Abstract
Background
Uterine artery embolization (UAE) is a minimally invasive technique well established for treating symptomatic uterine fibroids. However, the post-procedure recovery for UAE involves a notable inflammatory process in response to ischemia known as post-embolization syndrome (PES). PES encompasses transient leukocytosis, low-grade fever, and can result in readmission of up to 10% of patients. In surgical settings, multiple studies have demonstrated the efficacy of glucocorticoids in reducing inflammation and associated pain. However, this approach has not yet been assessed in predominantly ischemia-driven PES.
Methods
This paper describes the protocol of a prospective randomized, double-blind, placebo-controlled, multi-center trial to test the efficacy and safety of single-dose dexamethasone on inflammatory responses, pain, nausea, and readmission rates after UAE. The study will enroll pre-menopausal patients between 25 and 55 years (planned enrollment, n = 60) with MRI confirmed symptomatic fibroids. Patients will be randomly allocated into two groups: single-dose intravenous dexamethasone plus standard of care or placebo (normal saline) plus standard of care.
Results
The primary endpoint is the patient pain score 4 h following the UAE procedure. Secondary endpoints include pain scores at 7 h and 24 h following UAE; narcotic usage in the first 24 h following UAE; and serum inflammatory markers (white blood cell count, C-reactive protein [CRP], interleukin-6 [IL-6], and cortisol) 24 h after UAE.
Conclusion
Given the high incidence of post-procedure pain and difficulty with pain control after uterine artery embolization, results of this trial may directly influence the standard of care in perioperative management of patients undergoing UAE.
Keywords: Uterine artery embolization, Post-embolization syndrome, Perioperative, Dexamethasone, Interventional radiology
1. Introduction
1.1. Background
Uterine fibroids are the most common benign neoplasm of the female pelvis and result in symptoms of heavy menstrual bleeding, pelvis pressure/bulk, pain, and infertility [1]. Uterine artery embolization (UAE) is a well-established, minimally invasive treatment option for symptomatic uterine fibroids, with comparable long-term symptom relief, health-related quality of life, and patient satisfaction reported when compared to hysterectomy or myomectomy [[2], [3], [4], [5]]. However, the post-procedure recovery for UAE involves a notable inflammatory process, with transient leukocytosis, fatigue, low-grade fever, lack of appetite, nausea, and vomiting. 86% of women treated with UAE demonstrate an increase in white blood cell count (WBC) in the first 24 h post-procedure [6]. Pain associated with the procedure is thought to be related to these inflammatory processes. Collectively, these symptoms are known as post-embolization syndrome (PES) and can result in readmission of up to 10% of patients, adding extraneous burden on the healthcare system [6,7].
The perioperative use of epidural analgesia [8], superior hypogastric nerve blocks [9,10], or intraarterial lidocaine [11,12] to control PES has been described. Current standard of care controls pain with nonsteroidal anti-inflammatory drugs (NSAIDs) as an adjunct to opioids. However, NSAIDs only inhibit the vascular phase of inflammation, reducing vasodilation and vessel wall permeability [13]. Glucocorticoids are considered to be more potent anti-inflammatory agents by inhibiting both the vascular phase and the cellular phase (leukocyte extravasation) of inflammation. By further reducing the inflammatory response secondary to ischemic changes, pain may also be better alleviated.
Multiple studies have demonstrated the efficacy of perioperative glucocorticoids, particularly dexamethasone, in reducing inflammation and pain in surgical settings [14]. By inhibition of the NF—kB pathway and upregulation of anti-inflammatory mediators such as IL-1 and NEP, glucocorticoids cause a substantial anti-inflammatory effect and are used in various autoimmune and inflammatory conditions.
1.2. Rationale and objectives
The use of dexamethasone in reducing post-operative inflammation and symptoms of PES after UAE has not been described extensively, with insufficient randomized controlled trial data. In addition, previous studies have been limited by lack of evaluation for resolution of fibroid symptoms or long-term follow-up to assess for readmissions or adverse events [14,15]. Given the high incidence of post-procedure pain and difficulty with pain control after UAE, our objective is to investigate the effects of single-dose intravenous (IV) dexamethasone on inflammatory responses, pain, nausea, and readmission rates after UAE. If proven that a single pre-procedure dose of dexamethasone can reduce pain and the inflammatory response, all patients undergoing UAE may benefit from this change in medical management.
2. Materials and methods
2.1. Overview
The UAE-dex study is a prospective randomized, double-blind, placebo-controlled, multi-center trial in patients undergoing UAE for the treatment of symptomatic uterine fibroids. A total of 6 sites will participate in this study, with no site enrolling more than 15 patients. The sites include: University of California, San Francisco, Stanford University, University of California, Irvine, University of Colorado, Denver, Georgetown University, and Miami Cardiac and Vascular Institute.
2.2. Eligibility criteria and randomization
The study will include pre-menopausal women between the ages of 25–55 years with symptomatic uterine fibroids confirmed by recent MRI. Each patient must also be able to provide informed consent and participate in all study activities. Exclusion criteria include history of pelvic malignancy, viable pregnancy, active pelvic infection, severe contrast allergy, or renal insufficiency. Due to exposure to fluoroscopy, patients with viable pregnancies will not be included in this study. Similarly, patients who report a serious allergic reaction to contrast agents as well as patients with renal insufficiency (serum creatinine > 1.5 mg/dL) will be excluded. Patients with active pelvic infection are not included due to relative contraindication of dexamethasone use.
Randomization will take place prior to the embolization procedure and will determine if the subject receives a single dose of dexamethasone or placebo (normal saline) prior to the UAE procedure. Block randomization will be used to ensure a balance in sample size across groups during the course of study enrollment [16]. The study coordinator at each site will record the randomization number on all documents. Because of the double blinded nature of the study, the study coordinator will communicate whether the patient has been randomized to receive dexamethasone or placebo to another interventional radiology physician who is not part of the study. This physician will then ensure that the subject receives either dexamethasone or placebo without the knowledge of the treating interventional radiologists. Subjects will be blinded to whether they received dexamethasone or placebo.
2.3. Outcomes
Patients will be assigned randomly to the treatment group (single pre-procedure dose of dexamethasone) or the control group (normal saline). The primary objective of this study is to evaluate the effect of a single-dose of IV dexamethasone and pain and inflammatory response following UAE. The primary endpoint is the patient pain score 4 h following the UAE procedure.
Secondary endpoints will include pain scores at 7 h and 24 h following UAE; narcotic usage in the first 24 h following UAE; and serum inflammatory markers (white blood cell count, C-reactive protein [CRP], interleukin-6 [IL-6], and cortisol) 24 h after UAE. Additional endpoints include change of symptom severity and quality of life evaluated with the Uterine Fibroid Symptom and Health-Related Quality of Life Questionnaire (UFS-QOL) and an imaging endpoint of the change in the volume of the uterus and dominant fibroid and extent of fibroid necrosis determined on MRI pelvis performed 3 months after the UAE.
The estimated timeline for this study is 12 months. The study will be halted when sufficient data for the primary endpoint are gathered and an interim analysis can be performed.
2.4. Power analysis
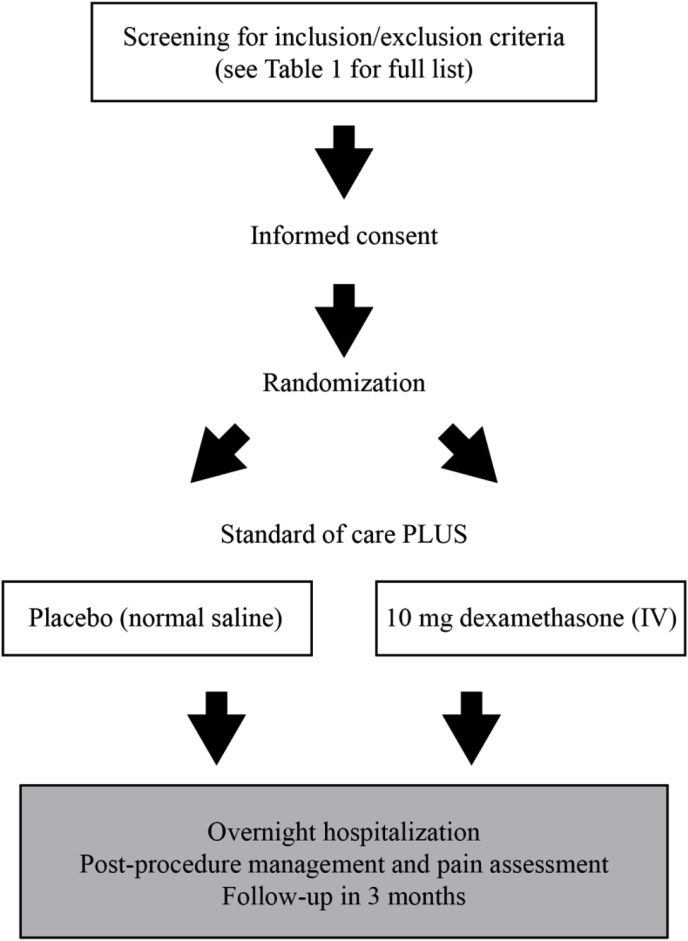
The primary outcome of this study is the pain score 4 h following the UAE procedure. With 60 patients randomized 1:1 to the two treatment arms, the two-sided two-sample t-test has sufficient (93%[80%]) power to detect a difference observed in prior study of 35 ± 22.6 vs. 59.4 ± 30.3 at the 0.05 [0.0085, Bonferroni adjustment for multiple testing] significance level. This study is sufficiently powered to detect an effect size of 0.5*SD at the two-sided 0.05 significance level and 1*SD at the two-sided 0.05/10 significance level. Effect of this magnitude was observed in a prior study in WBC count. Analyses anticipate 15% drop-out rate (see Fig. 1) (see Table 1).
Fig. 1.
Study flow.
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| 30–50 years of age at enrollment | History of pelvic malignancy |
| Pre-menopausal | Viable pregnancy |
| Able to provide informed consent | Active pelvic infection |
| Uterine fibroids documented by MRI | Sever contrast allergy |
| Symptomatic uterine fibroids causing one or more symptoms such as: heavy menstrual bleeding, bulk symptoms with bladder or bowel dysfunction or abdominal protrusion, dysmenorrhea, dyspareunia, infertility | Renal insufficiency |
2.5. Pre-treatment assessment
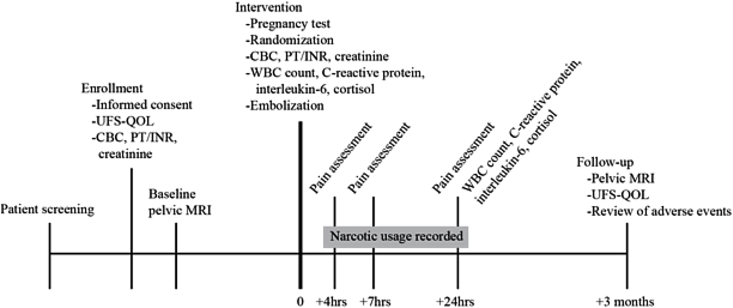
Each subject will be seen in a clinic setting by an interventional radiologist prior to enrollment. During the visit, the physician will determine if the patient meets the inclusion criteria to be included in this clinical trial. Informed consent, medical and surgical history, medication history, physical examination, and laboratory values, including complete blood count (CBC), prothrombin time and international normalized ratio (PT/INR), and serum creatinine, will be completed at this time. The UFS-QOL will also be administered at this time to assess for presenting symptoms and effect on quality of life. A baseline contrast-enhanced pelvic MRI will be performed. A summary of all study activities can be found in Table 2 and a timeline in Fig. 2.
Table 2.
Study activities.
| Visit 1 |
Visit 2 |
Visit 3 |
Visit 4 |
Visit 5 |
|---|---|---|---|---|
| Enrollment | Imaging | Intervention | Post-UAE Hospital Course | Follow-up |
| Informed consent | Baseline pelvic contrast-enhanced MRI | Pregnancy Test | Pain assessment at 4, 7, and 24 h | Contrast-enhanced pelvic MRI |
| Medical and surgical history | Randomization | Narcotic usage | UFS-QOL | |
| Medication history | Laboratory values | Inflammatory markers | Review of adverse events | |
| Physical examination | Inflammatory markers | |||
| UFS-QOL | Embolization | |||
| Laboratory values |
*Laboratory values include: CBC, PT/INR, and serum creatinine.
*Inflammatory markers include: WBC count, C-reactive protein, interleukin-6, cortisol.
Fig. 2.
Study timeline.
2.6. Description of the intervention
The day of the UAE procedure will be considered Day 0 of the study. Laboratory tests performed on Day 0 include a pregnancy test, CBC, and inflammatory markers including CRP, IL-6, and cortisol.
One hour prior to the UAE procedure, the patient will receive either dexamethasone (10 mg, IV) or placebo (normal saline). The UAE procedure will be performed under moderate sedation with fentanyl and midazolam. UAE will be performed using standard technique for each subject. Standard medications given intra- and post-operatively are summarized in Table 3. Depending on the preference of the interventional radiologist, a left transradial, unilateral common femoral artery or bilateral common femoral artery access will be obtained. The uterine artery will be catheterized with a 4, 5 French or a microcatheter depending on the preference of the interventional radiologist. Attention will be given to begin embolization distal to the origin of the cervicovaginal branch of the uterine artery, if visualized.
Table 3.
Standard medications given over the course of the study.
| Pre-procedure | Post-procedure | ||
|---|---|---|---|
| Dexamethasone 10 mg | IV 1 h prior to procedure (treatment group only) | Hydromorphone PCA | Loading dose 0.5 mg, 0 basal, 0.5 mg incremental, lock out interval 10 min, max 6 boluses/hr |
| Percocet (acetaminophen/oxycodone) 5–325 mg | 1-2 tablets PO q4h PRN pain | ||
|
Intra-procedure |
Toradol 30 mg | IV q6h, start 6hr after last intraoperative dose | |
| Prophylactic antibiotics | Single dose, at discretion of physician | Ibuprofen 600 mg | PO q6h, convert next morning |
| Fentanyl and versed | For moderate sedation, titrated to patient comfort | Phenergan (promethazine) 12.25–25 mg | IV q6h PRN nausea |
| Ketorolac 30 mg | IV, per side prior to embolization | Zofran (ondansetron) 4 mg | IV q8h |
| Lidocaine 50 mg | IA, delivered over 3–5 min, per side after embolization | Senokot | 2 tablets PO bid |
The embolic agent used for embolization will be trisacryl gelatin microspheres (Embosphere Microspheres, Merit Medical Systems, INC, South Jordan, UT) and the particle sizes will vary from 500-700 μm to 700–900 μm. The recommended endpoint for embolization will be near-stasis as defined by the visualization of contrast within the transverse segment of the target uterine artery for duration of time equivalent to 5 heartbeats. Total number of beads required to achieve near-stasis will be recorded for each procedure. At the completion of the procedures, all catheters will be removed and hemostasis will be achieved with manual compression or an arterial closure device used at the discretion of the interventional radiologist.
2.7. Post-procedure management and pain assessment
Following the intervention, patients will be admitted to the hospital for overnight observation. Pain will be assessed using a validated visual analog scale (VAS). Subjects’ pain will be assessed at 4 h, 7 h, and 24 h after the completion of the UAE. The total dose of in-hospital narcotic agents used following completion of the UAE until discharge will be recorded and converted to an equivalent of milligrams of morphine (1 mg oral oxycodone = 0.5 mg morphine; 1 mg IV hydromorphone = 5 mg morphine) [11]. All other medications administered during the hospital stay will be recorded. 24 h after the UAE procedure, laboratory tests including CBC, CRP, IL-6, and cortisol will be performed.
A follow-up visit at 3 months will be scheduled. At that time, the UFS-QOL questionnaire and review of adverse events will be performed. A contrast-enhanced pelvic will also be completed at this time to evaluate for the change in the volume of the uterus and dominant fibroid and the extent of fibroid necrosis.
2.8. Statistical analysis
An intention-to-treat analysis will be used, in addition to an analysis that compares patients who actually receive dexamethasone versus those who receive placebo. Sociodemographic and clinical measures taken at the baseline will be compared in their central tendency and variability across the two treatment arms. Continuous measures such as age will be examined using t-test and categorical using Chi-square or Fisher's exact. The pain score 4 h following the UAE procedure will be compared using two-sided two-sample t-test and analyses of variance (ANOVA) with adjustment for potential confounders measures at the baseline. The tendency in the pain score measured at 4, 7 and 27 h will be compared using the analyses of variance (ANOVA) and mixed-effects models or their generalized version. The mixed effects models might be more robust in presence of missing data. Similarly, trajectory in the degree of fibroid necrosis and changes in uterine and dominant fibroid volume will be examined in mixed effects models. All analyses will include assessing difference across centers, e.g. using treatment arm by center interaction term.
All assumptions will be checked and non-parametric alternatives and transformations considered.
3. Results
A flowchart will be included to detail the enrollment process, including assessment for eligibility, randomization, allocation, follow-up, and analysis. Patients who did not receive allocated treatment or were lost to follow-up will be noted. Baseline patient characteristics will also be compared. No significant difference is expected between the two groups at baseline.
The primary endpoint, patient pain score 4 h post-UAE, along with pain scores at 7 h and 24 h will be described using mean and SD and assessed for significance. We hypothesize that pain scores will be lower for patients in the dexamethasone group as compared to the placebo group.
Change in symptom severity and quality of life, assessed by the UFS-QOL questionnaire, will be analyzed as an ordinal variable. Post-UAE uterine volume, volume reduction of uterus, and extent of fibroid necrosis will be analyzed as mean and SD. We hypothesize that no significant difference should be seen between the two groups in symptom severity, quality of life, uterine volume, and fibroid necrosis 3 months post-UAE.
Total narcotic dosage in the first 24 h will also be recorded and analyzed as mean and SD, as well as change in serum inflammatory markers from pre-to post-UAE. We hypothesize that at 24 h post-UAE, a significantly lower CRP, IL-6, and cortisol level will be seen. WBC may show no significant difference due to the well-known side effect of leukocytosis during steroid therapy. Subgroup analysis will be performed, including by size of fibroid, original presenting symptoms, number of beads required to achieve near-stasis, and required dosage of non-narcotic analgesics.
4. Discussion
UAE serves an important role as a minimally invasive treatment option for symptomatic fibroids but does involve post-procedural pain in almost all patients. This study aims to evaluate the effects of a single-dose IV dexamethasone in controlling post-procedure pain, nausea, and vomiting. The efficacy of dexamethasone in reducing pain, nausea, vomiting, and inflammation has been demonstrated in surgical settings [14] but is less well-studied in UAE.
A strength of this study's design is the assessment following the UAE, including not only pain scores at multiple time points during hospital admission but also quality of life questionnaires and MRI imaging 3 months post-UAE. The economic impact of interventions for the purpose of treatment uterine fibroids and subsequent post-operative care and hospital admissions is substantial, and this study may help to minimize some of those costs An additional strength of our study is its multi-center design, allowing for increased geographical diversity and a resulting study population that closely reflects the target population. Enrollment of women of varying ages, size of individual fibroids, volume of fibroids, and ethnicity will allow for subgroup analysis. Finally, this protocol utilizes a double-blind approach, an approach not always possible when conducting clinical trials involving procedural interventions.
Limitations of our study include obtaining sufficient enrollment and standardizing procedures across participating centers. The study population will be limited to tertiary medical care centers. Notable confounding factors include actual volume of fibroid that undergoes embolization and use of non-narcotic analgesics.
5. Conclusion
This prospective randomized, double-blind, placebo-controlled, multi-center trial, will formally test the hypothesis that use of single-dose dexamethasone in the perioperative setting will decrease the inflammatory responses, pain, and nausea collectively known as post-embolization syndrome following UAE. In addition to addressing the use of dexamethasone in patients undergoing UAE, this protocol may also be used to assess the efficacy of glucocorticoids in reducing other inflammatory processes, particularly other embolization procedures common in interventional radiology.
Acknowledgements
We thank Drs. Theresa Caridi, Adam Geronemus, Gloria Hwang, Kari J. Nelson, and Paul Rochon for their contributions.
References
- 1.Baird D.D., Dunson D.B., Hill M.C., Cousins D., Schectman J.M. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am. J. Obstet. Gynecol. 2003;188(1):100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 2.Spies J.B. Current evidence on uterine embolization for fibroids. Semin. Intervent. Radiol. 2013;30(4):340–346. doi: 10.1055/s-0033-1359727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masciocchi C., Arrigoni F., Ferrari F. Uterine fibroid therapy using interventional radiology mini-invasive treatments: current perspective. Med. Oncol. 2017;34(4):52. doi: 10.1007/s12032-017-0906-5. [DOI] [PubMed] [Google Scholar]
- 4.de Bruijn A.M., Ankum W.M., Reekers J.A. Uterine artery embolization vs hysterectomy in the treatment of symptomatic uterine fibroids: 10-year outcomes from the randomized EMMY trial. Am. J. Obstet. Gynecol. 2016;215(6):745. doi: 10.1016/j.ajog.2016.06.051. e741-745.e712. [DOI] [PubMed] [Google Scholar]
- 5.Moss J.G., Cooper K.G., Khaund A. Randomised comparison of uterine artery embolisation (UAE) with surgical treatment in patients with symptomatic uterine fibroids (REST trial): 5-year results. BJOG. 2011;118(8):936–944. doi: 10.1111/j.1471-0528.2011.02952.x. [DOI] [PubMed] [Google Scholar]
- 6.Ganguli S., Faintuch S., Salazar G.M., Rabkin D.J. Postembolization syndrome: changes in white blood cell counts immediately after uterine artery embolization. J. Vasc. Intervent. Radiol. 2008;19(3):443–445. doi: 10.1016/j.jvir.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 7.Parker W.H. Uterine myomas: management. Fertil. Steril. 2007;88(2):255–271. doi: 10.1016/j.fertnstert.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 8.van der Kooij S.M., Moolenaar L.M., Ankum W.M., Reekers J.A., Mol B.W.J., Hehenkamp W.J.K. Epidural analgesia versus patient-controlled analgesia for pain relief in uterine artery embolization for uterine fibroids: a decision analysis. Cardiovasc. Interv. Radiol. 2013;36(6):1514–1520. doi: 10.1007/s00270-013-0607-1. [DOI] [PubMed] [Google Scholar]
- 9.Rasuli P., Jolly E.E., Hammond I. Superior hypogastric nerve block for pain control in outpatient uterine artery embolization. J. Vasc. Intervent. Radiol. 2004;15(12):1423–1429. doi: 10.1097/01.RVI.0000137406.09852.A4. [DOI] [PubMed] [Google Scholar]
- 10.Binkert C.A., Hirzel F.C., Gutzeit A., Zollikofer C.L., Hess T. Superior hypogastric nerve block to reduce pain after uterine artery embolization: advanced technique and comparison to epidural anesthesia. Cardiovasc. Interv. Radiol. 2015;38(5):1157–1161. doi: 10.1007/s00270-015-1118-z. [DOI] [PubMed] [Google Scholar]
- 11.Noel-Lamy M., Tan K.T., Simons M.E., Sniderman K.W., Mironov O., Rajan D.K. Intraarterial lidocaine for pain control in uterine artery embolization: a prospective, randomized study. J. Vasc. Intervent. Radiol. 2017;28(1):16–22. doi: 10.1016/j.jvir.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Keyoung J.A., Levy E.B., Roth A.R., Gomez-Jorge J., Chang T.C., Spies J.B. Intraarterial lidocaine for pain control after uterine artery embolization for leiomyomata. J. Vasc. Intervent. Radiol. 2001;12(9):1065–1069. doi: 10.1016/s1051-0443(07)61592-9. [DOI] [PubMed] [Google Scholar]
- 13.Wick E.C., Grant M.C., Wu C.L. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 2017;152(7):691–697. doi: 10.1001/jamasurg.2017.0898. [DOI] [PubMed] [Google Scholar]
- 14.Waldron N.H., Jones C.A., Gan T.J., Allen T.K., Habib A.S. Impact of perioperative dexamethasone on postoperative analgesia and side-effects: systematic review and meta-analysis. Br. J. Anaesth. 2013;110(2):191–200. doi: 10.1093/bja/aes431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim S.Y., Koo B.N., Shin C.S., Ban M., Han K., Kim M.D. The effects of single-dose dexamethasone on inflammatory response and pain after uterine artery embolisation for symptomatic fibroids or adenomyosis: a randomised controlled study. BJOG. 2016;123(4):580–587. doi: 10.1111/1471-0528.13785. [DOI] [PubMed] [Google Scholar]
- 16.Suresh K. An overview of randomization techniques: an unbiased assessment of outcome in clinical research. J. Hum. Reprod. Sci. 2011;4(1):8–11. doi: 10.4103/0974-1208.82352. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]