Abstract
Objective
The rhizome of Tectaria cicutaria has been used in Indian traditional medicine for the treatment of various disorders. The objective of present investigation is to screen various extracts of the rhizomes of Tectaria cicutaria for anti-cancer activity and to investigate the mechanism involved.
Materials and methods
The rhizomes of Tectaria cicutaria were extracted with different solvents. In vitro anti-cancer activity of different rhizome extracts were studied in Human cancer Cell Lines using Sulphorodamine B (SRB) colorimetric cytotoxicity assay. The effect of ethanolic extract (TCe) on cell growth inhibition, modulation in gene expression, and induction of apoptosis using the K562 human leukemia cell line were studied. The extract was analyzed by GC-MS to identify their major chemical compounds.
Results
TCe shows antioxidant potential in both DPPH scavenging assay and reducing capacity. Flow cytometric analysis showed that 11 μg/ml of TCe arrested cell cycle progression at the G0/G1 phase. In the TCe treated K562 cells, the mRNA and protein expression level of p53 was strongly up-regulated in reverse transcription polymerase chain reaction. Furthermore, its downstream target p21 level was also increased. The GC-MS study has depicted results with the presence of twelve different compounds which will require significant further efforts for structure and putative identification.
Conclusion
The present work has for the first time, tried to elucidate the anti leukemic potential of Tectaria cicutaria. TCe was more potent in K562 cells, altering the cell cycle progression and inducing apoptosis.
Keywords: Tectaria cicutaria, Apoptosis, Cell cycle analysis, p53, GC-MS
Graphical abstract
List of abbreviations
- RT-PCR
Reverse transcription polymerase chain reaction
- TCe
Ethanolic extract of Tectaria cicutaria rhizome
- TGI
Total growth inhibition
- GI50
Growth inhibition of 50%
- PBS
Phosphate-buffered saline
- PS
Phosphatidylserine
- FACS
Fluorescence activated cell sorting
- TE buffer
Tris/EDTA buffer
- Annexin FITC
fluorescein isothiocyanate
1. Introduction
For prevention and treatment of several diseases plant and plant extracts have been widely used all over the world. According to recent studies conducted by the World Health Organization (WHO), about 80% of the world's population relies on traditional medicine.1 Traditional medicine involves the use of plant extracts as well as their active principles. Indeed, several anti-cancer agents derived from plants have been discovered in the last decades.2, 3 Besides, many anticancer agents obtained from plant source have achieved pre-clinical or clinical development.4, 5
Tectaria cicutaria (L.) Copel is a species of fern in the Tectariaceae family. Its rhizomes are short (Fig. 1), erect and dark brown or black in color with distinctive hard thick leaf bases. This fern is found all over in India as well as in the tropics throughout the world. It is known as Kombadnakhi in the local language. In Ayurveda rhizomes of Tectaria cicutaria has been used for the treatment of wide variety of disorders and conditions such as chest complaints, sprain, rheumatic pain, burns, poisonous bites,6 toothache, gum complaints, and diarrhea.7 In folklore medicine, it is also used in clinical conditions like tonsillitis, mental disorders, and obesity. Decoction as well as an infusion is used for the treatment of different types of gynecological disorders and inflammatory conditions in Ayurvedic medicine. So far there is no studies available pertaining to anticancer activity of Tectaria cicutaria.
Fig. 1.

Rhizome of Tectaria cicutaria.
In view of this fact, the present study was aimed to investigate the phytochemicals present in the Tectaria cicutaria, in vitro anti cancer activity of rhizome extracts of Tectaria cicutaria on different human cancer cell lines and further to understand the underlying molecular mechanism.
2. Materials and methods
2.1. Collection of plant material and preparation of extracts
Tectaria cicutaria was collected from Koyana Wildlife Sanctuary, Western Ghats of Sahyadri hills, Satara, India. The species of fern were identified with the help of experts using the standard floras.8, 9, 10 Identification of the fern was confirmed by Dr. S. S. Sathe, Head/Dept of Botany, Padmabhushan Dr. Vasantraodada Patil Mahavidyalaya, Tasgoan, Maharashtra, India. Washed rhizomes were dried under shade, ground into powder and used for successive extraction. Coarse powder was subjected to hot successive continuous extraction in Soxhlet apparatus. 50 gm of powder was extracted with petroleum ether (400 mL). It was sequentially extracted with solvents of increasing polarity (ethyl acetate, ethanol and water). Each time before extracting with the next solvent, the material was dried at room temperature. The extracts were filtered and evaporated to dryness. Yield of the extract was recorded and extracts were stored at 4°, until analysis. These extracts were tested for the presence of active phytochemicals viz: tannins, alkaloids, phytosterols, triterpenoids, flavonoids, glycosides, saponins, carbohydrates, proteins, amino acids and fixed oils & fats using standard tests.11
2.2. DPPH radicals scavenging and reducing power assay
DPPH radical is a widely used method to evaluate the free radical scavenging ability of natural compounds. This assay is based on the measurement of the scavenging ability of antioxidant sub-stances toward the stable radical. 1.0 mL of various concentrations of extracts (10–50μg/mL) was mixed with 1.0 mL of 0.8 mmol/L DPPH solution. Each mixture was kept in the dark for 30 min and the absorbance was measured at 517 nm against a blank.12 Ascorbic acid was used as standard. The inhibition percentage for scavenging DPPH radical was calculated according to the equation:
| (1) |
The reducing power ability of the extracts was evaluated by the method described by Oyaizu. The reaction mixture contained 1.0 mL of TCe (20–100 μg/mL), 2.5 mL of 1% potassium ferricyanide and 2.5 mL of 0.2 mol/L sodium phosphate buffer. The mixture was incubated at 50 °C for 30 min and the reaction was terminated by the addition of 2.5 mL of 10% trichloroacetic acid, followed by centrifugation at 3000 rpm for 10 min. 2.5 mL of the upper layer was mixed with 2.5 mL of deionized water and 0.5 mL of 0.1% ferric chloride and absorbance was measured at 700 nm against blank that contained distilled water and phosphate buffer. Increase in absorbance indicates increased reducing power of the sample. BHT was used as standard.13
2.3. Determination of cell proliferation in vitro (sulphorodamine B assay)
K562 (Human Leukemia Cell Line), KB (Human Nasopharyngeal Cancer Cell Line), HT29 (Human Colon Cancer Cell Line) and Colo205 (Human Colon Cancer Cell Line) were used for screening the anticancer activity of the different extracts of Tectaria cicutaria using the Sulphorodamine B (SRB) colorimetric cytotoxicity assay. The extracts were dissolved in DMSO and tested at concentrations of 10, 20, 40, 80 μg/ml. Adriamycin (Doxorubicin) was used as a positive control at the same concentration range. Cytotoxicity was expressed as the percent viable cells relative to cells incubated in the presence of 0.1% DMSO vehicle control. Growth inhibition of 50% (GI50) was calculated from drug concentration resulting in a 50% reduction in the net protein increase. Each measurement was performed in triplicate.14
2.4. Apoptosis detection
The cells (1 × 106) were harvested and washed twice with ice-cold PBS. Subsequently, the cells were labeled with Annexin V and Dead Cell assay kit according to the manufacturer's instructions. This assay is based on the phosphatidylserine (PS) detection on the apoptotic cells surface, using fluorescently labeled Annexin V. The samples were determined by the Muse Cell Analyzer (Millipore, USA) and analyzed by software provided by Merck Millipore.
2.5. Cell cycle analysis
Analysis of DNA content and cell cycle distribution was done using muse cell cycle kit by flow cytometry. For fluorescence activated cell sorting (FACS) analysis, the treated K562 cells were incubated for 24 h. After overnight incubation cells were detached and pipetted out and spun at 300 × g for 5 min and washed once with 1 × Phosphate-buffered saline (PBS). The cells were fixed with 1 ml of ice cold ethanol and incubated at -20 °C for overnight. The ethanol fixed cells were washed once with PBS followed by 200 μL of Muse cell cycle reagent. The tubes were incubated for 30 min at dark and analyzed on Muse flow cytometer (Millipore, USA)
2.6. Gene expression
2.6.1. Isolation of total RNA (trizol method)
Total RNA was isolated using the total RNA isolation kit according to the manufactures instruction (Invitrogen). Addition of Trizol solution causes the disruption of cells and the release of RNA. Chloroform extraction following centrifugation, exclusively in the aqueous phase whereas proteins are in the inter phase and organic phase. 80% confluent cells were treated with sample and incubated overnight. On mixing with isopropanol RNA gets precipitated as a white pellet on the side and the bottom of the tube. 1 ml of trizol reagent was added to the cultured plate and homogenized until it formed a fine paste and transferred it into an eppendorf tube. 200 μl of chloroform was added and shaking was done vigorously for 15 s and incubated for 2–3 min at room temperature. The sample was then centrifuged at 14000 rpm for 15 min at 4 °C. The aqueous layer was collected and 100% 500 μl of isopropanol was added. It was incubated for 10 min at room temperature. Supernatant was discarded and pellet was collected, washed with 1 ml of 75% of ethanol (Merck, Germany). It was then centrifuged at 10000 rpm for 5 min at 4 °C. The RNA pellet was dried and dissolved in Tris/EDTA (TE) buffer.
2.6.2. Reverse transcriptase PCR (RT-PCR)
cDNA synthesis was performed as per thermo scientific verso cDNA synthesis kit manual and amplification was performed using Invitrogen Thermo Script RT-PCR System manual. After the amplification, the PCR product was separated by agarose gel electrophoresis. Sequences of primers used in real-time PCR analysis are as below.
ERK2 FORWARD-GAGCACCAGACCTACTGCCAG
ERK2 REVERSE- AATTTCTGGAGCCCTGTACCA
P53 F FORWARD - CCACCATGAGCGCTGCTCA
P53 F REVERSE - GCAGGGAGGGAGAGATG
P21 FORWARD- GCAGATCCACAGCGATATCC
P21 REVERSE – CAACTGCTCACTGTCCACGG
Agarose gel electrophoresis method was used for separating and visualizing DNA fragments.
2.7. GC-MS analysis
GC-MS analysis of the active ethanol extract of Tectaria cicutaria was carried out by using the GCMS instrument equipped with a capillary column DB-%MS (30 m × 0.25mm × 0.25 mm length). The detector temperature was 300 °C. The carrier gas used was helium at a flow rate of 1.5 ml/min. The oven temperature was initially programmed at 50 °C (isothermal for 2 min) and then increased to 300 °C at 7 °C/min. The identification of compounds from the spectral data was based on the available mass spectral records (NIST05 libraries).
3. Result
3.1. Phytochemical investigation
Phytochemical screening of rhizomes of Tectaria cicutaria confirmed the presence of triterpenoid, flavanoids, saponins, tannins, phenols, and carbohydrate.
3.2. DPPH radicals scavenging and reducing power assay
The DPPH method is fast and reliable method. Standards and extracts showed a dose dependent inhibition of the DPPH radicals. TCe exhibited strongest DPPH radical scavenging activity compared to other extracts. Radical scavenging activity of different extracts were in the order TCe > TCet > TCaq. Concentration of the extract necessary to decrease the initial concentration of DPPH by 50% (IC50) was calculated. A lower IC50 value indicates a higher antioxidant activity. Ascorbic acid was used as standard at the concentration 10–50 μg/mL. IC50 value was found to be 26.50 and 27.02 μg/mL for standard and TCe respectively.
Presence of reductones causes breaking of free radical chain by donating a hydrogen atom and shows reducing power.15 Like antioxidant activity, reducing power of TCe increases with the increase in concentration. TCe showed good reducing power ability in a dose dependent manner. This activity was comparable to that of standards. The antioxidant principles present in the extracts caused the reduction of Fe3+/ferricyanide complex to the ferrous form and thus proved the reducing power ability.
3.3. Cell proliferation in vitro
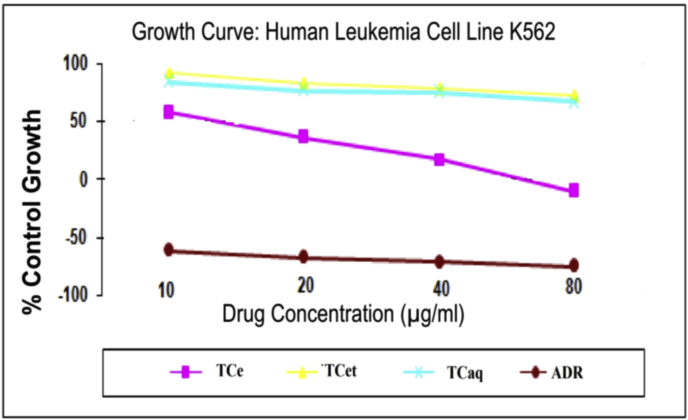
The anticancer activity of different extracts of Tectaria cicutaria was conducted against four different Human Cancer Cell Line using sulforhodamine B (SRB) assay. Adriamycin was used as the positive control. Fig. 2 shows activity of different extracts against K562 Cell Line (Data for other cell lines not shown). The results showed that (Table 1) TCe extract was the most potent extract in the K562 (GI50 value 11.9 μg/mL and LC50 value 53.2 μg/mL). This is probably due to the different chemical composition of the extract hence; further analyses were performed with the TCe.
Fig. 2.
Growth curve: The plot of percentage control growth vs. Molar drug concentration shows the effective drug concentration on the Human Leukemia Cell Line K562.
Table 1.
Effect of extracts of Tectaria cicutaria on Human Leukemia Cell Line K562.
| Drug Concentrations (μg/ml) | % Growth Control Average Values |
Drug concentrations (μg/ml) calculated |
|||||
|---|---|---|---|---|---|---|---|
| 10 | 20 | 40 | 80 | LC50 | TGI | GI50 | |
| TCe | 57.6 | 36.0 | 17.4 | −10.2 | 53.2 | 32.6 | 11.9 |
| TCet | 92.1 | 82.5 | 77.9 | 72.0 | >80 | >80 | >80 |
| TCaq | 84.2 | 76.8 | 74.7 | 67.0 | >80 | >80 | >80 |
| ADR | −61.5 | −67.1 | −71.1 | −74.3 | 21.5 | <10 | <10 |
TCe- Ethanolic Extract of Tectaria cicutaria, TCet –ethyl acetate extract of Tectaria cicutaria, TCaq-aqueous Extract of Tectaria cicutaria, ADR- Adriamycin (Doxorubicin).
Average Values are average of three sets of experiments.
3.4. Mode of cancer cell death (apoptosis)
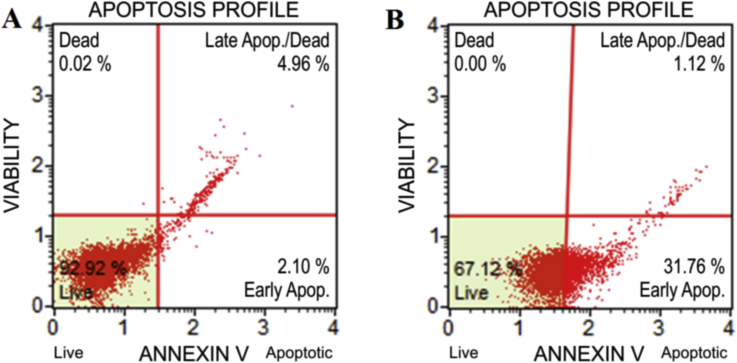
Cytotoxic anticancer drugs have potential to elicit cancer cell death by apoptosis or by necrosis.16 After staining the treated cells with Annexin V-FITC/PI, the mode of cell death was determined from the transition paths of the cells in the dot plot diagrams. The plots are divided into four quadrants, so that viable cells fall into the lower left quadrant (Annexin V-negative, PI-negative), early apoptosis is at lower right (Annexin V-positive, PI-negative), late apoptosis is at upper right (Annexin V-positive, PI-positive), and the nonviable cells are at upper left (Annexin V-negative, PI-positive). After treatment the highest early apoptotic populations was 31.76 % with the populations of viable cells 67.12% [Fig. 3 B] as compared to control [Fig. 3A].
Fig. 3.
Flow cytometry with FITC-Annexin V. analysis of the effect of TCe on cell cycle of Cell Line K562 A- Control and B- Test.
3.5. Cell cycle analysis
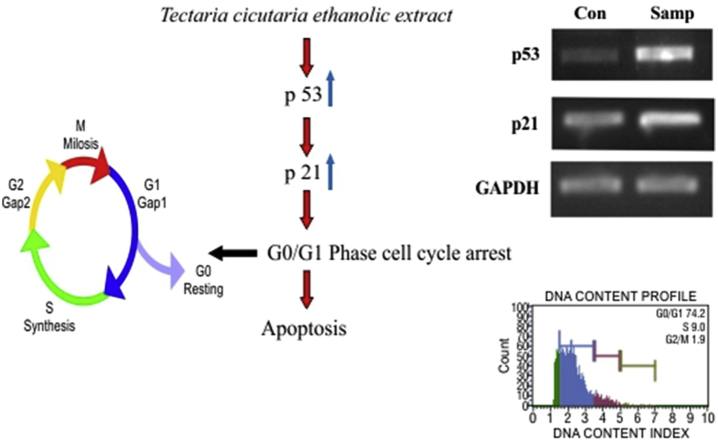
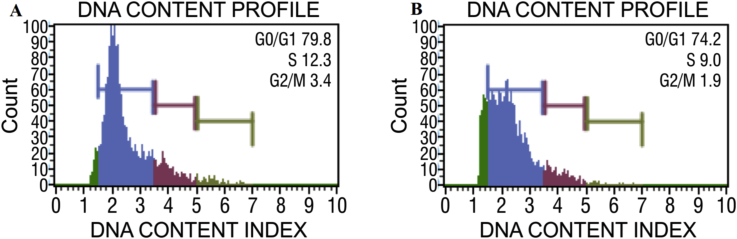
Importance of phytochemicals that function as cell-cycle modulators is increased due to the recent confirmation of concomitant involvement of apoptosis and cell cycle inhibition.17 Flow cytometry analysis was performed to analyze the various cell cycle checkpoints. We performed an experiment to evaluate the effect of TCe on the DNA content of K-562 cells by cell cycle phase distribution (G0, G1, S, G2 and M). This data suggested that cells exposed to 11 μg/ml of TCe significantly increased the accumulation of the DNA contents up to 74.2% in the G0/G1 phase. Both S and G2/M phase cells decreased in the treatment [Fig. 4 B]. It has been observed that different plant extracts with known antitumor activity such as ginseng extract, the extract of Tinospora cordifolia, or the Marsdenia tenacissima extract have similar effects; arresting cells in the G0/G1 phase of the cell cycle.18
Fig. 4.
Population and DNA content profile of A- Control and B- Test.
3.6. Gene expression
Tumor suppressor action of p53 is by preventing the uncontrolled proliferation of cells. The p53 is found to mediates cell cycle arrest and apoptosis in response to DNA damage.19 As mentioned in earlier studies p53 mediate cell arrest at late G1phase, G0/G1 phase as well as G2/M phase. P53 functions as a transcription factor and up-regulates a number of genes involved in these processes.20 Analysis of p53 protein expression and of its downstream transcription targets, p21 revealed a p53 associated growth arrest after extract treatment and apoptosis within 24 h. Our results demonstrate for the first time that TCe induces cell cycle arrest and apoptosis via p53-dependent mechanisms [Fig. 5].
Fig. 5.

Effect of TCe on the regulation of p53 and p21 mRNA expression.
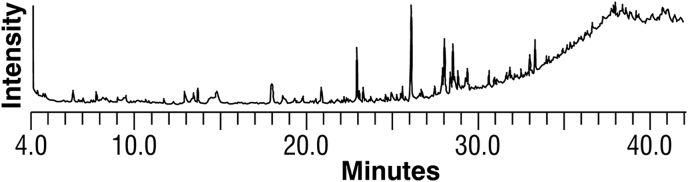
3.7. GC-MS analysis
The mass of the compounds and fragments recorded were matched with NIST database for identification of probable compounds present in the sample. The present communication describes preliminary analysis of the TCe as it showed good antileukemic activity. It is for the first time that the composition of extract has been investigated through GC-MS analysis [Fig. 6]. It revealed 12 probable compounds which are mentioned in Table 2 which indicates the active principles with their retention time, name of compound, concentration (% peak area), molecular formula and molecular weight. The most prevailing compounds are 9-Octadecenoic acid, oleic acid, n-hexadecanoic acid (palmitic acid), octadecanoic acid (stearic acid) and phthalic acid dibutyl ester. Hexadecanoic acid, octadecanoic acid and oleic acids are among the fatty acids known to have potential antibacterial and antifungal activity.21 Hexadecanoic acid is responsible for analgesic, anesthetic, antibacterial, anti inflammatory, antioxidant and antifungal activity activity.22 Oleic acid has been found to be fungistatic against a wide spectrum of moulds and yeasts. The compound 9-Octadecenoic acid is unsaturated fatty acid present in several plants. It lowers the level of cholesterol and the risk of heart problem. It is also responsible for hypotensive, antherosclerosis and aids in cancer prevention.23 Further studies are in process for the isolation and identification of individual compounds from the plant crude extracts.
Fig. 6.
GCMS chromatogram of TCe.
Table 2.
Probable compounds in TCe identified by GCMS.
| Sr. No. | Retention time | Name of the compound | Area % | Molecular formula | Molecular weight |
|---|---|---|---|---|---|
| 1 | 4.092 | 2-propanone | 3.37 | C3H6O2 | 74 |
| 2 | 4.133 | Silanediol | 3.90 | C2H8O2Si | 92 |
| 3 | 4.169 | Dihydroxydimethylsilane | 10.71 | C2H8O2Si | 92 |
| 4 | 20.899 | Hexasiloxane | 2.90 | C14H42O5Si6 | 458 |
| 5 | 22.974 | Phenol, 2-methyl-5-(1,2,2- trimethyl cyclopentyl)-, (S) | 11.40 | C15H22O | 218 |
| 6 | 26.042 | Phthalic acid, dibutyl ester | 2.95 | C16H22O4 | 278 |
| 7 | 26.116 | n-Hexadecanoic acid | 17.13 | C16H32O2 | 256 |
| 8 | 28.054 | Oleic acid, Methyl ester | 9.21 | Cl9H36O2 | 296 |
| 9 | 28.401 | Steric acid. Methyl ester | 3.57 | Cl9H38O2 | 298 |
| 10 | 28.536 | 9-Octadecenoic acid | 10.89 | C16H30O2 | 254 |
| 11 | 28.608 | l3-Octadecenal | 4.13 | C18H34O | 266 |
| 12 | 29.385 | Heptadecane | 2.95 | C17H36 | 240 |
4. Discussion
Herbs, herbal extracts and herbal formulations are known to possess curative potential.24, 25, 26 Tectaria cicutaria was reported to be rich in polyphenols.27 Advanced Centre for Treatment, Research & Education in Cancer (ACTREC Mumbai) recommended that GI50 value ≤ 20 μg/mL is considered to demonstrate activity.
Present study implicit the observation that Tecatria cicutaria (GI50 11 μg/mL) has a promising anti-cancer activity against the selected cell line. Flow cytometric analysis of apoptosis showed that the TCe induced apoptosis in K562 cell line though early apoptosis. The increased proportions of cells in the G0/G1 phase confirmed that TCe induced apoptosis in K-562 cells, resulting in DNA degradation. The percentage of cells at the G2/M phase decreased, which corresponded to an increase in the percentage of cells at the G0/G1 phase, indicating an arrest of the cell cycle at the G0/G1 phase by extracts after treatment for 24 h. The above results reveal that TCe showed considerable inhibitory activity on cellular growth, more by apoptosis than by necrosis in the K562 cells. Inactivation of p53 is commonly seen in tumors. P53 has become the focus on most intensive cancer based research. Interestingly, we observed an increase in the levels of p53 upon treatment with TCe. The increased p53 level also coincided with increase in its downstream target p21. Based on the preliminary phytochemical tests and antileukemic results of different extracts, only ethanol fraction was selected for GC-MS studies.
The presence of various bioactive compounds justifies the use of the plant rhizomes for various ailments by traditional practitioners. However, isolation of individual phytochemical constituents and subjecting it to pharmacological activity will definitely give fruitful results.
Conflict of interest statement
The authors declare no conflicts of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
We are extremely thankful to Rajesh Ramachandran, Director Biogenix Research Center, Thiruvananthapuram, Kerala for Cell cycle analysis and Gene expression.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Mahomoodally M.F. 2013. Traditional Medicines in Africa: An Appraisal of Ten Potent African Medicinal Plants Evidence-based Complementary and Alternative Medicine; pp. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cragg G.M., Newman D.J. Plants as a source of anti-cancer agents. J Ethnopharmacol. 2005;100:72–79. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Cragg G.M., Newman D.J. Natural products: a continuing source of novel drug leads. Biochim Biophys Acta. 2014;1830:3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mondal S., Bandyopadhyay S., Ghosh M.K. Natural products: promising resources for cancer drug discovery. Anticancer Agents Med Chem. 2012;12:49–75. doi: 10.2174/187152012798764697. [DOI] [PubMed] [Google Scholar]
- 5.Prakash O., Kumar A., Kumar P. Ajeet. Anticancer potential of plants and natural products: a review. Am J Pharmacol Sci. 2013;1:104–115. [Google Scholar]
- 6.Upadhye A., Kumbhojkar M.S., Vartak V.D. Observations on wild plants used in folk medicine in the rural areas of the Kolhapur district. Anc Sci Life. 1998;6:119–121. [PMC free article] [PubMed] [Google Scholar]
- 7.Dash S., Padhye S. Review on ethnomedicines for diarrhea diseases from Orissa: prevalence versus culture. J Hum Ecol. 2006;20:59–64. [Google Scholar]
- 8.Irudayaraj V., Bir S.S. Notes on some pteridophytic from the western Ghats of Goa state South India. Indian Fern J. 1997;14:113–117. [Google Scholar]
- 9.Beddome R.H. Thacker Spink & Co.; Calcutta: 1892. A Handbook to the Ferns of British India, Ceylon and Malaya Peninsula. [Google Scholar]
- 10.Bhardwaja T., Gena C., Verma S. Status survey of pteridophytic flora of Rajasthan with special reference to endangered fern and fern allies. Indian Fern J. 1987;4:47–50. [Google Scholar]
- 11.Khandelwal K. Practical pharmacognosy techniques and experiments. Nirali Prakashan. 2010;25:1–25. [Google Scholar]
- 12.Shimada K., Fujikawa K., Yahara K., Nakamura T. Antioxidative proper-ties of xanthan on the autioxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40:945–948. [Google Scholar]
- 13.Oyaizu M. Studies on products of browning reaction: antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J.Nutr. 1986;44:307–315. [Google Scholar]
- 14.Qian J. Development of a K562 cell-based assay for screening anticancer agents. Acta Pharmacol Sin. 2001;22:821–826. [PubMed] [Google Scholar]
- 15.Meir S., Kanner J., Akiri B., Hadar S.P. Determination andinvolvement of aqueous reducing compounds in oxidative systems of various senescing leaves. J Agric Food Chem. 1995;43:1813–1817. [Google Scholar]
- 16.Ozmen A., Madlener S., Bauer S. In vitro anti-leukemic activity of the ethno- pharmacological plant Scutellaria orientalis ssp. Carica endemic to western Turkey. Phytomedicine. 2010;17:55–62. doi: 10.1016/j.phymed.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Ding X., Zhu F.S., Li M., Gao S.G. Induction of apoptosis in human hepatoma SMMC-7721 cells by solamargine from Solanum nigrum L. J Ethnopharmacol. 2011;139:599–604. doi: 10.1016/j.jep.2011.11.058. [DOI] [PubMed] [Google Scholar]
- 18.Pereira J.M., Lopes-Rodrigues V., Xavier C. An aqueous extract of Tuberaria lignosa inhibits cell growth, alters the cell cycle profile, and induces apoptosis of NCI-H460 tumor cells. Molecules. 2016;21:595. doi: 10.3390/molecules21050595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen R., Oren M. p53: from inductive signal to cellular effect. Curr Opin Gene Dev. 1997;7:46–51. doi: 10.1016/s0959-437x(97)80108-6. [DOI] [PubMed] [Google Scholar]
- 20.Ko L.J., Prives C. p53: puzzle and paradigm. Genes Dev. 1996;10:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 21.McGraw L.J., Jager A.K., Van Staden J. Isolation of antibacterial fatty acids from Schotia brachypetala. Fitoterapia. 2002;73:431–433. doi: 10.1016/s0367-326x(02)00120-x. [DOI] [PubMed] [Google Scholar]
- 22.Dinesh M.G., Subbarayan R., Rallapalli S., Kansrajh C., Kalaivani R. Terminalia bellerica leaf extracts induce apoptosis in Hep G2 cells and regulates cell cycle progression by inducing G2/M cell cycle arrest. Indian J Res Pharm Biotechnol. 2014;2:1044–1056. [Google Scholar]
- 23.Ogunlesi M., Okiei W., Ademoye M., Osibote E.A. Analysis of essential oil from the stem of Chansmanthera dependens. J Nat Prod. 2010;3:47–53. [Google Scholar]
- 24.Ichikawa H., Nakamura Y., Kashiwada Y. Anticancer drugs designed by mother nature: ancient drugs but modern targets. Curr Pharm Des. 2007;13:3400–3416. [PubMed] [Google Scholar]
- 25.Sun J., Liu B., Hu W. In vitro anticancer activity of aqueous extracts and ethanol extracts of fifteen traditional Chinese medicines on human digestive tumor cell lines. Phytothe Res. 2007;21:1102–1104. doi: 10.1002/ptr.2196. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y., Xun K., Wang Y. A systematic review of the anticancer properties of berberine, a natural product from Chinese herbs. Anti-Canc Drugs. 2009;20:757–769. doi: 10.1097/CAD.0b013e328330d95b. [DOI] [PubMed] [Google Scholar]
- 27.Ghoghari A., Bagul M., Anandjiwala S. Free radical scavenging activity of Aspidium cicutarium rhizome. J Nat Remedies. 2006;6:131–134. [Google Scholar]