Abstract
Aims:
Visceral adiposity measured by computed tomography (CT) as intra-abdominal fat area (IAFA) predicts metabolic diseases. Existing adiposity surrogates have not been systematically compared to a regression-based model derived in individuals of Japanese ancestry. We developed and validated a method to estimate IAFA in individuals of Japanese ancestry and compared it to existing adiposity surrogates.
Methods:
We assessed age, BMI, waist circumference (WC), fasting lipids, glucose, smoking status, grip strength, mid-thigh circumference (MTC), humeral length, leg length, and IAFA by single-slice CT at the umbilicus for 622 Japanese Americans. We used stepwise linear regression to predict IAFA and termed the predicted value the Estimate of Visceral Adipose Tissue Area (EVA). For men, the final model included age, BMI, WC, high-density lipoprotein cholesterol (HDLc), glucose, and MTC; for women, age, BMI, WC, HDLc, low-density lipoprotein cholesterol, glucose, and MTC. We compared goodness-of-fit (R2) from linear regression models and mean-squared errors (MSE) from k-fold cross-validation to compare the ability of EVA to estimate IAFA compared to an estimate by Despres et al., waist-to-height ratio, WC, deep abdominal adipose tissue index, BMI, lipid accumulation product, and visceral adiposity index (VAI). We classified low/high IAFA using area under receiver-operating characteristic curves (AUROC) for IAFA dichotomized at the 75th percentile.
Results:
EVA gave the least MSE and greatest R2 (men: 1244, 0.61; women: 581, 0.72). VAI gave the greatest MSE and smallest R2 (mean 2888, 0.08; women 1734, 0.14).
Conclusions:
EVA better predicts IAFA in Japanese-American men and women compared to existing surrogates for adiposity.
Keywords: visceral adiposity, body mass index, waist-to-height ratio, abdominal obesity, waist circumference, anthropometrics
1. INTRODUCTION
Excess visceral adipose tissue, quantified as intra-abdominal fat area (IAFA), is a major risk factor for metabolic disease in Japanese Americans1-6. Surrogate measures of visceral adiposity derived from anthropometric measures including body mass index (BMI) and waist circumference (WC) are routinely used7 in both clinical practice and epidemiologic research, as direct measurement of IAFA is often prohibitively expensive or logistically difficult to obtain. Measures incorporating blood-based biomarkers have also been used to estimate IAFA, including the Lipid Accumulation Product8 and the Visceral Adiposity Index9.
When compared to White populations, Asians have more visceral fat10,11 and a higher body fat percentage for a given BMI12. Adiposity surrogates derived in non-Asian populations might therefore perform poorly when applied to Japanese, Japanese-American, and other Asian populations10. We therefore examined whether an alternate adiposity surrogate might more closely parallel the quantity of IAFA among individuals of Japanese ancestry. We hypothesized that an adiposity surrogate incorporating widely available demographic information, anthropometric data, and blood-based biomarkers could predict computed tomography (CT)-measured IAFA better in Japanese Americans better than existing visceral adiposity surrogates. These include anthropometric measures such as BMI, WC, and WHtR; indices experimentally derived from clinical and anthropometric measurements such as the Deep Abdominal Adipose-Tissue index13 and an index calculated by Després14; and indices derived from clinical, anthropometric and blood-based measurements such as the Visceral Adiposity Index9 and the Lipid Accumulation Product8.
2. MATERIALS/SUBJECTS & METHODS
2.1. Study design and setting
We measured demographic, metabolic and body composition variables in 622 Japanese-American men and women from the Japanese-American Community Diabetes Study (JACDS), a community-based cohort of second- and third-generation Japanese Americans of 100% Japanese ancestry who lived in King County, Washington State. These individuals were representative of Japanese-American residents of the county in age, residential distribution and parental immigration pattern. JACDS was initially designed to investigate risk factors for and prevalence of type 2 diabetes among Japanese Americans. Individuals were recruited between 1983 and 1991. Selection and recruitment have been described previously15. Of an initial sample of 658 individuals, 36 were missing data on covariates, leaving an analytic sample of 622 participants. The study was approved by the University of Washington Human Subjects Division, and all participants provided written informed consent.
2.2. Data collection
Evaluations were done at the General Clinical Research Center at the University of Washington, Seattle. Information on age, sex, and current and former tobacco use was obtained by interview. Trained staff measured height, weight and WC. Weight was measured using a digital scale after shoes and outer clothing were removed. BMI was defined as weight in kg divided by height in meters squared. WC was measured twice using a flexible but non-stretchable tape measure and averaged. In male participants, the measurement was obtained at the midpoint between the iliac crest and the inferior border of the lowest rib. In female participants, the minimal circumference between iliac crest and lowest rib was used. WHtR was defined as waist circumference in cm divided by height in cm. Serum glucose, triglycerides (TG), high-density lipoprotein cholesterol (HDLc) and low-density lipoprotein cholesterol (LDLc) were measured in specimens taken after at least a 10-hour fast.
For comparison to our new index, we chose seven existing measures that have been used as IAFA surrogates. Measures included BMI, WC, and WHtR; indices experimentally derived from clinical and anthropometric measurements such as the Deep-Abdominal Adipose Tissue index13 and an index calculated by Després14; and indices derived from clinical, anthropometric and blood-based measurements such as the Visceral Adiposity Index9 and the Lipid Accumulation Product8. We calculated sex-specific values of each index for all participants. The Deep-Abdominal Adipose Tissue index was defined as −382.9 + [1.09 × weight (kg)] + [6.04 × WC (cm)] + (−2.29 × BMI) for men, and −278 + [−0.86 × weight (kg)] + [5.19 × WC (cm)] for women13. The Després index was defined as −225.39 + 2.125 × age (years) + 2.843 × WC (cm)14.Because the Després index was derived in a male cohort, we restricted the analysis of this index to the men in our dataset. The Visceral Adiposity Index was defined as {WC (cm)/(39.68 + [1.88 × BMI (kg/m2)]} × (triglycerides/1.03) × (1.31/HDL) for men, and {WC (cm)/(36.58 + [1.89 × BMI (kg/m2)]} × (triglycerides/0.81) × (1.52/HDL) for women9, with both triglycerides and HDL specified in mmol/L. The Lipid Accumulation Product was defined as [(WC (cm) − 65) × TG (mmol/L)] in men, and [(WC (cm) − 58) × TG (mmol/L)] in women8.
To estimate IAFA, single (1cm) CT-scan slices were obtained at the level of the umbilicus. Scans were analyzed for cross-sectional area of fat using automated density contour software. Areas corresponding to a density of −250 to −50 Hounsfield units were classified as adipose tissue16. IAFA was defined as fat within the confines of the transversalis fascia and reported in cm2. Intra-observer variability for multiple measurements by a single observer of a single CT scan ranged from 0.2% to 1.4%.
2.3. Statistical analysis
We used number (%) and mean (standard deviation) for categorical and normally distributed continuous variables, respectively, to describe study population characteristics, both overall and stratified by sex. For TG, we reported median (inter-quartile range), as the distribution was skewed. To derive a novel adiposity estimate, we used backward stepwise linear regression to fit models using easy-to-obtain demographic, anthropometric, and blood-based measurements (age, BMI, WC, HDLc, LDLc, TG, fasting glucose, and smoking status) to predict IAFA. A p-value ≤0.05 allowed entry into the model, while a p-value of 0.1 or greater prompted removal. Because there was evidence of a multiplicative first-order interaction of sex with WC, we chose to perform analyses stratified by sex. We fit linear regression models looking for evidence of nonlinearity in associations of metabolic variables and body composition with IAFA using the Stata command mfp. We also performed model diagnostics to identify influential outliers and for evidence of violations of model assumptions17. We termed the new index the Estimate of Visceral Adipose Tissue Area (EVA). We dichotomized IAFA at the 75th percentile values and plotted receiver-operating characteristic (ROC) curves to estimate ability of EVA to categorize IAFA as below or above the 75th percentile.
We compared performance of EVA to the seven previously described adiposity surrogates. Because mid-thigh circumference may be assessed less frequently than the other anthropometric measures that were collected, we also compared performance of EVA to a version of our model that did not include mid-thigh circumference, which we called EVA-R (reduced). We regressed IAFA on each adiposity surrogate in a univariate linear regression model. We compared R2 values among the models and identified the model with the lowest Akaike Information Criterion (AIC). We validated results internally using k-fold cross-validation to compare mean-squared errors (MSE)18. For these analyses, data were partitioned into five subsamples repeatedly. Four sub-samples were combined as the training dataset and the last was left out as a validation dataset. Prediction errors (mean squared errors) were obtained on the left-out group. For each round this was repeated with each of the sub-samples used as the validation dataset. This was repeated 500 times. Errors were averaged, and 95% confidence intervals were calculated. Lastly, we compared performance of the seven visceral adiposity surrogates to EVA in discriminating low vs. high categories of IAFA. We used sex-specific 75th percentile cutpoints taken from the training dataset for all analyses (140 cm2 [men] and 100cm2 [women]). We compared the area under the ROC curves (AUROC) using chi-squared tests19.
For AUROC comparisons, a p-value of <0.05 was considered statistically significant. MSE with non-overlapping 95% confidence intervals were considered statistically significantly different. Analyses were performed with Stata (version 15.0; StataCorp, College Station, TX).
3. RESULTS
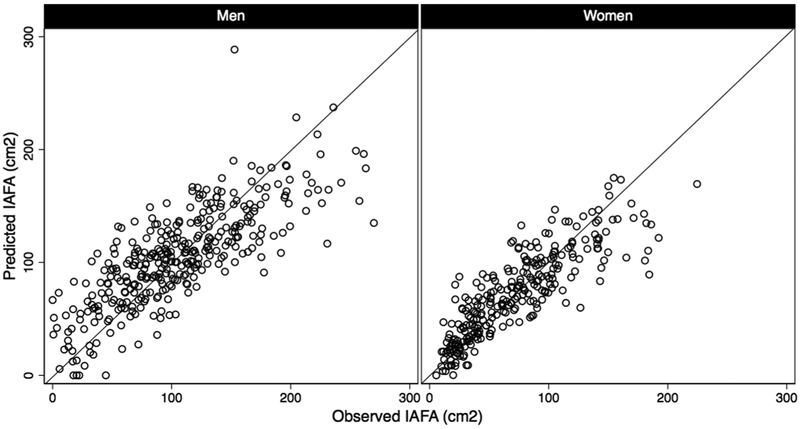
Table 1 shows participant characteristics overall and stratified by sex. Coefficients and p-values from sex-specific linear regression models testing associations of metabolic risk factors with IAFA in the derivation cohort are shown in Table 2. All the variables shown in Table 1 with the exception of IAFA and diabetes status were considered in the multivariable models described in Table 2. Using the stepwise methods described above, LDLc, triglycerides, smoking status, grip strength, humeral length and leg length were dropped from the model in men, leaving age, BMI, WC, HDLc, fasting glucose and mid-thigh circumference. In the model for women, triglycerides, smoking status, grip strength, humeral length, and leg length were dropped from the model, leaving age, BMI, WC, HDLc, LDLc, fasting glucose, and mid-thigh circumference. A test of regression assumptions found that for the model in women, the linear form of IAFA violated regression assumptions, while the square root transformation did not; however, nearly identical results were obtained with either the linear or square root transformation of IAFA as the dependent variable, so the linear version was used to permit easier interpretation of our findings. An examination of best fitting transformations of the independent variables using mfp revealed that the best fit was obtained with the linear versions of these covariates as shown in Table 2. Model diagnostics did not identify influential outliers, and there was no further evidence of violations of model assumptions. Sex-specific scatter plots of IAFA vs. fitted values of EVA for men and women are presented in Figure 1.In sensitivity analyses, we replaced BMI with height and weight as separate terms and BMI and waist circumference with weight and WHtR in our primary models. We also performed analyses including fasting insulin level as a covariate. The performance characteristics of the models obtained did not differ substantially from the primary models. Results comparing R2 and AIC for linear regression models, MSE from k-fold cross-validation, and AUROC for prediction of IAFA above the 75th percentile comparing existing adiposity surrogates to EVA in prediction of IAFA are shown in Table 3. EVA exhibited the highest R2 and lowest AIC in multiple regression models with continuous IAFA as the dependent variable for both sexes. It had significantly lower MSE for both men and women than all other models compared. For men, the AUROC for EVA in discriminating IAFA above the 75th percentile was significantly higher than all other measures compared, with the exception of the Després estimate (p=0.091). For women, the AUROC for EVA in discriminating IAFA above the 75th percentile was significantly higher than all other measures compared, with the exception of the EVA-R (p=0.771). In a “partial validation,” we validated our limited model (the EVA-R) in a cohort of Japanese participants (n=1033 men, n=612 women), but we were unable to validate the full model because the validation cohort did not have measurements of mid-thigh circumference available. R2 and AUROC were similar in the validation cohort. (Details of the methods and results for the partial validation are available in Supplementary Online Methods and Supplementary Table 1).
Table 1.
Baseline characteristics of Japanese American Community Diabetes Study participants with complete data on all covariates
| Overall n = 622 |
Men n = 344 |
Women n = 278 |
p-value* | |
|---|---|---|---|---|
| Mean age, years (SD) | 53.7 (11.8) | 54.2(11.4) | 53.1 (12.4) | 0.25 |
| Sex, % male (n) | 55(344) | — | — | — |
| Mean body mass index, kg/m2 (SD) | 24.5 (3.3) | 25.5 (3.0) | 23.2 (3.2) | <0.0001 |
| Mean waist circumference, cm (SD) | 83.3 (10.5) | 88.8 (8.2) | 76.5 (9.0) | <0.0001 |
| Mean HDL cholesterol, mg/dL (SD) | 57(17) | 50 (13) | 65 (16) | <0.0001 |
| Mean LDL cholesterol, mg/dL (SD) | 141(37) | 146 (38) | 135 (34) | 0.0002 |
| Median triglyceride level, mg/dL (IQR) | 118 (79-167) | 133 (95-207) | 95 (67-135) | <0.0001 |
| Mean fasting glucose, mg/dL (SD) | 106 (37) | 114 (43) | 97 (25) | <0.0001 |
| Smoking status | <0.0001 | |||
| Current smoker, % (n) | 13 (80) | 15 (50) | 11 (30) | |
| Former smoker, % (n) | 42 (261) | 58 (198) | 23 (63) | |
| Mean grip strength, lbs (SD) | 49.9 (14.0) | 59.1 (10.7) | 37.6(6.5) | <0.0001 |
| Mean mid-thigh circumference, cm (SD) | 49.2 (4.5) | 50.1 (4.2) | 48.1 (4.7) | <0.0001 |
| Mean humeral length, cm (SD) | 29.0 (2.2) | 30.2(1.9) | 27.4(1.6) | <0.0001 |
| Mean leg length, cm (SD) | 81.2 (6.8) | 86.0(4.3) | 75.3 (3.9) | <0.0001 |
| Diabetes at baseline, % yes (n) | 20(122) | 24 (84) | 14 (38) | 0.004 |
| Mean intraabdominal fat area, cm2 (SD) | 90.6 (53.3) | 105.3 (55.3) | 72.4 (44.7) | <0.0001 |
Abbreviations: SD (standard deviation), HDL (high-density lipoprotein), LDL (low-density lipoprotein)
p-value for t-test (continuous) or Chi-squared test (categorical) comparing distributions for men and women
Table 2.
Coefficients from sex-specific multiple regression models testing associations of metabolic risk factors with intraabdominal fat area (cm2)
| Men n = 344 |
Women n = 278 |
|||
|---|---|---|---|---|
| β | p-value | β | p-value | |
| Age, years | 0.80 | <0.001 | 1.07 | <0.001 |
| Body mass index, kg/m2 | 3.06 | 0.05 | 3.73 | 0.003 |
| Waist circumference, cm | 3.96 | <0.001 | 1.96 | <0.001 |
| HDL cholesterol, mg/dL | −0.54 | 0.001 | −0.40 | <0.001 |
| LDL cholesterol, mg/dL | — | — | 0.01 | 0.041 |
| Triglyceride level, mg/dL | — | — | — | — |
| Fasting glucose level, mg/dL | 0.10 | 0.03 | 0.14 | 0.023 |
| Smoking status | — | — | — | — |
| Grip strength, lbs | — | — | — | — |
| Mid-thigh circumference, cm | −2.92 | <0.001 | −1.29 | 0.016 |
| Humeral length, cm | — | — | — | — |
| Leg length, cm | — | — | — | — |
| Constant term | −205.91 | <0.001 | −160.02 | <0.001 |
On first-order multiplicative interaction testing, there was a significant interaction of waist circumference with sex in the full model (β= −1.98, p=0.003; male=1, female=2). We therefore chose to present sex-stratified results.
We built reverse stepwise prediction models using all covariates above. P-value for entry was set at 0.05. P-value for removal was set at 0.1.
Abbreviations: HDL (high-density lipoprotein), LDL (low-density lipoprotein)
Figure 1.
Sex-stratified scatter plots of intra-abdominal fat area (cm2) vs. fitted values (cm2) of EVA for men and women.
Table 3.
Model R2 from linear regression models predicting continuous intrabdominal fat area, mean squared errors from k-fold cross validation, and AUROC and 95% confidence intervals for prediction of high (above 75th percentile) vs. low visceral adiposity category, comparing a novel estimate of visceral adipose tissue area (EVA) to selected existing visceral adiposity surrogates
| Men n = 344 |
Women n = 278 |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | AIC | MSEa | 95% Cl | AUROCb | 95% Cl | p-value | R2 | AIC | MSEa | 95% Cl | AUROCb | 95% Cl | p-value | ||
| EVAc | 0.61 | 3421 | 1244 | 1243 - 1246 | 0.90 | 0.86 - 0.93 | — | 0.72 | 2554 | 582 | 581 - 583 | 0.94 | 0.92 - 0.96 | — | |
| EVA without mid-thigh circumferenced | 0.59 | 3433 | 1267 | 1265 - 1268 | 0.88 | 0.84 - 0.92 | 0.013 | 0.72 | 2558 | 589 | 588 - 590 | 0.94 | 0.92 - 0.97 | 0.771 | |
| Després estimatee | 0.50 | 3501 | 1544 | 1543 - 1545 | 0.85 | 0.80 - 0.89 | 0.004 | — | — | — | — — | — | — | — | |
| WHtR | 0.48 | 3515 | 1613 | 1611 - 1614 | 0.86 | 0.82 - 0.90 | 0.091 | 0.58 | 2664 | 852 | 852 - 853 | 0.88 | 0.84 - 0.92 | <0.0001 | |
| WC, cm | 0.48 | 3513 | 1603 | 1602 - 1605 | 0.84 | 0.80 - 0.88 | 0.009 | 0.56 | 2678 | 898 | 897 - 898 | 0.88 | 0.84 - 0.92 | 0.003 | |
| DAAT indexf | 0.46 | 3529 | 1680 | 1678 - 1681 | 0.82 | 0.77 - 0.87 | 0.001 | 0.58 | 2665 | 857 | 856 - 858 | 0.88 | 0.84 - 0.92 | 0.0003 | |
| BMI, kg/m2 | 0.36 | 3584 | 1968 | 1966 - 1970 | 0.82 | 0.76 - 0.87 | 0.003 | 0.40 | 2762 | 1215 | 1214 - 1216 | 0.84 | 0.79 - 0.89 | <0.0001 | |
| LAPg | 0.17 | 3674 | 2607 | 2602 - 2612 | 0.81 | 0.75 - 0.86 | 0.0002 | 0.22 | 2834 | 1793 | 1785 - 17801 | 0.86 | 0.81 - 0.90 | <0.0001 | |
| VAIh | 0.08 | 3710 | 2888 | 2882 - 2893 | 0.73 | 0.68 - 0.80 | <0.0001 | 0.14 | 2861 | 1734 | 1733 - 1736 | 0.78 | 0.72 - 0.84 | <0.0001 | |
Abbreviations: AIC (Akaike information criterion), AUROC (area under the receiver-operating characteristics curve), CI (confidence interval), EVA (estimate of visceral adiposity), WHtR (waist-to-height ratio), WC (waist circumference), DAAT index (deep abdominal adipose tissue index), BMI (body-mass index), LAP (lipid accumulation product), VAI (visceral adiposity index).
For k-fold cross validation, data were partitioned into 5 training sets, repeated 500 times
The 75th percentile of visceral adiposity was 140 cm2 for men and 100 cm2 for women; p-value compared to AUROC for VAE with fasting insulin
EVA (men) = [0.80 × age (years)] + [3.06 × BMI (kg/m2)] + [3.96 × WC (cm)] − [0.54 × HDL-cholesterol (mg/dL)] + [0.10 × fasting glucose (mg/dL)] − [2.92 × mid-thigh circumference (cm)] − 205.91;
EVA (women) = [1.07 × age (years)] + [3.73 × BMI (kg/m2)] + [1.96 × WC (cm)] − [0.40 × HDL-cholesterol (mg/dL)] + [0.01 × LDL-cholesterol (mg/dL)] + [0.14 × fasting glucose (md/dL)] − [1.29 × mid-thigh circimference (cm)] − 160.02
without mid-thigh circumference: EVA-C (men) = [1.28 × age (years)] + [4.12 × WC (cm)] − [0.53 × HDL-cholesterol (mg/dL)] + [0.14 × fasting glucose (mg/dL)] − 319.50;
EVA-C (women) = [1.26 × age (years)] + [1.89 × BMI (kg/m2)] + [2.16 × WC (cm)] − [0.43 × HDL-cholesterol (mg/dL)] + [0.11 × LDL-cholesterol (mg/dL)] + [0.18 × fasting glucose (md/dL)] − 207.22
Després estimate: calculated using coefficients derived by Després et al. (1991) in 110 men: IAFA = −225.39 + 2.125 × age(years) +2.843 × WC(cm); it was derived in a male cohort, so we chose not to calculate values for women
Deep abdominal adipose tissue (DAAT) index: calculated using coefficients derived by Brundavani et al. (2006). Men = −382.9 + [1.09 × weight -(kg)] + [6.04 × WC-(cm)] + (−2.29 × BMI), women = −278 + [−0.86 × weight -(kg)] + [5.19 × WC-(cm)]
Lipid accumulation product (LAP): men [(WC (cm)-65) × TG (mmol)], women [(WC (cm)-58) × TG (mmol)], in the manner of Kahn H. et al. (2005)
Visceral adiposity index (VAI): calculated using coefficents derived by Amato et al. (2010): VAI = (WC/(39.68+(1.88*BMI)))*(triglycerides/1.03)*(1.31/HDL) for men, and (WC/(36.58+(1.89*BMI)))*(triglycerides/0.81)*(1.52/HDL) for women, with both triglycerides and HDL specified in mmol/L
4. DISCUSSION
We have developed and internally validated a novel adiposity surrogate, the Estimate of Visceral Adipose Tissue Area, to predict visceral adiposity in a Japanese-American cohort of men and women, and demonstrated that it performed better than existing adiposity surrogates incorporating clinical, anthropometric and laboratory measurements in this population. It relies on readily available clinical and laboratory measurements and can easily be calculated for men and women using the regression coefficients and constants in Table 2. Whether EVA would be similarly accurate in other Asian and non-Asian populations is not known but possible and should be the subject of future research.
In this analysis, we compared anthropometric measures and indices that did and did not incorporate blood-based biomarkers in estimation of IAFA. The anthropometric measures and indices that did not incorporate blood-based biomarkers were the Despres index, WHtR, WC, the Deep-Abdominal Adipose Tissue index, and BMI. To our knowledge, discriminative ability of the Despres index and the Deep-Abdominal Adipose Tissue index for CT- or MRI-measured IAFA have not been previously validated in external cohorts. The ability of WHtR, WC, and BMI to estimate IAFA has been studied extensively, and trends in our results are generally consistent with previous studies20,21. In analyses stratified by both sex and age≥60 years, Roriz et al. calculated AUROCs for WHtR in discrimination of IAFA >130cm2 in a Latino sample20 that were higher (0.90-0.91) than our AUROC for men and lower (0.81-0.87) than our AUROC for women, although the differences were not large. These discrepancies may be due to the different cutpoints that were chosen for high vs. low IAFA in the two analyses, especially among women, where their cutpoint was substantially higher (130 cm2 vs. 100 cm2).
Mid-thigh circumference was included in the model for men and women. In comparisons of models without and without mid-thigh circumference (EVA vs. EVA-R), MSE were lower in models including mid-thigh circumference for both men and women. The AUROC for EVA vs. EVA-R was significantly better for men (p=0.013) but not women (p=0.771). Despite this, both performed well in comparison to other measures tested; therefore, in datasets where mid-thigh circumference is not available, we believe use of the EVA-R could be considered. Regression equations for both versions are provided in Table 3. Unlike mid-thigh circumference, the other anthropometrics we tested (grip strength, humeral length, and leg length) did not meet criteria for inclusion. These results are consistent with previous analyses in this dataset. Mid-thigh circumference has previously been associated with lower odds of incident diabetes independent of age, sex and body fat depots, but mid-thigh subcutaneous fat area was not, suggesting that thigh muscle mass and not fat is the key component underlying the metabolic associations with thigh circumference 22. Weaker grip strength is positively associated with diabetes only among the leanest individuals in this cohort23.
The addition of blood-based biomarkers might be expected to improve model fit, given the associations between IAFA and multiple metabolic measurements4-6. We evaluated two existing indices that included blood-based biomarkers: the Lipid Accumulation Product and the Visceral Adiposity Index. Both performed relatively poorly in estimation of IAFA in our cohort. It is important to note that neither index was designed for this purpose, although both have been used as surrogates for IAFA24,25. In previous studies, the association of both the Lipid Accumulation Product and the Visceral Adiposity Index with IAFA are variable20,21,26,27, but different analytic methods make direct comparisons somewhat difficult. In their Latino sample, Roriz et al. calculated AUROC for the Lipid Accumulation Product and the Visceral Adiposity Index that were quantitatively similar to the AUROC we obtained for these surrogates: Lipid Accumulation Product 0.81-0.88 in men and 0.78-0.80 in women; Visceral Adiposity Index 0.73-0.83 in men and 0.65-0.71 in women20. In an Australian study of 39 men and women with nonalcoholic fatty liver disease, Visceral Adiposity Index was modestly correlated with IAFA on MRI (r=0.39) 21. In our Japanese-American population, the correlation of Visceral Adiposity Index with IAFA was 0.29 for men and 0.38 for women. In a study of 99 overweight post-menopausal Canadian women enrolled in a weight reduction randomized controlled trial, baseline Visceral Adiposity Index was less well correlated with CT-measured IAFA than it was among the Japanese-American women in our dataset (r=0.28)27. A ROC analysis of the ability of the Visceral Adiposity Index to classify these study participants into the lowest or highest tertile of CT-measured IAFA demonstrated an AUROC of 0.61 that was of significantly lower magnitude than the AUROCs for BMI (0.84) or WC (0.86)27. In a study of 180 premenopausal Korean women with polycystic ovary syndrome, the Visceral Adiposity Index displayed an AUROC of 0.88 in the prediction of CT-measured VAT >100 cm26, which was considerably greater than corresponding values in our cohort, or in the analysis by Roriz et al. Notably, the Korean cohort had a relatively narrow age range.
The relatively poor performance of the Lipid Accumulation Product and the Visceral Adiposity Index in our cohort, as well as the better performance of Visceral Adiposity Index in a cohort of younger female Koreans with a relatively narrow age range may be explained by a single unifying phenomenon. In our analysis, the two measures with the best overall performance characteristics (EVA and the Després estimate) both include age as a covariate, suggesting a potential mechanism for some of the underlying differences in the relative performance of these measures. In 1991, Després et al. used all-possible-regressions selection methods to arrive at an adiposity surrogate to predict IAFA in men that incorporated age and WC14. Similarly, in an analysis that used stepwise regression to generate sex- and race-stratified equations for IAFA prediction in Europeans, South Asians and African Caribbeans, age was included in the models that were derived for every population group28. Older age is a recognized determinant of visceral adiposity29, potentially due to metabolic changes in visceral fat depots30. A prospective study of this Japanese-American population demonstrated that weight gain at older ages was more likely to accumulate fat in the visceral depot31. Age may therefore be a key feature associated with visceral fat deposition, which merits consideration for inclusion in surrogate measures of this depot. The other adiposity surrogates that we tested did not directly account for age.
This study has several strengths. Chief among them, we used a cohort of Japanese-American participants with well-characterized measurements of anthropometric and metabolic features. Secondly, the novel index that we developed was validated using robust statistical methods. Lastly, the small number of readily available covariates that were used in the model allows generalizability to external datasets and routine clinical use. There are also some limitations. First, measurements of IAFA were derived from single CT-scan slices; however, although the strength of the relationship varies somewhat depending on the location of the CT slice32, a high correlation has been demonstrated between a single CT slice and direct measurement of visceral fat volume, which limits the potential for bias33. We did not assess the contribution of menopausal status on IAFA in women. In addition, the performance characteristics of EVA in non-Asian populations is unknown and requires further study. Lastly, we were not able to validate the full model in an external dataset. We did validate the EVA-R in an external cohort. In addition, to validate the full model, we used k-fold cross-validation to simulate an independent dataset in an effort to decrease overfitting and give a reasonable assessment of the new model’s performance.
In conclusion, accurate estimation of IAFA is of special importance in individuals of Asian ancestry, as they may have a higher body fat percentage for a given BMI10-12 and are at greater risk to develop diabetes at any given body-mass index34. Measures incorporating age may provide better estimates of IAFA in individuals of Japanese ancestry and potentially in other Asian and perhaps non-Asian populations as well. Further testing in other populations would demonstrate its potential value to achieve this aim.
Supplementary Material
ACKNOWLEDGEMENTS:
This manuscript is dedicated to the memory of Marguerite J. McNeely who was an active investigator in the JACDS. Her critical contributions will be missed. We are grateful to the King County Japanese-American Community for their involvement and support in this and prior studies. VA Puget Sound supported the participation of Drs. Kahn and Boyko in this research. We gratefully acknowledge the support of Malcolm Wolff, B.S., and the University of Washington Statistical Consulting Service. Research reported in this manuscript was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number DK-103945, as well as NIH grants DK-031170, HL-049293, DK-002654, DK-017047, DK-035816 and RR-000037. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
FUNDING: NIH DK-103945, DK-031170, HL-049293, DK-002654, DK-017047, DK-035816 and RR-000037; JSPS KAKENHI Grant Numbers 17390177, 20390187, and 23390177.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST: None
REFERENCES
- 1.Hayashi T, Boyko EJ, McNeely MJ, Leonetti DL, Kahn SE, Fujimoto WY. Visceral adiposity, not abdominal subcutaneous fat area, is associated with an increase in future insulin resistance in Japanese Americans. Diabetes. 2008;57(5):1269–1275. [DOI] [PubMed] [Google Scholar]
- 2.Fujimoto WY, Bergstrom RW, Boyko EJ, et al. Visceral adiposity and incident coronary heart disease in Japanese-American men. The 10-year follow-up results of the Seattle Japanese-American Community Diabetes Study. Diabetes care. 1999;22(11):1808–1812. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi T, Boyko EJ, Leonetti DL, et al. Visceral adiposity is an independent predictor of incident hypertension in Japanese Americans. Annals of internal medicine. 2004;140(12):992–1000. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi T, Boyko EJ, Leonetti DL, et al. Visceral adiposity and the risk of impaired glucose tolerance: a prospective study among Japanese Americans. Diabetes care. 2003;26(3):650–655. [DOI] [PubMed] [Google Scholar]
- 5.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes care. 2000;23(4):465–471. [DOI] [PubMed] [Google Scholar]
- 6.Tong J, Boyko EJ, Utzschneider KM, et al. Intra-abdominal fat accumulation predicts the development of the metabolic syndrome in non-diabetic Japanese-Americans. Diabetologia. 2007;50(6):1156–1160. [DOI] [PubMed] [Google Scholar]
- 7.Nazare JA, Smith J, Borel AL, et al. Usefulness of measuring both body mass index and waist circumference for the estimation of visceral adiposity and related cardiometabolic risk profile (from the INSPIRE ME IAA study). The American journal of cardiology. 2015;115(3):307–315. [DOI] [PubMed] [Google Scholar]
- 8.Kahn HS. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: a population-based comparison. BMC Cardiovasc Disord. 2005;5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amato MC, Giordano C, Galia M, et al. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes care. 2010;33(4):920–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park YW, Allison DB, Heymsfield SB, Gallagher D. Larger amounts of visceral adipose tissue in Asian Americans. Obesity research. 2001;9(7):381–387. [DOI] [PubMed] [Google Scholar]
- 11.Lim U, Ernst T, Buchthal SD, et al. Asian women have greater abdominal and visceral adiposity than Caucasian women with similar body mass index. Nutrition & diabetes. 2011;1:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2002;3(3):141–146. [DOI] [PubMed] [Google Scholar]
- 13.Brundavani V, Murthy SR, Kurpad AV. Estimation of deep-abdominal-adipose-tissue (DAAT) accumulation from simple anthropometric measurements in Indian men and women. European journal of clinical nutrition. 2006;60(5):658–666. [DOI] [PubMed] [Google Scholar]
- 14.Despres JP, Prud’homme D, Pouliot MC, Tremblay A, Bouchard C. Estimation of deep abdominal adipose-tissue accumulation from simple anthropometric measurements in men. The American journal of clinical nutrition. 1991;54(3):471–477. [DOI] [PubMed] [Google Scholar]
- 15.Fujimoto WY, Leonetti DL, Kinyoun JL, et al. Prevalence of diabetes mellitus and impaired glucose tolerance among second-generation Japanese-American men. Diabetes. 1987;36(6):721–729. [DOI] [PubMed] [Google Scholar]
- 16.Shuman WP, Morris LL, Leonetti DL, et al. Abnormal body fat distribution detected by computed tomography in diabetic men. Investigative radiology. 1986;21(6):483–487. [DOI] [PubMed] [Google Scholar]
- 17.Moran JL, Solomon PJ. Statistics in review Part I: graphics, data summary and linear models. Critical care and resuscitation : journal of the Australasian Academy of Critical Care Medicine. 2007;9(1):81–90. [PubMed] [Google Scholar]
- 18.Osten DW. Selection of optimal regression models via cross-validation. Journal of Chemometrics. 1988;2(1):39–48. [Google Scholar]
- 19.Pepe MS. The statistical evaluation of medical tests for classification and prediction. Oxford ; New York: Oxford University Press; 2003. [Google Scholar]
- 20.Roriz AK, Passos LC, de Oliveira CC, Eickemberg M, Moreira Pde A, Sampaio LR. Evaluation of the accuracy of anthropometric clinical indicators of visceral fat in adults and elderly. PloS one. 2014;9(7):e103499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vongsuvanh R, George J, McLeod D, van der Poorten D. Visceral adiposity index is not a predictor of liver histology in patients with non-alcoholic fatty liver disease. Journal of hepatology. 2012;57(2):392–398. [DOI] [PubMed] [Google Scholar]
- 22.Hoyer D, Boyko EJ, McNeely MJ, Leonetti DL, Kahn SE, Fujimoto WY. Subcutaneous thigh fat area is unrelated to risk of type 2 diabetes in a prospective study of Japanese Americans. Diabetologia. 2011;54(11):2795–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wander PL, Boyko EJ, Leonetti DL, McNeely MJ, Kahn SE, Fujimoto WY. Greater handgrip strength predicts a lower risk of developing type 2 diabetes over 10 years in leaner Japanese Americans. Diabetes research and clinical practice. 2011. ;92(2):261–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang YM, Jung CH, Cho YK, et al. Visceral adiposity index predicts the conversion of metabolically healthy obesity to an unhealthy phenotype. PloS one. 2017;12(6):e0179635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abulmeaty MM, Almajwal AM, Almadani NK, et al. Anthropometric and central obesity indices as predictors of long-term cardiometabolic risk among Saudi young and middleaged men and women. Saudi Med J. 2017;38(4):372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh JY, Sung YA, Lee HJ. The visceral adiposity index as a predictor of insulin resistance in young women with polycystic ovary syndrome. Obesity (Silver Spring). 2013;21(8):1690–1694. [DOI] [PubMed] [Google Scholar]
- 27.Elisha B, Messier V, Karelis A, et al. The Visceral Adiposity Index: Relationship with cardiometabolic risk factors in obese and overweight postmenopausal women - A MONET group study. Appl Physiol Nutr Metab. 2013;38(8):892–899. [DOI] [PubMed] [Google Scholar]
- 28.Eastwood SV, Tillin T, Wright A, et al. Estimation of CT-derived abdominal visceral and subcutaneous adipose tissue depots from anthropometry in Europeans, South Asians and African Caribbeans. PloS one. 2013;8(9):e75085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bray GA, Bouchard C. Handbook of obesity: epidemiology, etiology, and physiopathology. 3rd ed. Boca Raton: CRC Press; 2014. [Google Scholar]
- 30.Park SE, Park CY, Choi JM, et al. Depot-Specific Changes in Fat Metabolism with Aging in a Type 2 Diabetic Animal Model. PloS one. 2016;11(2):e0148141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee CG, Fujimoto WY, Brunzell JD, et al. Intra-abdominal fat accumulation is greatest at younger ages in Japanese-American adults. Diabetes research and clinical practice. 2010;89(1):58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen W, Punyanitya M, Wang Z, et al. Visceral adipose tissue: relations between single-slice areas and total volume. The American journal of clinical nutrition. 2004;80(2):271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. American journal of physiology Endocrinology and metabolism. 2000;278(5):E941–948. [DOI] [PubMed] [Google Scholar]
- 34.McNeely MJ, Boyko EJ. Type 2 diabetes prevalence in Asian Americans: results of a national health survey. Diabetes care. 2004;27(1):66–69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.