Abstract
Most available therapies for endometriosis are hormone-based and generally broadly used without taking into consideration the ovarian hormone receptor expression status. This contrasts strikingly with the standard of care for other hormone-based conditions such as breast cancer. We therefore aimed to characterize the expression of ovarian steroid hormone receptors for estrogen alpha (ESR1), estrogen beta (ESR2), and progesterone (PGR) in different types of endometriotic lesions and eutopic endometrium from women with endometriosis and controls using a tissue microarray (TMA). Nuclear expression levels of the receptors were analyzed by tissue (i.e., ectopic vs. eutopic endometrium) and cell type (i.e., glands vs. stroma). Ovarian lesions showed the lowest expression of ESR1 and PGR, and the highest expression of ESR2, while the fallopian tube lesions showed high expression of the three receptors. Differences among endometria included lower expression of ESR1 and higher expression of ESR2 in stroma of proliferative endometrium from patients vs. patients, and a trend towards loss of PGR nuclear positivity in proliferative endometrium from patients. The largest ESR2:ESR1 ratios were observed in ovarian lesions and secretory endometrium. The highest proportion of samples with >10% Ki67 positive nuclei was in glands of fallopian tube (54%) and extra-pelvic lesions (75%); 60% of glands of secretory endometrium from patients had >10% Ki67 positivity compared to only 15% in controls. Our results provide a better understanding of endometriosis heterogeneity by revealing lesion type-specific differences and case-by-case variability in the expression of ovarian hormone receptors. This knowledge could potentially predict individual responses to hormone therapies, and set the basis for the application of personalized medicine approaches for women with endometriosis.
Keywords: Endometriosis, Tissue Microarray, Estrogen Receptor, Progesterone Receptor, Ki67
INTRODUCTION
Endometriosis, defined as the growth and development of endometrial-like tissue (i.e., glands and stroma) outside the uterine cavity, is a gynecological disease characterized by chronic pelvic pain, dyspareunia, dysmenorrhea, and infertility, that affects 10 percent (%) of women of reproductive age [1]. The currently available treatments aim to decrease endometriotic lesion burden and to reduce inflammation and pain. Treatment options for endometriosis include non-hormonal [non-steroidal inflammatory drugs (NSAIDs)], and hormonal [gonadotropin-releasing hormone (GnRH), oral contraceptives (OCs) and progestins] treatments [2]. However, inadequate responses and resistance to treatment are common, and lead to symptom recurrence [2]. In addition, patients often report negative side effects that impair compliance with these treatments [3]. Many of these factors have contributed to the consensus that endometriosis has neither a pharmacologic cure nor high therapy response rates, resulting in poor quality of life for women suffering from this incapacitating disease [4–6].
The pathophysiology of endometriosis is not well understood, but estrogen dependence and progesterone resistance are known to be important players in the establishment and maintenance of endometriotic lesions [7]. The primary action of these ovarian steroid hormones is mediated through their cognate receptors, the estrogen receptors alpha and beta (ESR1 and ESR2) and two progesterone receptor isoforms A and B (PGR-A and PGR-B) [8]. Previous studies have shown aberrant expression of these hormone receptors in endometriosis lesions, including high ESR2 to ESR1 ratios and loss of expression of PGR [9]. These observations suggest that dysregulation in the signaling cascades mediated by these receptors and in the cellular behaviors they activate (e.g., proliferation) are important factors not only in the etiology of endometriosis, but also in the observed inconsistency in therapeutic responses to the hormone treatments commonly used for this condition [10]. This variability in responses to treatment could be due to differences in the underlying pathophysiology of various disease presentations (e.g., ovarian vs. peritoneal lesions) but also to differential expression of the drug’s target due to physiological factors or therapeutic stimuli [11]. Although it should be standard of care to ensure that all lesion types express the receptor being targeted (as in other hormonal conditions such as breast cancer), this is not currently taken into consideration in the treatment plan for endometriosis. Therefore, there is still a need to investigate the association between clinical responses and individual heterogeneity in the expression of steroid ovarian hormone receptors in tissues that are biopsied during surgery for endometriosis. As a first step in this process, we aimed to characterize the expression profile of ESR1, ESR2 and PGR in tissues from diverse lesion types based on their localization (e.g., ovarian, peritoneal, fallopian, appendix, cecum, and skin & subcutaneous) as well as in eutopic endometrium (from patients and controls) representing the diversity of patients commonly encountered in a clinical setting.
The present study was undertaken to expand and validate previous observations of hormonal receptor heterogeneity among endometriotic lesions and endometrial samples, taking advantage of the availability of a Tissue Microarray (TMA) containing samples from endometriosis cases and controls. Immunohistochemical (IHC) analysis of this endometriosis-focused TMA allowed the identification of specific cells (e.g., stroma, gland, endothelium, infiltrating inflammatory cells) and cellular localization (e.g., cytoplasmic vs. nuclear) in a large sample size, and enabled direct comparisons while avoiding technical issues (e.g., decreasing variability on staining) [12]. We report here our characterization of different expression patterns of ESR1, ESR2, PGR, and Ki67 (a marker of heightened cellular proliferation) in eutopic and ectopic endometrium, and discuss the potential implications for the clinical management of individual patients with endometriosis.
MATERIALS AND METHODS
Population Characteristics
Using protocols approved by our institution IRB committee (IRB #050207, IF), a total of 164 cores from 83 de-identified archived formalin fixed paraffin-embedded endometrium and endometriosis tissue blocks were obtained from a Pathology Laboratory from Southern Puerto Rico between the years of 2000-2009. Tissues were evaluated by two pathologists (MG and AM) to confirm the diagnosis of endometriosis, to select areas of the block where endometriosis (defined as glands and stroma) was present, and to determine the menstrual cycle phase of the endometrial samples. There were 5 cases of matched samples (lesion and endometrium); only for those we could determine menstrual cycle phase (proliferative n=2; secretory n=3). The blocks were used to construct a tissue microarray (TMA) at the Moffitt Cancer Center (Moffitt) Tissue Core that has previously been described and used for various IHC studies [13–15]. Duplicate core biopsies in the TMA came from the following tissue types: endometriosis lesions localized in the ovaries (n=29), fallopian tubes (n=16), peritoneum (n=34), skin (umbilical region) (n=4), and gastrointestinal tract [cecum and appendix] (n=7). Due to lower number of tissue samples in the skin (n=4), appendix (n=4) and cecum (n=3) groups, these were pooled for the analysis and categorized as extra-pelvic endometriosis. Additionally, the TMA included eutopic endometrial samples from women with endometriosis and controls in either proliferative or secretory phases. Control endometrial samples were obtained from women undergoing hysterectomy for benign gynecological conditions such as uterine fibroids of dysfunctional bleeding, who had normally cycling endometrium (not hyperplastic, not menopausal, in either proliferative or secretory phase) as per pathological analysis. The mean age of the endometriosis cases was 40.0 ±7.0 years (endometrial samples) and 35.8 ± 8.7 years (lesions) compared to 46.1 ± 7.7 of controls, which was not significantly different.
Immunohistochemistry (IHC) for ESR1, ESR2, PGR, and Ki67
Immunostaining for ESR1, ESR2, PGR, and Ki67 was conducted at Moffitt’s Tissue Core following standardized protocols. Antibody dilutions and experimental conditions were determined after standardization and normalization procedures prior to the experiments. Individual TMA 10 μm slides were incubated with the following primary antibodies: ESR1 (Genentech, cat# GTX29269), ESR2 (Genentech, cat# GTX70174), PGR (Abcam, cat# ab131486) and Ki67 (Novus, cat# NB110-57147). Differences between PGR-A and PGR-B were not assessed because the antibody employed for the staining does not discriminate between isoforms. Thus, nuclear immunostaining for PGR is reported as global PGR expression. The slides were scanned at Moffitt’s Analytical Microscopy Core using the Aperio Digital Pathology Scanner (Buffalo Grove, IL). The Image Scope Software (Aperio) was used to select areas representative of glands and stroma of the tissues in the TMA to be analyzed digitally for nuclear immunostaining intensity of ESR1, ESR2, PGR, and Ki67. Data were analyzed as percent (%) of positive nuclei presented as average per tissue type (glands vs stroma).
Statistical Analyses
Statistical analysis included non-parametric Mann Whitney test to analyze differences in age among groups and non-parametric ANOVA (Kruskal-Wallis test) followed by a Dunn’s Post-Hoc test to analyze the significance of differences in the percentage of positive nuclei for the hormone receptors and Ki67 among the groups (lesion type, proliferative and secretory endometrium from cases and controls). All the statistical analyses were performed using SPSS v20 and GraphPad Prism was used for graphical representation of data. Statistical significance was set at p<0.05.
RESULTS
ESR1 nuclear expression in endometriotic and endometrial tissues
Ovarian lesions showed the lowest glandular ESR1 expression, which was significant compared to fallopian (p<0.001), peritoneal (p<0.05), and extra-pelvic lesions (p<0.01). (Figure 1 panel A). In the stromal compartment of the lesions, we observed similar results with ovarian lesions showing the lowest level of ESR1 expression, significant when compared to fallopian tube (p<0.001) and extrapelvic lesions (p<0.001) (Figure 1 panel B). In stroma of lesions, 10.8% of the samples show loss or very low (>10%) ESR1 positivity (16 out of 83).
Figure 1. ESR1 nuclear immunostaining in endometrial and endometriotic samples on a TMA.
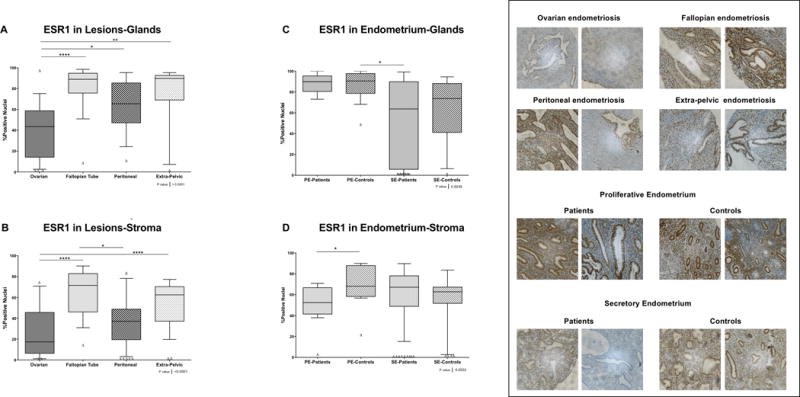
A) and C) Nuclear immunostaining in glands. A total of 145 of 164 for ESR1 formalin-fixed paraffin-embedded samples were analyzed by immunohistochemistry (IHC). B) and D) Nuclear immunostaining in stroma. A total of 154 of 164 for ESR1 formalin-fixed paraffin-embedded samples were analyzed by IHC. Using the Image Scope Program and Aperio Digital Pathology Slide Scanner, the immunostaining of ESR1 in both compartments (i.e., stroma and glands) was evaluated. Significant differences between groups are shown in the figure (p>0.05). Δ Outliers (<−2SD of the mean).
Regarding endometrial tissues, significant differences were observed in ESR1 nuclear expression levels between the stroma of proliferative endometrium from patients and controls (p <0.05) (Figure 1 panels C and D). Also, we observed a high number of secretory endometrium samples from patients with loss or very low (>10%) ESR1 positivity in glands (45.5%; 10/22) and stroma (45%; 9/20) compared to controls (glands: 10.5%, 2/19; stroma: 26.3%, 5/19).
ESR2 nuclear expression in endometriotic and endometrial tissues
We observed high levels of ESR2 nuclear positivity in the glandular compartment of all lesions. The highest percentage of positive nuclei was seen in the ovarian and fallopian tube lesions (significantly different from peritoneal and extra-pelvic lesions) (Figure 2 panel A). In stroma, the only significant difference observed in ESR2 protein levels was between extra-pelvic and fallopian tube lesions (Figure 2 panel B).
Figure 2. ESR2 nuclear immunostaining in endometrial and endometriotic samples on a TMA.
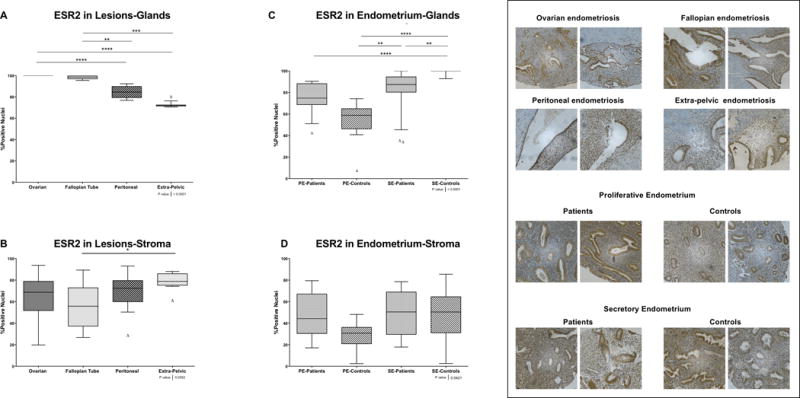
A) and C) Nuclear immunostaining in glands. A total of 137 of 164 for ESR2 formalin-fixed paraffin-embedded samples were analyzed by immunohistochemistry (IHC). B) and D) Nuclear immunostaining in stroma. A total of 136 of 164 for ESR2 formalin-fixed paraffin-embedded samples were analyzed by IHC. Using the Image Scope Program and Aperio Analysis, the immunostaining of ESR2 in both compartments (i.e., stroma and glands) was evaluated. Significant differences between groups are shown in the figure (p>0.05). Δ Outliers (<−2SD of the mean).
In the glandular compartment of the endometrial tissues, secretory endometrium from controls expressed the highest percentage of ESR2 positive nuclei, which was significantly different from secretory endometrium from patients (p<0.01) (Figure 2 panel C). Statistical significance was also observed between secretory and proliferative endometrium from controls (p<0.0001); however, no significant differences were observed when comparing proliferative and secretory endometrium from patients (Figure 2 panel C). Although the percentage of ESR2 positivity was lower in proliferative endometrium from controls compared to patients in both glands and stroma, these differences did not reach statistical significance (Figure 2 panel D).
PGR nuclear expression in endometriotic and endometrial tissues
In glands, the highest percentage of nuclear positivity for PGR was observed in extra-pelvic endometriotic lesions, followed by fallopian tube and peritoneal lesions. Ovarian lesions were characterized by a wide range of PGR positivity, from 99% to 3% (Figure 3 panel A). Significant differences were observed between extra-pelvic lesions compared to ovarian (p<0.001). Loss of PGR expression was most striking in ovarian lesions, which had the highest proportion of lesions with null or very low (>10%) PGR nuclear positivity in both glands (56.3%; 9/16) and stroma (68.8%; 11/16) (Figure 3 panels A and B).
Figure 3. PGR nuclear immunostaining in endometrial and endometriotic samples on a TMA.
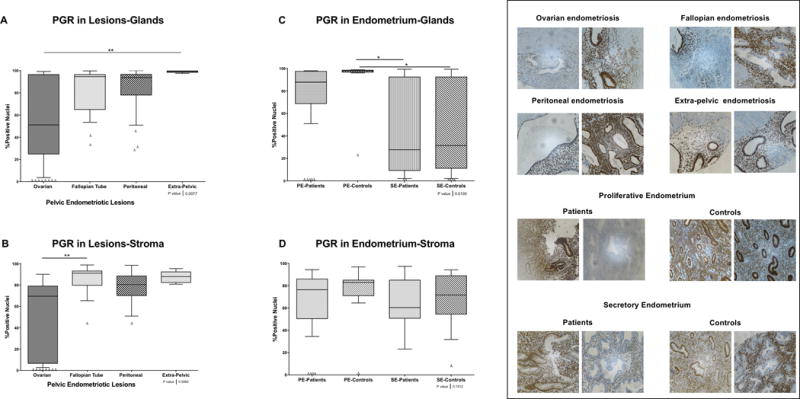
A) and C) Nuclear immunostaining in glands. A total of 123 of 164 for PGR formalin-fixed paraffin-embedded samples were analyzed by immunohistochemistry (IHC). B) and D) Nuclear immunostaining in stroma. A total of 124 of 164 for PGR formalin-fixed paraffin-embedded samples were analyzed by IHC. Using the Image Scope Program and Aperio Analysis, the immunostaining of PGR in both compartments (i.e., stroma and glands) was evaluated. Significant differences between groups are shown in the figure (p>0.05). Δ Outliers (<−2SD of the mean).
Expression of PGR in glands was higher in the proliferative endometrium of both patients and controls compared to secretory endometrium; however, endometrium from patients had a higher proportion of null or very low (>10%) PGR expression in glands (33.3; 5/15) and stroma (28.6%; 4/14), and a broader range of PGR positivity (glands: 97-51%; stroma: 94 to 35%). (Figure 3 panels C and D). No differences were observed in PGR expression in stroma of the endometrial samples (Figure 3 panel D).
ESR2 to ESR1 ratios of nuclear positivity in endometriotic and endometrial tissues
We next calculated the ratio of percent positive nuclei in ESR2 compared to ESR1 in all lesions and endometrial samples in the TMA (Figure 4). The largest ESR2 to ESR1 ratio was observed in ovarian lesions compared to peritoneal (p<0.001), fallopian tube (p<0.001), and extra-pelvic lesions (p<0.0001) in glands, and compared only to fallopian tube (p<0.0001) in stroma (Figure 4 panels A and B). ESR2 to ESR1 ratios were highest in secretory vs. proliferative endometrium from both patients and controls in glands and stroma (Figure 4 panel C and D). No significant differences were observed when comparing endometria obtained at the same menstrual cycle phase from patients and controls.
Figure 4. ESR2 to ESR1 ratios of nuclear positivity in endometrial and endometriotic samples on a TMA.
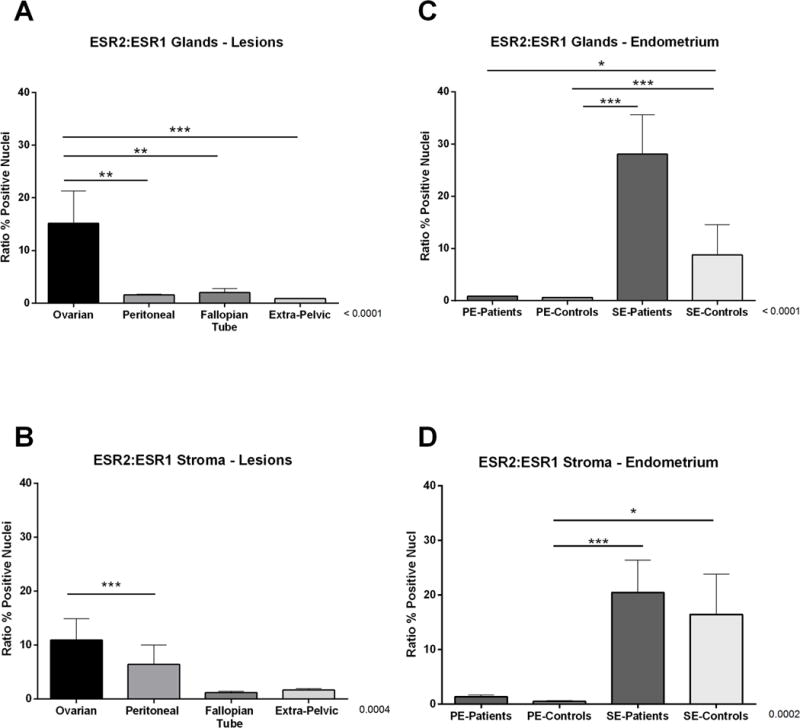
Ratios of nuclear immunostaining in endometriotic lesions are depicted for A) glands and B) stroma. Similarly, the nuclear immunostaining ratios in endometrial samples are depicted for C) glands and D) stroma. Ratios were calculated by dividing the averages of percent positive nuclei for ESR2 between the averages of percent positive nuclei for ESR1. Using the Image Scope Program and Aperio Digital Pathology scanner, the immunostaining of ESR2 and ESR1 in both compartments (i.e., stroma and glands) was evaluated. Significant differences between groups are shown in the figure (p>0.05).
Ovarian hormone receptor profile in matched eutopic and ectopic endometrium
Five cases had matched eutopic and ectopic endometrium samples in the TMA. For those we were able to compare the pattern of receptor expression. Although the number of cases is small, analysis of samples from matched ectopic and eutopic endometrium uncovered interesting trends (Figure 5). Ovarian endometriosis cases (1 and 2) were characterized by higher levels of ESR2 vs. the other receptors regardless of menstrual phase. The most striking difference between the two cases of ovarian endometriosis was the negligible ESR1 expression in eutopic endometrium seen only in the patient at the secretory phase, which was not seen in the two other cases at secretory phase (case 3 with fallopian tube and case 5 with peritoneal lesions). Fallopian endometriosis cases (3 and 4) differed only on the higher positivity for ESR1 seen in proliferative endometrium. Peritoneal endometriosis (5) showed a tendency for lower levels of ESR1 than the other receptors that can be attributed to the menstrual phase (secretory). One case (6) with multiple lesions showed low PGR expression only in ovarian lesions (n.s.).
Figure 5. Receptor expression in matched ectopic and ectopic samples.
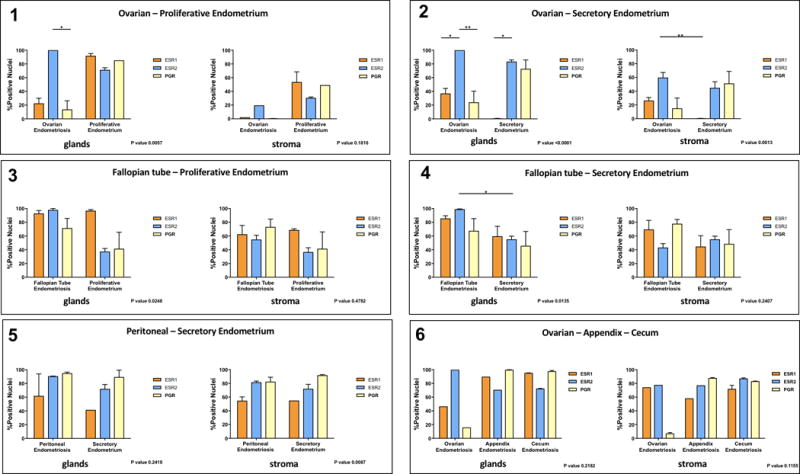
Analysis of samples from matched ectopic and eutopic endometrium (Cases 1 to 5) or one case with multiple lesions (case 6). Percent positive nuclei for ESR1, ESR2 and PGR in glands and stroma was measured and compared in each case were more than one tissue was present.
Ki67 nuclear staining in endometriotic and endometrial tissues
The percentage of Ki67 positive nuclei was analyzed based on cutoffs commonly used in determining cancer prognosis [favorable if staining <10% (low), borderline from 10% to 20%, and unfavorable if staining >20% (high)] [16]. We observed that extra-pelvic (75%) and fallopian tube (54%) lesions had the highest (>50%) proportion of samples with >10% Ki67 positivity (Figure 6). Differences in Ki67 positivity were statistically significant only in glands of extra-pelvic vs. ovarian and vs. peritoneal lesions (p<0.05). In glands of the endometrial tissues, the secretory endometrium from patients had a higher proportion of samples with >10% Ki67 positive nuclei compared to the secretory endometrium from controls (60% vs. 15%).
Figure 6. Ki67 percent positivity in endometrial and endometriotic samples on a TMA.
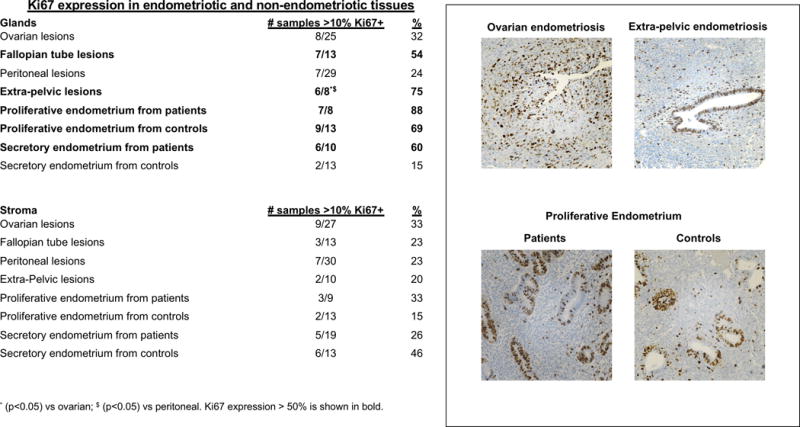
The percentage of Ki67 positive nuclei was examined in all tissues on the TMA and analyzed based on cutoffs commonly used in determining cancer prognosis [favorable if staining <10% (low), borderline from 10% to 20%, and unfavorable if staining >20% (high)]. Columns show % of tissues per group with >10% positive nuclei for Ki67. Representative pictures shown demonstrate that immunostaining is specific to endometrial or endometriotic glandular and stromal cells.
DISCUSSION
Aberrant expression and signaling of ESR1, ESR2 and PGR have been associated with the development and progression of endometriosis, which is commonly referred to as estrogen-dependent and progesterone-resistant [17]. Consequently, most treatments used today for endometriosis target these nuclear receptors by either blocking their action or promoting signaling actions. Thus, immunohistochemical characterization of the nuclear expression of ESR isoforms and PGR in target tissues that are biopsied during surgery could help determine response to commonly prescribed hormonal treatments. This is standard of care in other hormonal conditions such as breast cancer. However, the association between therapeutic responses, recurrence rates, and expression of ESR1/2 and PGR in endometriosis has not been well characterized. Using IHC and automated image analysis of percentage of positive nuclei, we observed significant differences in the pattern of hormone expression based on lesion localization and also by endometrial cell compartment (e.g., glands vs. stroma). Ovarian lesions showed the lowest expression of ESR1 and PGR in both glands and stroma, and the highest expression of ESR2 in glands only. All ovarian implants had 100% of ESR2 nuclear immunostaining in glands. Fallopian tube lesions in general showed high expression of ESR1/2 and PGR. Extra-pelvic endometriotic lesions showed the highest ESR1 and PGR expression and the lowest ESR2 expression (only in glands). The most striking differences among endometria were observed for ESR2 positivity, which was highest in the glandular epithelium of both proliferative and secretory endometrium from patients. We also observed a range in the levels of PGR in the glands and stroma of proliferative endometria from cases, compared to controls that showed much less variability in the staining; however, the physiological implications of these differences are still unknown. Although endometrium from patients and controls as well as endometriotic lesions have been shown to differ in their capability of responding to P4 due to low level expression of the PGR isoforms A and B [18], this study showed that not all lesion types are characterized by loss of PGR expression (one example being extra-pelvic lesions). Therefore, ‘P4 resistance’ may not be a universal characteristic of the disease in its varying manifestations and across all patients at a given time depending on their lifetime history of disease. Whether the observed heterogeneity in hormone receptor expression in different types of tissues could explain differences in patient responses to hormonal treatments still needs to be evaluated.
It has been speculated that high levels of ESR2 in endometriotic tissues suppresses ESR1 and PGR expression, thus contributing to increased proliferation in response to E2, as well as to P4 resistance [19, 20]. Therefore, we next evaluated the ESR2 to ESR1 ratios of nuclear positivity in all tissues in the TMA. We show here that some, but not all, endometriosis lesion types are characterized by high ESR2:ESR1. Ovarian endometriotic lesions in particular had high levels of ESR2 positivity compared to ESR1 in both glands and stroma. Higher ESR2 to ESR1 ratios were also observed in secretory endometria from both patients and controls. However, no significant differences in the ESR2 to ESR1 ratio were observed in the proliferative phase endometrial tissues. The majority of published studies have shown overexpression of ESR2 relative to ESR1 in tissues from endometriosis patients [9, 20, 21], except two studies showing that 1) both eutopic endometrium from patients and ovarian endometriosis had predominantly higher levels of ESR1 than ESR2 at the mRNA level [22] and 2) endometriotic stromal cells derived from ovarian chocolate cysts expressed the same levels of ESR1 and ESR2 mRNA [23]. These studies suggest that ESR1 and ESR2 may contribute independently to the estrogenic cues that trigger specific cellular actions or that estrogen receptor heterodimerization could lead to a state of co-dependency that mediates the estrogen-induced phenotype observed in endometriosis. In hormone-dependent malignancies such as breast, prostate, and endometrial cancers, skewed ESR2:ESR1 have been correlated with tumor grade and clinical outcomes including overall survival rates [24, 25]; however, clinical correlations of ESR2:ESR1 in endometriosis still need to be conducted that should take in consideration the type of lesions present.
Immunohistochemical assessment of Ki67 positive cells has been widely employed to assess the cellular proliferation capacity in benign and malignant diseases [26, 27], and as a biomarker of poor prognosis, lower survival, and therapy resistance in cancer [28, 29]. Clinically, if >10% of nuclei are positive the cancer is considered to have borderline to unfavorable prognosis [30]. Meanwhile, data regarding Ki67 expression in eutopic and ectopic endometrium are contradictory [31, 32], although it is generally accepted that Ki67 positivity is higher in eutopic proliferative endometrium than in endometriotic lesions [33, 34]. In this study, we observed that Ki67 nuclear positivity of over >10% was most prominent in glands of fallopian tube and extra-pelvic lesions, as well as in secretory endometrium from patients compared to the same tissues from controls. As expected, we also observed higher levels of Ki67 nuclear staining in proliferative endometrium from both cases and controls. In view of the fact that a diagnosis of endometriosis has been associated with increased risk of cancer (endometrioid and clear cell subtypes) [35], and that carcinogenic transformation of fallopian tube epithelium is associated to ovarian cancer [36], it would be important to assess the Ki67 index of lesions that are surgically removed in order to have a better understanding of the clinical picture and prognosis of individual patients.
There are few studies using TMAs to elucidate hormone receptor status of endometriosis [37–39]. To our knowledge, this is the first study using a TMA that includes pelvic endometriotic lesions (ovarian, peritoneal, and fallopian tube), extra-pelvic endometriotic lesions (skin & subcutaneous, cecum and appendix), as well as endometria from patients and controls to evaluate hormone receptor expression. Our results highlight 1) the value of the TMA technology to screen for potential biomarkers with diagnostic, prognostic, and therapeutic value (while controlling for potential experimental variables), and 2) the importance of analyzing different lesion types. Side by side analysis of hormone receptor expression in matched eutopic and ectopic endometrium uncovered interesting trends and lesion-specific differences in hormone receptor expression. For instance, ovarian endometriosis was the only tissue type showing predominant expression of ESR2 regardless of menstrual phase. In contrast, ESR1 levels were lower in ovarian endometriosis compared to fallopian tube, although both patients were in the proliferative phase. And ESR1 levels were almost negligible only in secretory endometrium from patients with ovarian endometriosis, not in those with fallopian or peritoneal disease. Evidently, these results need to be validated in larger studies.
Our study is limited by the nature of the TMA as it was constructed with archived lesions and endometrium biopsies obtained in a de-identified fashion. Therefore, there is no information on previous treatments and natural history of the disease. Also, while some cases had matched lesions and eutopic endometrium allowing for important comparisons, for others information about menstrual cycle phase was lacking. However, apart from known changes in ESR1 level between phases, no apparent differences were observed in ESR2 and PGR expression in lesions from which menstrual cycle phase was known. Our study is also limited in that it does not take into account differences in expression of ESR1 in mid-late luteal vs. late follicular phase, of PGR levels between early and late proliferative phase, or between PGR-A and PGR-B in early vs late secretory phase, for which significant differences have been reported [40, 41]. Despite these limitations, this study is valuable as it includes samples from patients with diverse clinical and treatment histories, as would typically be encountered in a clinical setting. Other strengths of our study include the characterization of receptor positivity by glands and stroma, and the use of an automated imaging analysis software that reduces the variability introduced by semi-quantitative analysis by independent scorers.
The differences in hormone receptor expression levels among lesions shown here and by others may be indicative of 1) different pathophysiological mechanisms underlying their development [42], ii) menstrual cycle phase [41], and iii) different lifetime history of hormonal and other treatments. For instance, it is well known that ESR1 levels will significantly decrease after prolonged exogenous therapy with progestins[43]. Regardless of the underlying reason, these differences could explain the observed variability in therapeutic responses to hormonal treatments among patients. The TMA used in this study includes a wide variety of subjects, likely to have variable lifetime experiences, treatments, exposures and clinical histories, akin to what a community gynecologist will have to treat in his/her general practice. Moving forward towards a personalized approach to patient management using hormonal treatments, heterogeneity in expression of ovarian steroid hormone receptor expression is not only expected, but imperative to test in order to customize the treatment. The wider the variability observed (refer to ESR1 levels in ovarian lesions vs. fallopian tube in Figure 1A) the greater the need to test the sample before prescribing any hormonal drug because wide variations in receptor expression are to be expected from one patient to the next. Also quite telling is to see surprisingly low variability across patients for ESR2 levels in glands seen in Figure 2, despite subjects likely having heterogeneous treatments and exposures. Whether this can be considered a tell-sign of endometriosis would need to be confirmed in larger studies.
The results of this study have high translational value as they suggest that a gynecologist would be able to select the most appropriate hormonal treatment [e.g., oral contraceptives, oral or IUD progestins] based on the particular pattern of expression of the ESR1, ESR2 and PGR of individual lesions that are surgically resected and characterized by IHC in a given patient. While these data could also suggest the potential use of selective [44](SERMs, e.g., raloxifene) and selective progesterone receptor modulators (SPERMs, e.g., mifepristone, asoprisnil), synthetic steroidal drugs that act as agonists or antagonists of ESR1/2 or PGR, respectively, it is important to note that these drugs have not yet been approved for the treatment of endometriosis [3, 44–46]. The results of this study would require of further clinical validation before widespread application, and follow-up studies to correlate receptor expression with previous treatments and clinical responses are warranted. Our data support the need to implement personalized medicine strategies in endometriosis that take in consideration biological differences of the lesions and their clinical implications.
Acknowledgments
These studies were funded in part by grant # R01-HD050559 to IF from the National Institute for Child Health and Human Development and RISE fellowship to MCC (grant # R25-GM082406). Construction of the Tissue Microarray (TMA) used for this study was funded by the U56 PSM-MCC Partnership grant # U56CA126379 from the National Cancer Institute (NCI). We thank Dr. Annelisse Santiago, Dr. Jennifer Marquez, and Dr. Candace Gonzalez for their role in the analysis of the TMA. We acknowledge the revision and editing of the manuscript by Patricia Casbas, PhD, and the support in statistical analysis provided by Himilce Vélez, MS, Public Health Program, Ponce Health Sciences University. Finally, we acknowledge the critical revision and editing of this manuscript during the American Physiology Association (APS) workshop entitled “Writing and Reviewing for Scientific Journals”, attended by MCC (NIGMS MARC travel awardee). We also acknowledge the support from the PhD Program in Biomedical Sciences of the Ponce Health Sciences University.
Disclosure
These studies were funded in part by NIH-NICHD grant #R01-HD050559 to IF; NIH-NIGMS grant #R25-GM082406 to MCC; and NIH-NCI grant #U56CA126379
Footnotes
AUTHORS’ CONTRIBUTIONS
Experimental design, data collection, and interpretation was performed by MCC and IF. Statistical analysis was performed by MCC and IF. Selection and pathological analysis of the samples in the TMA were performed by MG and AM. Manuscript was drafted by MCC and IF. All authors contributed in the final revision. Final approval of the manuscript by IF and MCC.
DECLARATION OF CONFLICTING INTERESTS
The authors declare no conflicts of interest.
References
- 1.Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98(3):511–9. doi: 10.1016/j.fertnstert.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giudice LC. Clinical practice. Endometriosis. The New England journal of medicine. 2010;362(25):2389–98. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bedaiwy MA, et al. New developments in the medical treatment of endometriosis. Fertil Steril. 2017;107(3):555–565. doi: 10.1016/j.fertnstert.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 4.Simoens S, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27(5):1292–9. doi: 10.1093/humrep/des073. [DOI] [PubMed] [Google Scholar]
- 5.Fourquet J, et al. Quantification of the impact of endometriosis symptoms on health-related quality of life and work productivity. Fertil Steril. 2011;96(1):107–12. doi: 10.1016/j.fertnstert.2011.04.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fourquet J, et al. Patients’ report on how endometriosis affects health, work, and daily life. Fertil Steril. 2010;93(7):2424–8. doi: 10.1016/j.fertnstert.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greene AD, et al. Endometriosis: where are we and where are we going? Reproduction. 2016;152(3):R63–78. doi: 10.1530/REP-16-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergqvist A, Ferno M. Estrogen and progesterone receptors in endometriotic tissue and endometrium: comparison according to localization and recurrence. Fertil Steril. 1993;60(1):63–8. [PubMed] [Google Scholar]
- 9.Shao R, et al. The elusive and controversial roles of estrogen and progesterone receptors in human endometriosis. Am J Transl Res. 2014;6(2):104–13. [PMC free article] [PubMed] [Google Scholar]
- 10.Wingfield M, et al. Cell proliferation is increased in the endometrium of women with endometriosis. Fertility and sterility. 1995;64(2):340–6. doi: 10.1016/s0015-0282(16)57733-4. [DOI] [PubMed] [Google Scholar]
- 11.Nisolle M. Ovarian endometriosis and peritoneal endometriosis: are they different entities from a fertility perspective? Current opinion in obstetrics & gynecology. 2002;14(3):283–8. doi: 10.1097/00001703-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Dekker TJ, et al. Quality assessment of estrogen receptor and progesterone receptor testing in breast cancer using a tissue microarray-based approach. Breast Cancer Res Treat. 2015;152(2):247–52. doi: 10.1007/s10549-015-3444-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colon-Caraballo M, Monteiro JB, Flores I. H3K27me3 is an Epigenetic Mark of Relevance in Endometriosis. Reprod Sci. 2015;22(9):1134–42. doi: 10.1177/1933719115578924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colon-Diaz M, et al. HDAC1 and HDAC2 are Differentially Expressed in Endometriosis. Reproductive sciences. 2012 doi: 10.1177/1933719111432870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruiz LA, et al. Dysregulation of Lysyl Oxidase Expression in Lesions and Endometrium of Women With Endometriosis. Reprod Sci. 2015;22(12):1496–508. doi: 10.1177/1933719115585144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stuart-Harris R, et al. Proliferation markers and survival in early breast cancer: a systematic review and meta-analysis of 85 studies in 32,825 patients. Breast. 2008;17(4):323–34. doi: 10.1016/j.breast.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Bulun SE, et al. Molecular biology of endometriosis: from aromatase to genomic abnormalities. Semin Reprod Med. 2015;33(3):220–4. doi: 10.1055/s-0035-1554053. [DOI] [PubMed] [Google Scholar]
- 18.Attia GR, et al. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85(8):2897–902. doi: 10.1210/jcem.85.8.6739. [DOI] [PubMed] [Google Scholar]
- 19.Bulun SE, et al. Role of estrogen receptor-beta in endometriosis. Semin Reprod Med. 2012;30(1):39–45. doi: 10.1055/s-0031-1299596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bulun SE, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Seminars in reproductive medicine. 2010;28(1):36–43. doi: 10.1055/s-0029-1242991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandenberger AW, et al. Oestrogen receptor (ER)-alpha and ER-beta isoforms in normal endometrial and endometriosis-derived stromal cells. Mol Hum Reprod. 1999;5(7):651–5. doi: 10.1093/molehr/5.7.651. [DOI] [PubMed] [Google Scholar]
- 22.Matsuzaki S, et al. Expression of estrogen receptor alpha and beta in peritoneal and ovarian endometriosis. Fertility and sterility. 2001;75(6):1198–205. doi: 10.1016/s0015-0282(01)01783-6. [DOI] [PubMed] [Google Scholar]
- 23.Izawa M, Taniguchi F, Harada T. Molecular Background of Estrogen Receptor Gene Expression in Endometriotic Cells. Reprod Sci. 2016;23(7):871–6. doi: 10.1177/1933719115623642. [DOI] [PubMed] [Google Scholar]
- 24.Zannoni GF, et al. The expression ratios of estrogen receptor alpha (ERalpha) to estrogen receptor beta1 (ERbeta1) and ERalpha to ERbeta2 identify poor clinical outcome in endometrioid endometrial cancer. Hum Pathol. 2013;44(6):1047–54. doi: 10.1016/j.humpath.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Miro AM, et al. 17beta-Estradiol regulates oxidative stress in prostate cancer cell lines according to ERalpha/ERbeta ratio. J Steroid Biochem Mol Biol. 2011;123(3–5):133–9. doi: 10.1016/j.jsbmb.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Alonso-Magdalena P, et al. A role for epithelial-mesenchymal transition in the etiology of benign prostatic hyperplasia. Proc Natl Acad Sci U S A. 2009;106(8):2859–63. doi: 10.1073/pnas.0812666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pathmanathan N, Balleine RL. Ki67 and proliferation in breast cancer. J Clin Pathol. 2013;66(6):512–6. doi: 10.1136/jclinpath-2012-201085. [DOI] [PubMed] [Google Scholar]
- 28.Liu P, et al. CD105/Ki67 coexpression correlates with tumor progression and poor prognosis in epithelial ovarian cancer. Int J Gynecol Cancer. 2012;22(4):586–92. doi: 10.1097/IGC.0b013e31823c36b8. [DOI] [PubMed] [Google Scholar]
- 29.Adisa JO, et al. Expression of some selected cytokeratins and Ki67 protein in prostatic tumor: can these be used as tumor markers. Pan Afr Med J. 2015;20:46. doi: 10.11604/pamj.2015.20.46.3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viale G, et al. Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol. 2008;26(34):5569–75. doi: 10.1200/JCO.2008.17.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park JS, et al. Endometrium from women with endometriosis shows increased proliferation activity. Fertil Steril. 2009;92(4):1246–9. doi: 10.1016/j.fertnstert.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 32.Nisolle M, Casanas-Roux F, Donnez J. Immunohistochemical analysis of proliferative activity and steroid receptor expression in peritoneal and ovarian endometriosis. Fertil Steril. 1997;68(5):912–9. doi: 10.1016/s0015-0282(97)00341-5. [DOI] [PubMed] [Google Scholar]
- 33.Franco-Murillo Y, et al. Unremitting cell proliferation in the secretory phase of eutopic endometriosis: involvement of pAkt and pGSK3beta. Reprod Sci. 2015;22(4):502–10. doi: 10.1177/1933719114549843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scotti S, et al. Reduced proliferation and cell adhesion in endometriosis. Mol Hum Reprod. 2000;6(7):610–7. doi: 10.1093/molehr/6.7.610. [DOI] [PubMed] [Google Scholar]
- 35.Heidemann LN, et al. The relation between endometriosis and ovarian cancer – a review. Acta obstetricia et gynecologica Scandinavica. 2014;93(1):20–31. doi: 10.1111/aogs.12255. [DOI] [PubMed] [Google Scholar]
- 36.McCluggage WG, et al. The Fallopian Tube Origin and Primary Site Assignment in Extrauterine High-grade Serous Carcinoma: Findings of a Survey of Pathologists and Clinicians. Int J Gynecol Pathol. 2016 doi: 10.1097/PGP.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 37.Calcagno A, et al. Expression patterns of Aurora A and B kinases, Ki-67 and the estrogen and progesterone receptors determined using an endometriosis tissue microarray model. Hum Reprod. 2011;26(10):2731–41. doi: 10.1093/humrep/der264. [DOI] [PubMed] [Google Scholar]
- 38.Kamat AA, et al. Protein expression profiling of endometriosis: Validation of 2-mm tissue microarrays. Fertility and Sterility. 2004;82(6):1681–1683. doi: 10.1016/j.fertnstert.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 39.Zanatta A, et al. The relationship among HOXA10, estrogen receptor alpha, progesterone receptor, and progesterone receptor B proteins in rectosigmoid endometriosis: a tissue microarray study. Reprod Sci. 2015;22(1):31–7. doi: 10.1177/1933719114549846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagenfeld A, et al. BAY 1002670: a novel, highly potent and selective progesterone receptor modulator for gynaecological therapies. Hum Reprod. 2013;28(8):2253–64. doi: 10.1093/humrep/det247. [DOI] [PubMed] [Google Scholar]
- 41.Ingamells S, et al. Endometrial progesterone receptor expression during the human menstrual cycle. J Reprod Fertil. 1996;106(1):33–8. doi: 10.1530/jrf.0.1060033. [DOI] [PubMed] [Google Scholar]
- 42.Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. 1997;68(4):585–96. doi: 10.1016/s0015-0282(97)00191-x. [DOI] [PubMed] [Google Scholar]
- 43.Mazur EC, Large MJ, DeMayo FJ. Human Oviduct and Endometrium: Changes over the Menstrual Cycle. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill’s Physiology of Reproduction. Elsevier; 2015. pp. 1077–1097. [Google Scholar]
- 44.Giannini A, et al. Selective steroid receptor modulators in reproductive medicine. Minerva Ginecol. 2015;67(5):431–55. [PubMed] [Google Scholar]
- 45.Stratton P, et al. Return of chronic pelvic pain from endometriosis after raloxifene treatment: a randomized controlled trial. Obstetrics and gynecology. 2008;111(1):88–96. doi: 10.1097/01.AOG.0000297307.35024.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chwalisz K, et al. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocrine reviews. 2005;26(3):423–38. doi: 10.1210/er.2005-0001. [DOI] [PubMed] [Google Scholar]


