Abstract
Horses are susceptible to developing large areas of pulmonary atelectasis during recumbency and anesthesia. The subsequent pulmonary shunt is responsible for significant impairment of oxygenation. Since ventilation perfusion mismatch persists into the post-operative period, hypoxemia remains an important concern in the recovery stall. This case report describes the diagnosis and supportive therapy of persistent hypoxemia in a 914 kg draft horse after isoflurane anesthesia. It highlights how challenging it can be to deal with hypoxemia after disconnection from the anesthesia machine and how life-threatening it can become if refractory to treatment. Furthermore, it stresses the point on the interactions between hypoxemia and other factors, such as residual drug effects and hypothermia, that should also be considered in the case of delayed recovery from general anesthesia.
Keywords: horse, anesthesia, recovery, hypoxemia, estimated shunt fraction, hypothermia, residual drug effects
Introduction
A 9-year-old Boulonnais gelding weighing 914 kg was referred to the Equine Clinic of the University of Liege for transpalpebral enucleation of the left eye under general anesthesia. Preoperative laboratory values including total protein, packed cell volume (PCV), total hemoglobin (tHb), total and differential white blood cell count, serum creatinine, total and conjugated bilirubin, and gamma glutamyl transpeptidase were within normal limits. Physical examination was unnoticeable unless a mild tachypnoea (24 breaths/min), the patient was graded II according to the American Society of Anesthesiologists physical status.
Food, but not water, was withheld for 10 h prior to surgery. Vitamin E acetate and sodium selenite pentahydrate (VMD, Belgium; 6500 mg and 130 mg respectively), were administered IM twice, the day before and the morning of the surgery. A 12-gauge intravenous catheter was placed in the left jugular vein. Procaine penicillin (Kela, Belgium; 19.2 M UI IM), gentamicin (Franklin Pharmaceuticals, Ireland; 6 g IV), and acepromazine (Kela, Belgium; 90 mg IM) were administered 120 min before induction. Flunixin meglumine (Ecuphar, Belgium; 950 mg IV) immediately followed by xylazine (Prodivet Pharmaceuticals, Belgium; 480 mg IV) were administered as preanaesthetic medication. Anesthesia was induced with midazolam (Mylan, Belgium; 55 mg IV) and ketamine (Ecuphar, Belgium; 2 g IV) and the trachea was intubated with a 30 mm cuffed endotracheal tube (ETT) as soon as the patient was recumbent. The horse was positioned in right lateral recumbency on a padded surface and conducted to the operating room where he was connected to a rebreathing circuit.
A 20-gauge catheter was placed in the left dorsal metatarsal artery for continuous direct arterial pressure measurement and repeated arterial blood sampling. Invasive arterial blood pressure, pulse oximetry, electrocardiogram, inspired and expired percentages of oxygen and isoflurane, inspired and expired carbon dioxide partial pressures, airway pressure, and flow-volume loops were continuously recorded using a multiparameter monitor (Tafonius, Vetronics, UK). Arterial partial pressure of oxygen (PaO2) and carbon dioxide (PaCO2), pH, PCV, plasma electrolytes, arterial saturation of hemoglobin (SaO2), tHb, oxyhemoglobin (O2Hb), carboxyhemoglobin, and methemoglobin were measured with co-oxymetry (Cobas b 123, Roche, Belgium).
Isoflurane (Zoetis, Belgium) was delivered in 100% oxygen and its end-tidal percentage was maintained between 0.9 and 1.3%. Intermittent positive pressure ventilation (IPPV) was provided during the whole procedure (Tafonius) to maintain end-tidal carbon dioxide partial pressure between 35 and 45 mmHg (respiratory rate: 6–8 breaths/min, tidal volume: 9.5–10 L, I:E ratio = 1:2 and maximum peak inspiratory pressure: 35 cmH2O). Retrobulbar nerve block was performed with 100 mg lidocaine (AstraZeneca, Belgium) and 100 mg mepivacaine (AstraZeneca, Belgium) and auriculopalpebral nerve block with 20 mg lidocaine. Ketamine was administered three times as IV bolus (300 mg) to increase the anesthetic depth and morphine (Sterop, Belgium; 90 mg) was injected IV to control pain 82 min after induction. Partial intravenous anesthesia using ketamine (1 mg/kg/h) and midazolam (0.02 mg/kg/h) was added 25 min after induction, lasted for 95 min to be finished 80 min before the end of anesthesia. Lactated Ringer's solution (Dechra, UK) was infused during anesthesia at a rate of 5.6 ml/kg/h. Standard transpalpebral enucleation of the left eye was completed within 150 min and the total anesthesia time was 200 min.
Heart rate was comprised between 35 and 45 beats/min. Systolic, mean and diastolic arterial blood pressures ranged between 85 and 120, 65 and 95, and 50 and 85 mmHg, respectively. The first arterial blood gas revealed mild hypoxemia (PaO2 64 mmHg). Salbutamol (Sandoz, Belgium; 1.9 mg) was administered through the ETT with a metered-dose inhaler 37 min after induction. However, the second arterial blood sample showed only a small improvement (PaO2 73 mmHg). Therefore, an alveolar recruitment maneuver (ARM) was performed 47 min after induction. Practically, it consisted in interrupting IPPV during inspiratory phase and applying a continuous positive airway pressure (CPAP) of 50 cmH2O during 45 s. Afterwards, IPPV was resumed and a positive end-expiratory pressure (PEEP) of 10 cmH2O was maintained until the end of the procedure. Ulterior arterial blood samples showed a progressive improvement and hypoxemia was solved (PaO2 80, 103, 153, and 203 mmHg at 3, 34, 61, and 90 min post-recruitment maneuver, respectively). Data from blood gas analysis are displayed in Table 1.
Table 1.
Peripheral arterial and venous blood samples analyzed during anesthesia (A), when recumbent during recovery (R) and after standing (S).
| Time | A/R/S | Patm | FiO2 | pH | Hba | PaO2 | SaO2 | PaCO2 | PvO2 | SvO2 | PvCO2 | Lact | CK | PAO2 | F-shunte |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 31 min | A | 742.6 | 0.66 | 7.396 | 10.5 | 64.3 | 94.2 | 49.3 | / | / | / | / | / | 397.5 | 35 |
| 42 min (after salbutamol) | A | 742.7 | 0.71 | 7.380 | 9.3 | 73.0 | 96.0 | 44.9 | / | / | / | / | / | 437.8 | 32 |
| 50 min (after ARM) | A | 742.8 | 0.80 | 7.379 | 9.9 | 79.7 | 96.6 | 49.8 | / | / | / | / | / | 494.4 | 33 |
| 1 h 21 min | A | 742.7 | 0.82 | 7.361 | 10.5 | 103.3 | 98.0 | 51.0 | / | / | / | / | / | 506.7 | 31 |
| 1 h 48 min | A | 742.7 | 0.84 | 7.364 | 9.8 | 153.1 | 98.8 | 52.0 | / | / | / | / | / | 519.4 | 27 |
| 2 h 17 min | A | 742.8 | 0.85 | 7.384 | 9.7 | 203.4 | 98.9 | 51.7 | / | / | / | / | / | 526.8 | 25 |
| 5 h 42 min | R | 742.0 | 0.30 | 7.501 | / | 40.5 | / | 36.4 | / | / | / | / | / | 163.0 | / |
| 5 h 46 min | R | 741.8 | 0.30 | 7.441 | / | / | / | / | 20.7 | 40.2 | 47.7 | 4.2 | 787 | / | / |
| 10 h 38 min | R | 741.8 | 0.30 | 7.401 | 13.2 | 48.1 | 84.9 | 46.0 | / | / | / | 3.2 | / | 150.9 | 46 |
| 13 h 27 min | R | 742.0 | 0.30 | 7.377 | 12.8 | 57.2 | 91.0 | 44.1 | / | / | / | / | / | 153.4 | 35 |
| 21 h 36 min | R | 740.7 | 0.21 | 7.340 | / | / | / | / | 25.5 | 43.0 | 51.4 | 2.8 | / | / | / |
| 23 h 15 min | R | 740.9 | 0.21 | 7.386 | 13.4 | 46.7 | 85.8 | 39.8 | / | / | / | / | / | 96.0 | 44 |
| 27 h 27 min | R | 740.8 | 0.30 | 7.401 | / | 61.6 | / | 34.1 | / | / | / | / | 13,000 | 165.5 | / |
| 34 h 44 min | R (after S) | 741.8 | 0.21 | 7.424 | 11.3 | 49.0 | 89.0 | 41.2 | / | / | / | 3.8 | / | 94.4 | 34 |
| 45 h 59 min | S | 743.4 | 0.21 | 7.454 | 11.8 | 38.1 | 78.6 | 41.2 | / | / | / | 0.9 | 3,788 | 94.7 | 51 |
Patm, atmospheric pressure (mmHg); FiO2, inspired oxygen fraction (mmHg); Hba, arterial hemoglobin concentration (g/dl); PaO2, arterial oxygen partial pressure (mmHg); SaO2, arterial hemoglobin oxygen saturation (%); PaCO2, arterial carbon dioxide partial pressure (mmHg); PvO2, venous oxygen partial pressure (mmHg); SvO2, venous hemoglobin oxygen saturation (%); PvCO2, venous carbon dioxide partial pressure (mmHg); Lact, lactates (mmol/L); CK, creatine kinase (UI/L); PAO2, alveolar oxygen partial pressure (mmHg); F-shunte, estimated shunt fraction (%); ARM, alveolar recruitment maneuver.
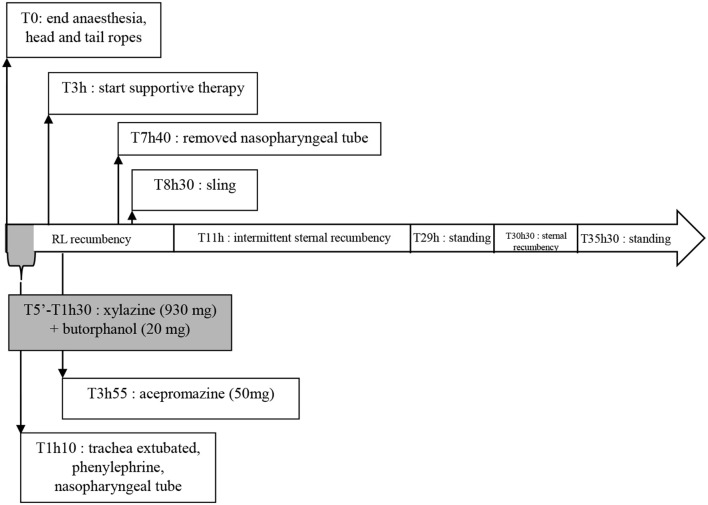
At the end of surgery, the horse was placed in right lateral recumbency in a rubber floored and heavily padded recovery stall. The ETT was secured and oxygen (15 L/min) was administered through it. The horse became extremely agitated 5 min after isoflurane discontinuation and needed to be sedated nine times with xylazine (total dose: 930 mg IV) and butorphanol (Ecuphar, Austria; 20 mg IV) to avoid self-inflicted traumas. The trachea was extubated 70 min after the end of anesthesia. Because of snoring, 10 ml of a solution of 0.5% phenylephrine (Bausch and Lomb, Belgium) was instilled in each nostril, a 16 mm nasopharyngeal tube was inserted through the left nostril and oxygen therapy was continued. He removed the nasopharyngeal tube during one of his violent uncoordinated movements but, because he was not snoring anymore, the oxygen hose (15 L/min) was placed directly in the nose to the pharynx and maintained in place whenever it was possible. Following the nine unsuccessful attempts to sedate the horse with xylazine, and as he was still not standing at that time, acepromazine was administered (50 mg IV), providing light but longer sedation (Figure 1).
Figure 1.
Time line of the main events that happened during recovery. RL, right lateral.
Supportive therapy was started in the recovery stall as the recovery period exceeded 180 min. It consisted in fluid therapy with lactated Ringer's solution (11 ml/kg/h) supplemented with calcium gluconate (Dechra, Belgium), magnesium sulfate (Sterop, Belgium), and potassium chloride (Braun, Germany). Alternatively, sodium chloride 0.9% (Aguettant, Belgium) was infused according to plasma electrolytes measurement (sodium 137.2 mmol/l; chloride 98.6 mmol/l). Furthermore, nutrition support was provided with glucose 50% (Baxter, Belgium; 2mg/kg/min). Inotropic support with dobutamine (Mylan, Belgium; 1 μg/kg/min) was also added as the pulse was weak. Analgesia was provided with morphine (90 mg IM) TID and flunixin meglumine (950 mg IV) BID. The horse was also given a single dose of dexamethasone (Eurovet, Holland; 92 mg IV) 285 min after isoflurane discontinuation. Regular physical examinations were performed: the pulse became stronger after fluid therapy and inotropic support were initiated. Mucosa were pink and refill capillary time was prolonged up to 4 s. He presented tachycardia (50–64 beats/min) with arrhythmias and mild tachypnoea (22–28 breaths/min). Hypothermia, up to 33.4°C, was efficiently treated by covering him with blankets. Furthermore, he showed nystagmus and an obtunded pupillary light reflex. As the recovery period abnormally prolonged, concern was raised about generalized post-anaesthetic myopathy. However, the soft and non-painful muscle palpation, the normal gross appearance of urine and serum creatine kinase levels (Table 1) did not advocate for this condition.
A sling was put on the horse 8.5 h after entering the recovery stall, in addition to head and tail ropes, which were attached from the beginning, to lift him up with the hoist. Unfortunately, we did not manage to get him to standing position after having tried several times. Because he was very agitated and heavy, we also failed to change recumbency and he spent 14.5 h (including surgery) in right lateral recumbency. At 11 h after anesthesia, he managed to get and stay intermittently in sternal recumbency. Twenty-nine hours after the end of anesthesia, the horse finally stood up without any assistance. The right front leg was painful and he could not bear any weight on it, he kept standing for 90 min before lying down again. The horse finally stood up 5 h later and he was moved out of the recovery stall. The likely radial neuropathy and/or triceps myopathy were treated by administering acepromazine (46 mg IM) TID, morphine (90 mg IM) TID, ketamine (460 mg IM) QID and flunixin meglumine (950 mg IV) BID. Low molecular weight heparin (Sanofi, Belgium; 150 mg) was administered SC SID and supportive hoof bandages were placed on both front legs to prevent laminitis. Moreover, the horse presented paralysis and distortion of the nose and lips to the right side, most likely due to paralysis of the buccal branch of the right facial nerve, which spontaneously returned to normal upon discharge. Furthermore, the horse developed a surgical wound dehiscence due to infection, which successfully healed after a second surgery under standing sedation.
Background
Perioperative respiratory complications
Horses are susceptible to quickly developing large areas of atelectasis and a consequent large pulmonary shunt causing significant impairment of gas exchange during recumbency and anesthesia (1–4). Pulmonary shunt has been estimated at 1% in standing horses, extending to 19%, and 33% in anesthetized laterally and dorsally recumbent horses, respectively (5, 6).
Compression atelectasis is the major type of atelectasis in anesthetized horses (6). Recumbency changes distribution of ventilation by reducing lung volume and altering the pleural pressure gradient to such an extent that the peripheral airways close in the dependent regions of the lung where closing volume exceeds functional residual capacity (2, 5). Body weight and body shape both influence PaO2 and alveolar arterial oxygen gradient (Aa gradient). Indeed, light-weight animals which are tall with large thoracic circumference and flat belly better maintain oxygenation (7–9).
Moreover, characteristics of the pulmonary artery in the caudodorsal regions of the lung alter the hypoxic vasoconstrictive response to alveolar hypoxia, leading to preferential caudodorsal lung perfusion and larger ventilation perfusion mismatch regardless of the posture (3, 10, 11). In addition, most anesthetics, and particularly inhalants, deeply reduce or even abolish hypoxic vasoconstriction and flow redistribution (12).
Consequently, there can be prolonged periods of hypoxemia during general anesthesia in horses (4, 13–16).
Since ventilation perfusion (V/Q) mismatch persists into the postoperative period (17), hypoxemia remains an important concern in the early recovery period, especially when breathing room air reduces the inspired oxygen fraction (FiO2), requiring vigilant monitoring and oxygen supplementation (18). Nasotracheal insufflation of oxygen at a flow rate of at least 15 L/min immediately after disconnection from the anesthesia machine efficiently improves PaO2 and relieves hypoxemia in the recovering horse (18). Furthermore, horses auto-recruit their lungs by inspiratory breath holding following recovery from general anesthesia, possibly reflecting a compensatory mechanism to counteract persistent atelectasis (19).
Residual drug effects
Ketamine infusion is commonly used to balance inhalational anesthesia and midazolam infusion is frequently added to reduce the central excitatory effects of ketamine (20). Nevertheless, common concerns are often raised about their negative influence on recovery quality after prolonged infusion and the difficulty to predict the pharmacokinetics and pharmacodynamics of drugs combinations.
The pharmacokinetics of midazolam has only been described in conscious horses (21). Redistribution is responsible for the termination of its clinical effect and accumulation in peripheral compartment is highly probable.
Two studies described the pharmacokinetics of racemic ketamine after infusion in conscious horses (22, 23). They both mentioned that the pharmacokinetics of ketamine after infusion was different from those described after single bolus. Moreover, both studies stressed the point that premedication or concurrent administration of inhalants or other anesthetics were known to influence the pharmacokinetics of ketamine. Ketamine undergoes rapid metabolism to norketamine, whose redistribution and metabolism are slower than for parent drug. Nevertheless, the contribution of ketamine's metabolite to its pharmacological effects is unknown in the horse.
Intraoperative administration of ketamine has been shown to be a significant predictor of faster recovery time, indicating that these horses were kept at a lighter plane of anesthesia, and consequently had less isoflurane accumulation (24).
Inadvertent perioperative hypothermia
Several studies reported that hypothermia prolong time to standing in horses (24–26).
Drug metabolism relies on enzymatic reactions that may be altered by hypothermia. Hypothermia can therefore prolong recovery time (27).
Discussion
Perioperative respiratory complications
Assessment of oxygenation
diagnostic tools
Usually, clinical assessment is sufficient to monitor most recoveries. However, different indices have been described to assess oxygenation. Venous admixture (Qs/Qt) is the most accurate but requires mixed venous blood collected from the pulmonary artery. Estimated shunt fraction (F-shunte) is a content-based index that is calculated from peripheral arterial blood and has the best agreement with Qs/Qt (28).
perioperative hypoxemia and atelectasis
Arterial blood gas analysis revealed mild hypoxemia at the beginning of the anesthesia period (PaO2 64 mmHg). Inhalation of salbutamol and ARM followed by PEEP reverted hypoxemia (PaO2 up to 203 mmHg at the end of the anesthesia period). During anesthesia, F-shunte lay between 25 and 35%. In the recovery stall, PaO2 was between 41 and 62 mmHg, corresponding to mild to moderate hypoxemia, and F-shunte lay between 34 and 46% (Table 1). These calculated values of shunt percentage were largely superior to the expected value of 19% in anesthetized laterally recumbent horses (5, 6), suggesting a large pulmonary shunt responsible for hypoxemia in that horse. Moreover, PaO2 10 h after standing was 38 mmHg, corresponding to severe hypoxemia and F-shunte was 51%.
Prevention and treatment of hypoxemia
oxygen supplementation
These measurements showed that, despite oxygen insufflation immediately after disconnection from the anesthesia machine, hypoxemia developed during recovery. Administering oxygen in ETT creates a combination of room air and oxygen leading to FiO2 between 30 and 70% (29). The further distal in the airway oxygen is delivered, the greater the increase in FiO2 for a particular oxygen flow rate (29). However, the horse could not bear the nasopharyngeal tube and was sometimes so agitated that the anesthetist did not manage to maintain the oxygen hose in his nose continuously, preventing from proper oxygen insufflation. Furthermore, the degree of improvement in PaO2 depends on the increment in FiO2 and on the degree of V/Q mismatch (29). Nevertheless, increasing FiO2 during anesthesia is generally unsuccessful in correcting hypoxemia since much of the impairment in gas exchange results directly from shunt (30). Unfortunately, we did not measure the real FiO2 during recovery and considered 30% for F-shunte calculation, which might be a potential source of imprecision.
lung recruitment
Open lung concept consists in, first, applying a high PIP to reinflate atelectatic lungs, which is also referred as ARM, and, second, maintaining a PEEP to prevent re-collapse. Indeed, once a critical amount of atelectasis is present in the equine lung, it is difficult to recruit that portion of the lung using traditional ventilation strategies (29) and PIP of up to 80 cmH2O and PEEP of up to 30 cmH2O are required (31–36). Two strategies are commonly used: either a stepwise incremental and decremental PIP and PEEP titration; or a sustained high-pressure maneuver followed by a predetermined PEEP. Although high airway pressures inevitably induce cardiovascular and pulmonary side effects, the first technique may present two main advantages: first, by allowing the cardiovascular system to better adapt to higher intrathoracic pressures; and second, by using the lowest PEEP required to keep recruited alveoli open (32, 34, 35, 37, 38). Optimal PEEP for each patient is best titrated by monitoring PaO2 or the compliance of the dependent lung assessed by electrical impedance tomography (35). Despite that the sustained high-pressure ARM followed by predetermined PEEP used in this case resulted in reduction of pulmonary shunt and resolution of hypoxemia, these improvements did not extend in the recovery period. These observations suggested re-collapse as soon as positive airway pressure is lost, which is conflicting with studies reporting applications of modified open lung concept techniques. It is technically difficult to provide PEEP after disconnection, but, in theory, it may have limited de-recruitment (32, 33, 36, 37).
auto-recruitment
It has been reported that horses auto-recruit their lungs by inspiratory breath holding until 5 h after standing (19). Nevertheless, this seemed not to be the case for this horse because pulmonary shunt and hypoxemia worsened after standing for 10 h. The reason why he did not manage to recruit his lungs is not clear. Obviously, the horse did not present a clinical picture compatible with a severe lung pathology such as pulmonary edema, pneumothorax, or pulmonary embolism. However, gene expression quantification has shown that mechanical ventilation, either IPPV (PIP of 20 cmH2O) or stepwise ARM combined with PEEP (PIP of up to 60 cmH2O and PEEP of 20 cmH2O), might be responsible for an early inflammatory state in the lungs. Indeed, these ventilation strategies both increased markers possibly associated with lung injuries without being related to any histological lesion nor any modification of total and differential cell counts in bronchoalveolar lavage fluid (39). Therefore, we cannot exclude that the ventilation strategy applied to this horse, and combining IPPV (PIP of up to 35 cmH2O) and sustained high-pressure ARM followed by predetermined PEEP (CPAP of 50 cmH2O and PEEP of 10 cmH2O), might not have caused alterations in the lungs that prevent from auto-recruitment.
Moreover, the higher weight of that draft horse might have played a role as for example prolonged atelectasis has been demonstrated in morbidly obese patients (40).
body weight
As body weight and body shape both influence PaO2 and Aa gradient (7–9), body weight might have played a role in the development of atelectasis, compression atelectasis being the major type of atelectasis in anesthetized horses (6).
duration of anesthesia
Duration of anesthesia is known to influence the incidence of episodes of hypoxemia in humans (41, 42). The total anesthesia time was 200 min, which is much more than usual for transpalpebral enucleation and might have contributed to hypoxemia.
anaesthetics
Anaesthetics may alter cardiorespiratory function and therefore negatively influence gas exchange. However, morphine and butorphanol have not produced clinically significant cardiorespiratory impairments, maintaining PaO2 (43, 44). Similarly, acepromazine has improved arterial oxygenation by reducing V/Q disturbance and fall in PaO2 associated with general anesthesia (45).
Hypoxemia-related postoperative complications
delayed recovery
Inhalants attenuate autoregulation of cerebral blood flow (46). Inhalants and hypoxemia can be involved in brain injury and consequent altered cognition (47), potentially explaining nystagmus and obtunded pupillary light reflex. They might therefore affect recovery quality. However, hypoxemia is aggravated by repeated attempts to stand (48). Moreover, hypoxemia reduces the strength of muscle contraction (49). In that case, reduced cardiac output, probably added to hypoxemia, led to further decrease in tissue oxygen delivery. Indeed, pulse became stronger after initiation of supportive therapy, suggesting improvement in circulatory function.
Residual drug effects
The use of midazolam (loading dose 0.04 mg/kg and infusion rate 0.02 mg/kg/h) and ketamine (loading dose 2.5 mg/kg and infusion rate 1 mg/kg/h) in sevoflurane-anesthetized horses has been described (50). All horses recovered satisfactorily but showed mild ataxia for 15–20 min, probably due to midazolam. As doses that we used were comparable to those described in this study; and as infusion lasted for a shorter duration (95 vs. 126–190 min) and was discontinued well before switching off inhalant (80 vs. 0 min), it is less likely that neither midazolam nor ketamine prevented our horse from standing.
Some studies described ketamine infusions in halothane-anesthetized horses (51–53), using loading doses ranging from 2.2 to 2.4 mg/kg and infusion rates from 2.0 to 2.8 mg/kg/h. All recoveries were considered acceptable and comparable of those observed with halothane only. We used comparable loading dose but our infusion rate was two to almost three times less than that described in these studies. Furthermore, the maximum infusion time was shorter (95 vs. 127 min) and it was stopped much more ealier than inhalant (80 vs. 0–15 min). Consequently, it is less likely that ketamine might have interfere with recovery in our case.
Although its pharmacological activity has not been described yet in horses, norketamine accumulation is still a concern. Its implication in the nystagmus and the obtunded pupillary light reflex that we observed can not be ruled out.
Inadvertent perioperative hypothermia
Hypothermia might be partly responsible for prolonged recovery. Indeed, it may play a part in midazolam and ketamine accumulation by reducing their metabolism. Moreover, by reducing baroreceptor function and cardiomyocytes contractility, hypothermia may be involved in the low cardiac output suspected in our case as well as in the cardiac arrhythmias that we noticed (54, 55). Furthermore, it may decrease central nervous system function and alter cerebral perfusion, potentially leading to the altered cognition that we observed (56). In addition, hypothermia and consequent shivering might have worsened hypoxemia by greatly increasing oxygen consumption. In our case, body temperature should have been more closely monitored during anesthesia and recovery; and hypothermia should have been aggressively treated as soon as it appeared.
Concluding remarks
When facing complicated recovery, all the potentially contributing factors should always be contemplated. In this case, hypoxemia, residual drug effects, and hypothermia are three relevant factors that might have prolonged the period of time elapsed before the horse stood up. Indeed, although hypoxemia is a common complication in equine anesthesia and should be considered, residual drug effects and hypothermia should not be misregarded as they might have been responsible for rough recovery even without hypoxemia.
Ethics statement
While signing the hospitalization contract, the owner is aware and accepts that data concerning his animal might be used for scientific purposes without further consent, guaranteeing confidentiality and anonimity of the owner.
Author contributions
JD, DS, and CS were involved in clinical management of the case including data treatment and interpretation. All authors were involved in the preparation of the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to acknowledge Dr. Alexandra Gougnard for the precious help she brought in clinical management of the case.
References
- 1.Hall LW. Disturbances of cardiopulmonary function in anesthetized horses. Equine Vet J. (1971) 3:95–8. 10.1111/j.2042-3306.1971.tb04447.x [DOI] [PubMed] [Google Scholar]
- 2.McDonnell WN, Hall LW, Jeffcott LB. Radiographic evidence of impaired pulmonary function in laterally recumbent anaesthetized horses. Equine Vet J. (1979) 11:24–32. 10.1111/j.2042-3306.1979.tb01290.x [DOI] [PubMed] [Google Scholar]
- 3.Dobson A, Gleed RD, Meyer RE, Stewart BJ. Changes in blood flow distribution in equine lungs induced by anaesthesia. Q J Exp Physiol. (1985) 70:283–97. 10.1113/expphysiol.1985.sp002909 [DOI] [PubMed] [Google Scholar]
- 4.Nyman G, Funkquist B, Kvart C, Frostell C, Tokics L, Strandberg A, et al. Atelectasis causes gas exchange impairment in the anaesthetised horse. Equine Vet J. (1990) 22:317–24. 10.1111/j.2042-3306.1990.tb04280.x [DOI] [PubMed] [Google Scholar]
- 5.Sorenson PR, Robinson NE. Postural effects on lung volumes and asynchronous ventilation in anaesthetized horses. J Appl Physiol. (1980) 48:97–103. 10.1152/jappl.1980.48.1.97 [DOI] [PubMed] [Google Scholar]
- 6.Nyman G, Hedenstierna G. Ventilation-perfusion relationships in the anaesthetised horse. Equine Vet J. (1989) 21:274–81. 10.1111/j.2042-3306.1989.tb02167.x [DOI] [PubMed] [Google Scholar]
- 7.Moens Y, Lagerweij E, Gootjes P, Poortman J. Distribution of inspired gas to each lung in the anaesthetised horse and influence of body shape. Equine Vet J. (1995) 27:110–6. 10.1111/j.2042-3306.1995.tb03045.x [DOI] [PubMed] [Google Scholar]
- 8.Mansel JC, Clutton RE. The influence of body mass and thoracic dimensions on arterial oxygenation in anaesthetized horses and ponies. Vet Anaesth Anal. (2008) 35:392–9. 10.1111/j.1467-2995.2008.00400.x [DOI] [PubMed] [Google Scholar]
- 9.Schauvliege S, Savvast I, Gasthuys F. The effect of the inspired oxygen fraction on arterial blood oxygenation in spontaneously breathing, isoflurane anaesthetized horses: a retrospective study. Vet Anaest Analg. (2015) 42:280–5. 10.1111/vaa.12208 [DOI] [PubMed] [Google Scholar]
- 10.Hlastala MP, Bernard SL, Erickson HH, Fedde MR, Gaughan EM, McMurphy R, et al. Pulmonary blood flow distribution in standing horses is not dominated by gravity. J Appl Physiol. (1996) 81:1051–61. 10.1152/jappl.1996.81.3.1051 [DOI] [PubMed] [Google Scholar]
- 11.Stack A, Derksen FJ, Williams KJ, Robinson NE, Jackson WF. Lung region and racing affect mechanical properties of equine pulmonary microvasculature. J Appl Physiol. (2014) 117:370–6. 10.1152/japplphysiol.00314.2014 [DOI] [PubMed] [Google Scholar]
- 12.Steffey EP, Wheat JD, Meagher DM, Norrie RD, McKee J, Brown M, et al. Body position and mode of ventilation influences arterial pH, oxygen, and carbon dioxide tensions in halothane- anesthetized horses. Am J Vet Res. (1977) 38:379–82. [PubMed] [Google Scholar]
- 13.Hall LW, Gillespie JR, Tyler WS. Alveolo-arearterial oxygen tension differences in anaesthetised horses. Br J Anaesth. (1968) 40:560–8. 10.1093/bja/40.8.560 [DOI] [PubMed] [Google Scholar]
- 14.Mitchell B, Littlejohn A. The effect of anaesthesia and posture on the exchange of respiratory gases and on the heart rate. Equine Vet J. (1974) 6:177–8. 10.1111/j.2042-3306.1974.tb03956.x [DOI] [PubMed] [Google Scholar]
- 15.Stegmann GF, Littlejohn A. The effect of lateral and dorsal recumbency on cardiopulmonary function in the anaesthetised horse. J S Afr Vet Assoc. (1987) 58:21–7. [PubMed] [Google Scholar]
- 16.Day TK, Gaynor JS, Muir WW, Bednarski RM, Mason DE. Blood gas values during intermittent positive pressure ventilation and spontaneous ventilation in 160 anesthetized horses positioned in lateral or dorsal recumbency. Vet Surg. (1995) 26:266–76. 10.1111/j.1532-950X.1995.tb01330.x [DOI] [PubMed] [Google Scholar]
- 17.Mason DE, Muir WW, Wade A. Arterial blood gas tensions in the horse during recovery from anesthesia. J Am Vet Med Assoc. (1987) 190:989–94. [PubMed] [Google Scholar]
- 18.McMurphy RM, Cribb PH. Alleviation of post-anesthetic hypoxemia in the horse. Can Vet J. (1989) 30:37–41. [PMC free article] [PubMed] [Google Scholar]
- 19.Mosing M, Waldmann AD, MacFarlane P, Iff S, Auer U, Bohm SH, et al. Horses auto-recruit their lungs by inspiratory breath holding following recovery from general anaesthesia. PLoS ONE (2016) 11:e0158080. 10.1371/journal.pone.0158080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bettschart-Wolfensberger R. Horses. In: Grimm KA, Lamont LA, Tranquilli WJ, Greene SA, Robertson SA. editors. Veterinary Anesthesia and Analgesia, The Fifth Edition of Lumb and Jones. Oxford, UK: Wiley Blackwell; (2015). p. 857–66. [Google Scholar]
- 21.Hubbell JA, Kelly EM, Aarnes TK, Bednarski RM, Lerche P, Liu Z, et al. Pharmacokinetics of midazolam after intravenous administration to horses. Equine Vet J. (2013) 45:721–5. 10.1111/evj.12049 [DOI] [PubMed] [Google Scholar]
- 22.Fielding CL, Brumbaugh GW, Matthews NS, Peck KE, Roussel AJ. Pharmacokinetics and clinical effects of subanesthetic continuous rate infusion of ketamine in awake horses. Am J Vet Res. (2006) 67:1484–90. 10.2460/ajvr.67.9.1484 [DOI] [PubMed] [Google Scholar]
- 23.Lankveld DP, Driessen B, Soma IR, Moate PJ, Rudy J, Uboh CE, et al. Pharmacodynamic effects and pharmacokinetic profile of a long-term continuous rate infusion of racemic ketamine in healthy conscious horses. J Vet Pharmacol Therap. (2006) 29:477–88. 10.1111/j.1365-2885.2006.00794.x [DOI] [PubMed] [Google Scholar]
- 24.Voulgaris DA, Hofmeister EH. Multivariate analysis of factors associated with post-anesthetic times to standing in isoflurane-anesthetized horses: 381 cases. Vet Anaesth Analg. (2009) 36:414–20. 10.1111/j.1467-2995.2009.00472.x [DOI] [PubMed] [Google Scholar]
- 25.Tomasic M. Temporal changes in core body temperature in anesthetized adult horses. Am J Vet Res. (1999) 60:556–62. [PubMed] [Google Scholar]
- 26.Mayerhofer I, Scherzer S, Gabler C, van den Hoven R. Hypothermia in horses induces by general anaesthesia and limiting measures. Equine Vet Educ. (2005) 17:53–6. 10.1111/j.2042-3292.2005.tb00336.x [DOI] [Google Scholar]
- 27.Clark-Price S. Inadvertent perianesthetic hypothermia in small animal patients. Vet Clin Small Anim. (2015) 45:983–94. 10.1016/j.cvsm.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 28.Araos JD, Larenza P, Boston R, De Monte V, De Marzo C, Grasso S, et al. Use of oxygen content-based index, Fshunt, as an indicator of pulmonary venous admixture at various inspired oxygen fractions in anesthetized sheep. Am J Vet Res. (2012) 73:2013–20. 10.2460/ajvr.73.12.2013 [DOI] [PubMed] [Google Scholar]
- 29.Kerr CL, McDonell WN. Oxygen supplementation and ventilatory support. In: Muir WW, Hubbell JAE. editors. Equine Anesthesia: Monitoring and Emergency Therapy. St. Louis, MI: Saunders; (2009). p. 332–52. [Google Scholar]
- 30.Benator SR, Hewlett AM, Nunn JF. The use of iso-shunt lines for control of oxygen therapy. Br J Anaesth. (1973) 45:711–8. 10.1093/bja/45.7.711 [DOI] [PubMed] [Google Scholar]
- 31.Levionnois OL, Iff I, Moens Y. Successful treatment of hypoxemia by an alveolar recruitment maneuver in a horse during general anaesthesia for colic surgery. Pferdeheilkunde (2006) 2:333–6. 10.21836/PEM20060314 [DOI] [Google Scholar]
- 32.Bringewatt T, Hopster K, Kästner SB, Rohn K, Ohnesorge B. Influence of modified open lung concept ventilation on the cardiovascular and pulmonary function of horses during total intravenous anaesthesia. Vet Rec. (2010) 167:1000–6. 10.1136/vr.c4172 [DOI] [PubMed] [Google Scholar]
- 33.Hopster K, Kästner SB, Rohn K, Ohnesorge B. Intermittent positive pressure ventilation with constant positive end-expiratory pressure and alveolar recruitment manoeuvre during inhalation anaesthesia in horses undergoing surgery for colic, and its influence on the early recovery period. Vet Anaesth Analg. (2011) 38:169–77. 10.1111/j.1467-2995.2011.00606.x [DOI] [PubMed] [Google Scholar]
- 34.Moens Y, Schrammel JP, Tusman G, Ambrisko TD, Solà J, Brunner JX, et al. Variety of non-invasive continuous monitoring methodologies including electrical impedance tomography provides novel insights into the physiology of lung collapse and recruitment – case report of an anaesthetized horse. Vet Anaesth Analg. (2014) 41:196–204. 10.1111/vaa.12098 [DOI] [PubMed] [Google Scholar]
- 35.Ambrisko TD, Schrammel J, Hopster K, Kästner S, Moens Y. Assessment of distribution of ventilation and regional lung compliance by electrical impedance tomography in anaesthetized horses undergoing alveolar recruitment manoeuvres. Vet Anaesth Analg. (2017) 44:264–72. 10.1016/j.vaa.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 36.Hopster K, Rohn K, Ohnesorge B, Kästner SB. Controlled mechanical ventilation with constant positive pressure and alveolar recruitment manouevres during anaesthesia in laterally or dorsally recumbent horses. Vet Anaest Analg. (2017) 44:121–6. 10.1111/vaa.12390 [DOI] [PubMed] [Google Scholar]
- 37.Wettstein D, Moens Y, Jaeggin-Schmucker N, Böhn SH, Rothen HU, Mosing M, et al. Effects of an alveolar recruitment maneuver on cardiovascular and respiratory parameters during total intravenous anesthesia in ponies. Am J Vet Res. (2006) 67:152–9. 10.2460/ajvr.67.1.152 [DOI] [PubMed] [Google Scholar]
- 38.Hospter K, Wogatzki A, Geburek F, Conze P, Kästner SB. Effects of positive end-expiratory pressure titration on intestinal oxygenation and perfusion in isoflurane anaesthetised horses. Equine Vet J. (2016) 49:250–256. 10.1111/evj.12555 [DOI] [PubMed] [Google Scholar]
- 39.Hopster K, Jacobson B, Hopster-Iversen C, Rohn K, Kästner SB. Histopathological changes and mRNA expression in lungs of horses after inhalation anaesthesia with different ventilation strategies. Res Vet Sci. (2016) 107:8–15. 10.1016/j.rvsc.2016.04.008 [DOI] [PubMed] [Google Scholar]
- 40.Eichenberger A, Proietti S, Wicky S, Magnusson L. Morbid obesity and postoperative pulmonary atelectasis: an underestimated problem. Anesth Analg. (2002) 95:1788–92. 10.1213/01.ANE.0000065081.21607.4C [DOI] [PubMed] [Google Scholar]
- 41.Smith DC, Crul JF. Early postoperative hypoxia during transport. Br J Anaesth. (1988) 61:625–7. [DOI] [PubMed] [Google Scholar]
- 42.Moller JT, Wittrup M, Johansen SH. Hypoxemia in the post-anesthesia care unit: an observer study. Anesthesiology (1990) 73:890–5. 10.1097/00000542-199011000-00016 [DOI] [PubMed] [Google Scholar]
- 43.Hofmeister EH, Mackey EB, Trim CM. Effect of butorphanol administration on cardiovascular parameters in isoflurane-anesthetized horses-a retrospective clinical evaluation. Vet Anaest Analg. (2008) 35:38–44. 10.1111/j.1467-2995.2007.00355.x [DOI] [PubMed] [Google Scholar]
- 44.Benmasour P, Husulak ML, Bracamonte JL, Beazley SG, Withnall E, Duke-Novakovski T. Cardiopulmonary effects of an infusion of remifentanil or morphine in horses anesthetized with isoflurane and dexmedetomidine. Vet Anaest Analg. (2014) 41:46–56. 10.1111/vaa.12149 [DOI] [PubMed] [Google Scholar]
- 45.Marntell S, Nyman G, Funkquist P, Hedenstierna G. Effects of acepromazine on pulmonary gas exchange and circulation during sedation and dissociative anaesthesia in horses. Vet Anaest Analg. (2005) 32:83–93. 10.1111/j.1467-2995.2004.00178.x [DOI] [PubMed] [Google Scholar]
- 46.Patel PM, Drummond JC, Lemkuil BP. Cerebral physiology and the effects of anesthetic drugs. In: Cohen NH, Eriksson LI, Fleisher LA, et al. editors. Miller's Anesthesia. Elsevier Saunders; (2015). p. 387–422. [Google Scholar]
- 47.Hopkins RO, Bigler ED. Pulmonary disorders. In: Tarter RE, Butters M, Beers SR. editors. Medical Neuropsychology. Alphen aan den Rijn: Kluwer Academic; (2001). p. 25–50. [Google Scholar]
- 48.Auckburally A, Nyman G. Review of hypoxaemia in anaesthetized horses: predisposing factors, consequences and management. Vet Anaesth Analg. (2017) 44:397–408. 10.1016/j.vaa.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 49.Romer LM, Dempsey JA, Lovering A, Eldridge M. Exercise-induced arterial hypoxemia: consequences for locomotor muscle fatigue. Adv Exp Med Biol. (2006) 588:47–55. 10.1007/978-0-387-34817-9_5 [DOI] [PubMed] [Google Scholar]
- 50.Kushiro T, Yamashita K, Umar MA, Maehara S, Wakaiki S, Abe R, et al. Anesthetic and cardiovascular effects of balanced anesthesia using constant rate infusion of midazolam-ketamine-medetomidine-with inhalation of oxygen-sevoflurane (MKM-OS) in horses. J Vet Med Sci. (2005) 67:379–84. 10.1292/jvms.67.379 [DOI] [PubMed] [Google Scholar]
- 51.Flaherty D, Nolan A, Reid J, Monteiro AM. The pharmacokinetics of ketamine after a continuous infusion under halothane anaesthesia in horses. J Vet Anaesth. (1998) 25:31–6. 10.1111/j.1467-2995.1998.tb00166.x [DOI] [Google Scholar]
- 52.Spadavecchia C, Stucki F, Moens Y, Schatzmann U. Anaesthesia in horses using halothane and intravenous ketamine-guaiphenesin: a clinical study. Vet Anaesth Analg. (2002) 29:20–8. 10.1046/j.1467-2987.2001.00060.x [DOI] [PubMed] [Google Scholar]
- 53.Kruger K, Stegmann GF. Partial intravenous anaesthesia in 5 horses using ketamine, lidocaine, medetomidine and halothane. J S Afr Vet Assoc. (2009) 80:233–6. 10.4102/jsava.v80i4.214 [DOI] [PubMed] [Google Scholar]
- 54.Kaul SU, Beard DJ, Millar MD. Preganglionic sympathetic activity and baroreceptor responses during hypothermia. Br J Anaesth. (1973) 45:433–9. 10.1093/bja/45.5.433 [DOI] [PubMed] [Google Scholar]
- 55.Maaravi Y, Weiss T. The effect of prolonged hypothermia on cardiac function in a young patient with accidental hypothermia. Chest (1990) 98:1019–20. 10.1378/chest.98.4.1019 [DOI] [PubMed] [Google Scholar]
- 56.Niwa K, Takizawa S, Takagi S, Shinohara Y. Mild hypothermia disturbs cerebrovascular autoregulation in awake rats. Brain Res. (1998) 789:68–73. 10.1016/S0006-8993(98)00013-4 [DOI] [PubMed] [Google Scholar]