al. Metaplastic Cells in the Stomach Arise, Independently of Stem Cells, via Dedifferentiation or Transdifferentiation of Chief Cells. Gastroenterology. 2018;154:839–843. e2. doi: 10.1053/j.gastro.2017.11.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Willet SG, Lewis MA, Miao ZF, et al. Regenerative proliferation of differentiated cells by mTORC1-dependent paligenosis. EMBO J. 2018:37. doi: 10.15252/embj.201798311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spechler SJ, Merchant JL, Wang TC, et al. A Summary of the 2016 James W. Freston Conference of the American Gastroenterological Association: Intestinal Metaplasia in the Esophagus and Stomach: Origins, Differences, Similarities and Significance Gastroenterology. 2017;153:e6–e13. doi: 10.1053/j.gastro.2017.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]