Abstract
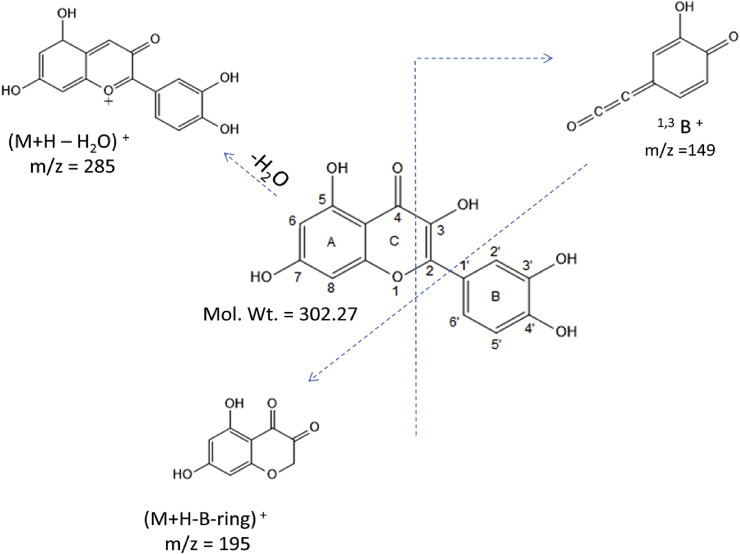
Chenopodium album L., (C. album) (family: Chenopodiaceae) is an annual shrub widely grown in Asia, Africa, Europe and North America. It is commonly known as Bathua (in Hindi), pigweed, fat hen or lamb-quarters. The leaves of C. album are applied as a poultice to bug bites, sunstroke, rheumatic joints and as mild laxative. The flavonoids contained in C. album aerial parts were extracted, identified and characterized. Sequential soxhlet extraction was subjected to preliminary phytochemical screening and flavonoid quantification. The results showed that maximum yield of the flavonoid (7.335 mg/g) were obtained from acetone extract. This acetone extract was subjected to flash chromatography for isolation of flavonoid. Characterization of isolated flavonoid was done by UV, IR, 1H & 13C NMR and MS. On the basis of chemical and spectral analysis structure was elucidated as 2-(3, 4-dihydroxyphenyl)-3, 5, 7-trihydroxy-4H-chromen-4-one, a flavonoid.
Keywords: Chenopodium album, Chenopodiaceae, Flavonoids, Flash chromatography, Quercetin
Graphical abstract
1. Introduction
Polyphenols appear to be important metabolic modulators by virtue of their ability to influence several cellular pathways and molecules that have been reported as potential targets for polyphenolic compounds. Flavonoids are “the most common group of polyphenolic compounds in the human diet and are found ubiquitously in plants”. Although more than 4000 flavonoids have been identified, several appear to be important components of many fruits and vegetables. According to the differences in functional groups and their relative positions of the 15-carbon skeleton (aglycons), flavonoids are classified into several subgroups such as flavone, flavanone, flavonol, isoflavonoid, anthocyanidin, and chalcones.1 Flavonols, the original bioflavonoids such as quercetin, are also found ubiquitously, but in lesser quantities. Flavonoids, a subclass of polyphenols, are a group of phytochemicals that are among the most potent and abundant antioxidants in our diet and also possesses various activities such as anti inflammatory, anti cancer etc.
Chenopodium album L., (C. album) (family: Chenopodiaceae) is an annual shrub widely grown in Asia, Africa, Europe and North America. It is commonly known as Bathua (in Hindi), pigweed, fat hen or lamb-quarters. The leaves of C. album are applied as a poultice to bug bites, sunstroke, rheumatic joints and as mild laxative.
The plant is used in folk medicine in different parts of the world as diuretic, laxative, sedative, hepato-protective and antiparasitic. The leaves possesses anthelmentic, antiphlogistic, antirheumatic, mildly laxative and odontalgic properties, applied as wash or poultice to bug bites, sunstroke, rheumatic joints and swollen feets.2 Additionally, decoction of its aerial parts mixed with alcohol was used in the rheumatism.3 Cinnamic acids amides.,4 flavonoids5, 6 and apocarotenoids7 have also been isolated from this species. Flavonoid from C. album has significant potential to scavenge free radicals, NF kappa B inhibition, anti-inflammatory, and hence have got antirheumatic potential.8
The aim of the study was to isolate the flavonoids using Flash Chromatography and was characterized through their spectral analysis like IR, 1H, 13C NMR and MS.
2. Material and methods
2.1. Chemicals and standard drugs
All the chemicals and solvents used were of analytical grade, Silica gel (G) 60 F and 0.25 readymade aluminum sheets (Merck, Germany), Rutin and Quercetin from SD fine Chem. Ltd. Mumbai.
2.2. Plant material and preparation of extract
The aerial parts were collected from Ramtek region in the month of August, authenticated by Dr. (Mrs.) Alka Chaturvedi, Department of Botany, R.T.M. Nagpur University, Nagpur. A voucher specimen has been deposited in the Herbarium of Department of Botany, with collection number RA 9576.
The aerial parts of C.album were dried under shade and pulverized to a coarse powder. The powdered crude material (1 kg) was defatted with petroleum ether and then extracted successively with ethyl acetate, acetone and methanol using Soxhlet extractor followed by cold maceration (7 days) with 50 % methanol. The extracts were concentrated using rotary vacuum evaporator to yield ethyl acetate extract (EACA; yield: 3.9 % w/w), acetone extract (ACCA; yield: 4.79 % w/w), methanolic extract (MECA; yield: 13.58 % w/w) and 50 % methanolic extract (HACA; yield: 12.76 % w/w). These extracts were subsequently subjected to phytochemical screening and quantitative estimation.
2.3. Phytochemical screening1, 9
The extracts were screened for the presence of different phytochemicals employing thin layer chromatographic (TLC) techniques. Thin layer plates precoated with silica gel G (Merck, 0.25 mm thickness) were used. Development was carried out with different solvent systems such as ethyl acetate: methanol: water (100:13.5:10, v/v/v), ethyl acetate: formic acid: acetic acid: water (100:11:11:26, v/v/v/v), chloroform: methanol: water (70:30:4, v/v/v), toluene: ethyl acetate: diethylamine (70:20:10, v/v/v) and ethyl acetate: methanol: water: acetic acid (65:15:15:10, v/v/v/v). After development of chromatogram in the solvents, the plates were dried and sprayed with AlCl3 reagents for the detection of flavonoids. Visualization was carried out under visible and UV light (λ: 366 nm). The quantitative estimation of flavonoid in EACA, ACCA, MECA and HACA extracts were carried out respectively.
2.4. Determination of flavonoids (TFA)
Flavonoid content was determined by the aluminium chloride method.10 Briefly, to 1 ml of test solution (1 mg/ml), 1.5 ml of 95 % alcohol, 0.1 ml of 10 % aluminum chloride hexahydrate (AlCl3.6H2O), 0.1 ml of 1 M sodium acetate (CH3COONa) and 2.3 ml of distilled water was added. After incubation at room temperature for 40 min, absorbance of the reaction mixture was measured at 435 nm against corresponding blank, prepared in the same manner without adding AlCl3. Rutin was used as a reference standard and results were expressed as mg of rutin equivalents (RE)/g of extract. All determinations were performed in triplicate.
2.5. Isolation using flash chromatography11
Flash column chromatography was performed on spherical silica gel, C-18; 40–63 micron (230–400 mesh), 60Å pore size, pH range of 6.5–7.5, in glass columns designed for FCC. Sorbent Technologies (Atlanta, GA, USA) supplied silica gel. Glass flash columns were manually dry packed using C-18 silica. After the column was packed and mounted on the instrument, a volume of initial solvent mixture was pushed through the silica to remove air and to wet and equilibrate the column. The sample (Extract or fractions) previously triturated with three times column silica and dried was placed in between Teflon disc in solid sample loading cartridge. Sample loaded cartridge was placed over head of the column before elution. Fractions were collected in test tubes, graduated with the fraction volume and number. The various flash chromatographic conditions used for the separation of compounds were mentioned in Table 1.
Table 1.
Method parameters of flash chromatographic separation of ACCA extract.
| Instrumentation | Teledyne Isco CombiFlash® Companion™ 4x | |
| Column | 24 g C-18 Reversed Phase RediSep Silica | |
| Run length | 270 min | |
| Flow Rate | 21 ml/min | |
| Equilibration Volume | 100.0 ml | |
| Peak Detection | Slope-based | |
| Sensitivity: High | ||
| Peak Width: 1 min or Thresholding: 0.05AU | ||
| Detection wavelength (red) | 254 nm | |
| Monitor wavelength (purple) | 267 nm | |
| Mobile phase | Solvent A: mixture of 0.1% formic acid in water (v/v) | |
| Solvent B: Acetonitrile | ||
| Gradienta | % of Solvent B | Column Vol. (CV) |
| 5% | 3 | |
| a linear gradient to 80% | 35 | |
| a linear gradient to 100% | 50 | |
| 100% | 55 | |
| Ramp back to 5% B | 55–60 | |
| 5% | 60–65 | |
Mobile phase composition was freeze during the peak elution and continued with the gradient elution after peak retaining base line.
2.6. Compound characterization
For characterization of compound UV, IR, NMR and Mass spectra were performed. The UV absorption of isolated constituent was determined in methanol over a scanning range of 200–800 nm. The compound was dissolved in methanol to obtain required concentration and spectrum was recorded. The infrared absorption spectrum of the isolated constituent as KBr disc has been determined on FTIR-8101A (Shimadzu) and the absorption peaks in the form of wave numbers (cm −1) were noted.
1H NMR spectra were acquired on a Bruker DSX-300 spectrometer, using the standard pulse program ‘lc1pnf2’, which is based on the one dimension version of the NOESY sequence and allows double pre-saturation, to suppress the water peaks. 32k data points were recorded over a sweep width of 9191 Hz, with 512 scans. An exponential line broadening of 1 Hz was imposed on the accumulated data before Fourier transformation. The 13C NMR experiments were obtained at 400.23 MHz on a Bruker Biospin Ultrashield plus AV-400 MHz instrument. The samples were dissolved in deuterated methanol. The mass spectroscopy of isolated constituent was carried out using a TOF MS ES + mass spectrometer and experiments were acquired using the potential LIFT technique based on post source and post-decay acceleration of fragment ions. MS spectra were annotated using Flex Analysis software and transferred to BioTools for sequence evaluation. The spectrometer was operated in reflection mode optimized for positive ions with masses from 0 to 2000 Da with 25 kV acceleration voltage. The nitrogen laser extinction frequency was set at 10 Hz. The laser power was optimized to obtain a good signal-to-noise ratio after averaging 100 signal-shot spectra. Mass spectra were acquired at National Institute of Pharmaceutical Education & Research, Kolkata, India.
3. Results and discussion
3.1. Phytochemical screening
The phytochemical screening of EACA extract showed the presence of tannins, sterol and flavonoids, while ACCA extract revealed the presence of mainly proteins, tannins and flavonoids. Further, MECA and HACA extracts also showed the presence of proteins, carbohydrates, tannins, saponin and flavonoids.
3.2. Determination of flavonoid
The content of flavonoids (mg/g) was determined by the regression equation of the calibration curve (y = 0.018x - 0.001, r2 = 0.990) and was expressed in rutin equivalents (RE) and was found to be in decreasing order of ACCA > HACA > EACA > MECA.
3.3. Flash chromatographic isolation of flavonoid
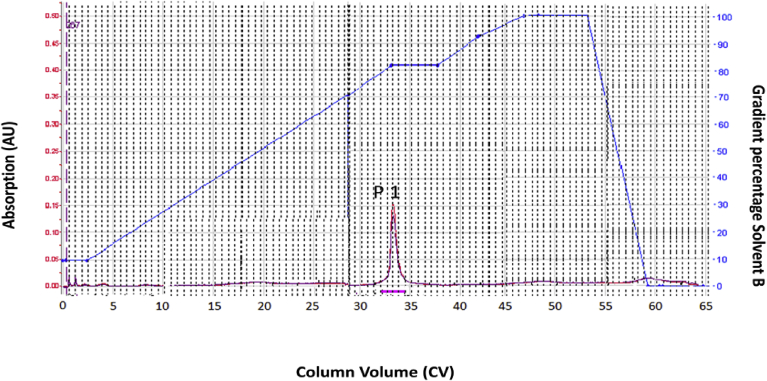
In the present study for the separation of bioactive molecules, the Flavonoid rich extract from C. album subjected to automated flash chromatography on ISCO-combiflash (Teledyne Isco, Inc. Nebraska, USA). For reversed phase separations, selection of mobile phase and optimization of gradient profiles was performed by selecting the mobile phase used for HPLC for separation of specific class of compound or plant under investigation, and increasing the gradient elution time by a factor of 4 or 5.12, 13, 14 RP-TLC mobile phase is of limited use due to the poor wettability of the plates. However, for normal phase separation flash separations on silica gel, mobile phase was selected on trial-and-error basis to give Rf values of compounds between 0.5 and more than 0.1. Thus depending upon reverse phase or normal phase separations different mobile phase was selected. The reverse-phase flash chromatographic separation of ACCA extract resulted in separation of single compounds, which are represented as peak 1 in flash chromatogram (Fig. 1). The peak 1 (P1) was collected as fraction 3–9; this fraction was pooled together and concentrated under vacuum. On drying, peak 1 yielded yellow colored powder (13 mg), gave positive test for flavonoid. The flash chromatographic method parameters used were listed in Table 1. The yellow colored powder compound has been given the code name ACCA 1. Pale yellow coloured powder compound were obtained after purification and melting point was 312–315 °C. UV-spectra, IR, Mass and 13C and proton NMR spectra were recorded.
Fig. 1.
The Flash chromatographic separation of ACCA extract.
3.4. Identification and spectral analysis of isolated flavonoid
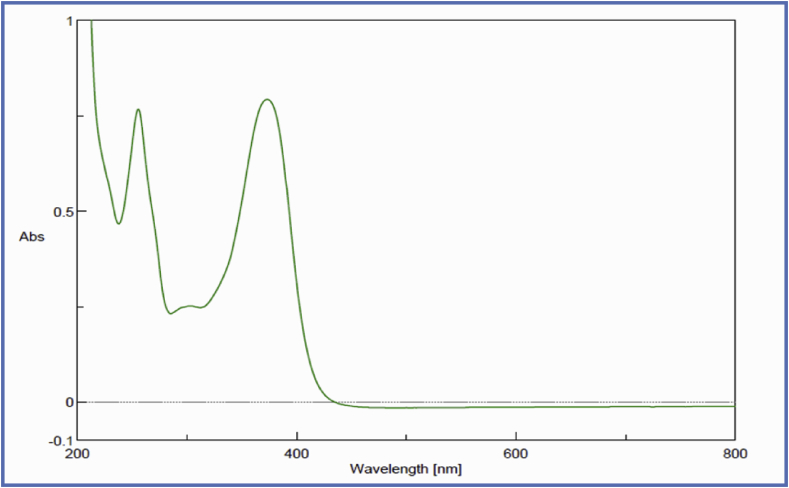
The UV spectrum of the ACCA-1 compound shows spectral maxima at 373 nm and 256 nm and λ max was found to be 373 nm. The typical UV-Vis spectra of flavonoids include two absorbance bands. Band A lies in the 310–350 nm range for flavones, while for flavonols it is between 350 and 385 nm. Band B, found in the 250–290 nm range.15 Thus the UV spectrum showed that the compound may belong to flavonol category of flavonoid. Consequently, these two subgroups cannot be distinguished by simple UV-Vis analysis. Flavonols show maximum absorbance at non specific wavelengths between 270 and 290 nm, at which many phenolics absorb, thus not allowing their selective detection (Fig. 2). The UV/Vis spectrum obtained was also in agreement with published UV spectrum of Quercetin.16
Fig. 2.
UV spectrum of isolated compound ACCA-1.
IR spectrum exhibits peaks at 3624, 3473, 3295 cm−1 (OH), 1616 cm−1 (Aromatic C C Bend), 1458 cm−1, (CH2 and CH3; RCH2CH3), 1165 cm−1 (C-CO-C Stretch and bending in Ketone), The peaks revealed the number of functional groups and nature of bonding.
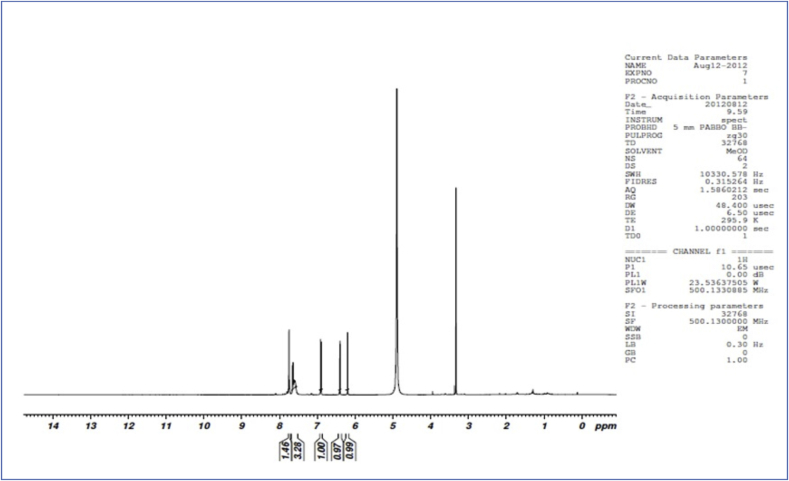
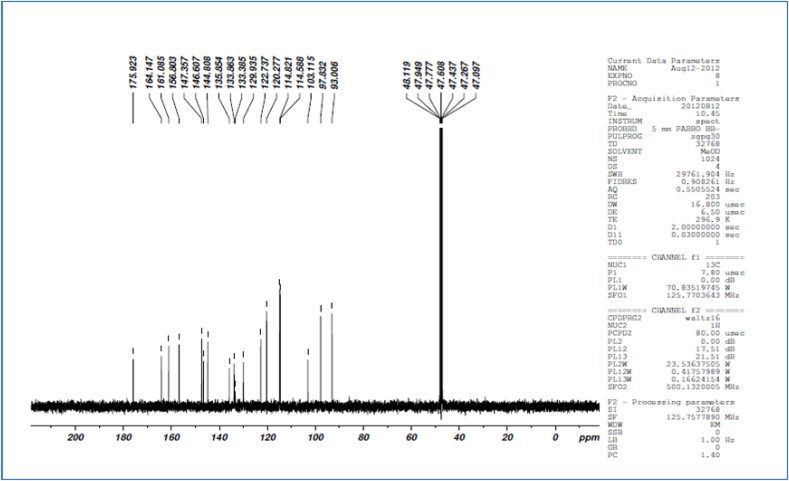
1H NMR spectra of ACCA-1 shows solvent peak observe at δ 5.1. Singlet peak at δ 6.20 and 6.40 indicates H-6 and H-8 proton of flavonol. Another doublet peak at δ 6.89 and 6.91 indicate presence of H-5′ proton of flavonol. Peak at δ 7.59 to 7.66 indicate H-2′ and H-6′ proton of flavonol (Fig. 3). While C13 NMR spectra revealed the Signal at 175.92 ppm indicate carbonyl carbon (C-4) at normal low field position. Another signal at 164.14, 161.08, 156.80 and 158.30 ppm indicate C-7, C-5, C-4′ and C-3′ position of flavonol carbons. Singlet at 147.35 and 146.60 ppm attributed for C-2′ and C-6′ position of the carbon, signal at 135.85 ppm indicate C-3 oxygenated aromatic carbon. Another signal at 122.73 ppm indicate C-1 carbon atom of flavonol. Signal at 114.82, 114.58 ppm showed the C-1 and C-1′ position of non oxygenated carbon atom. Signal at 103.11 ppm showed C-10 carbon position. Another signal at 97.83 and 93.06 ppm indicate C-6 and C-8 position of carbon. (Fig. 4).
Fig. 3.
1H NMR spectra of compound ACCA 1 isolated from C. album plant.
Fig. 4.
13C NMR spectra of compound ACCA 1 isolated from C. album plant.
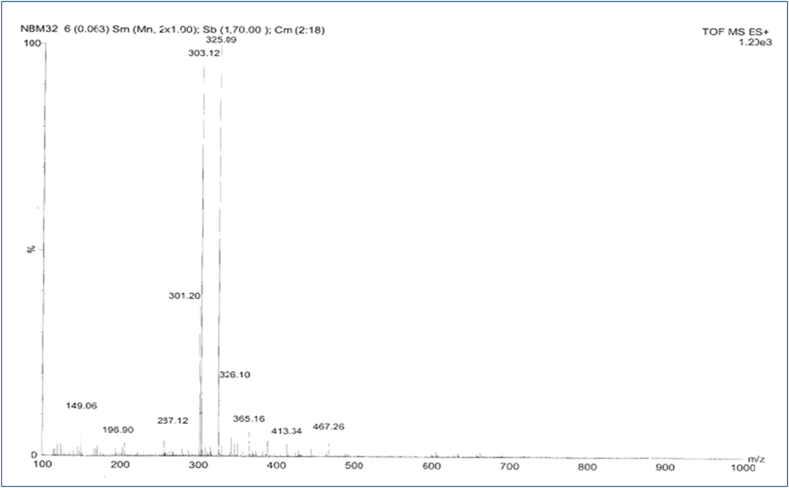
The TOF MS ES + spectra of isolated compound ACCA 2 was performed in positive ion mode and molecular ion peak was observed at m/z 303.12 (M + H)+ (Fig. 5). The molecular weight of the compound is 302.12. While fragment at m/z 325 represents (M + Na)+peak. Other fragments were observed at m/z 287, 196, 149. Fragment at m/z 287 (Corresponding to 285 m/z) is of (M + H-H2O)+represent the loss of one water molecule or (M + H)+product ions dehydrated to (M + H-H2O)+.16 Fragment peak at m/z 196 (Corresponding to 193 m/z) represent the (M + H-B-ring)+ion, confirmed the dihydroxy-substitution of A-ring. Fragments at m/z 149 indicate that fission of ring C results in the fragments 1, 3B + ones with m/z = 149. (B represents the intact ring while the superscript on the left indicates the broken bonds of the protonated molecule)15, 17 and the other fragments at 365, 413 and 467 were results from the multiple adduct formation. Mass spectral assignment and its Identification were reported in Table 2 and Fig. 6.
Fig. 5.
MASS spectra of compound ACCA 1 isolated from C. album plant.
Table 2.
MS spectral assignment of isolated constituent and its identification.
| Sr. No. | Mass/Abundance | Identification |
|---|---|---|
| 1 | 303.12 | (M + H)+ |
| 2 | 325.09 | (M + Na) + |
| 3 | 287.12 | (M + H-H2O) + |
| 4 | 196.90 | (M + H-B-ring) + |
| 5 | 149.06 | 1, 3B+ |
Fig. 6.
Schematic fragmentation of ACCA 1 compound.
Concluding the aforementioned studies it was analyzed that the studied plant contained flavonoids. The phytochemical screening of isolated compound suggested it to be a flavonoidal molecule. TLC with standard was also performed and it showed same Rf value. The melting point of isolated compound was same as that of Quercetin. From the above spectral data isolated compound was resemblance to Quercetin.
Conflicts of interest
None to declare.
Funding
This work was supported by the Council of Scientific and Industrial Research, New Delhi, India (09/128/(0083)/2012/EMR-I).
Acknowledgement
Authors are thankful to the Department of Pharmaceutical Sciences, R. T. M. Nagpur University for providing all the necessary facilities related to the present research work.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Harborne J.B. 3rd ed. Springer Pvt. Ltd.; New Delhi, India: 1998. Phytochemical Methods, a Guide to Modern Techniques of Plant Analysis. [Google Scholar]
- 2.Kokanova-Nedialkova Z., Nedialkov P., Nikolov S. The genus chenopodium: phytochemistry, ethnopharmacology and pharmacology. Pharmacog Rev. 2009;3:280–306. [Google Scholar]
- 3.Gupta K., Wagle D.S. Nutritional and antinutritional factors of green leafy vegetables. J Agric Food Chem. 1988;36:472–474. [Google Scholar]
- 4.Cutillo F., D'Abrosca B., DellaGreca M., et al. Cinnamic acid amides from Chenopodium album: effects on seeds germination and plant growth. Phytochemistry. 2003;64:1381–1387. doi: 10.1016/s0031-9422(03)00511-9. [DOI] [PubMed] [Google Scholar]
- 5.González J.A., Gallardo M., de Israilev L.A. Leaf flavonoids in Chenopodium hircinum schrad and Chenopodium album L. (Chenopodiaceae) Phyton. 1998;63:279–281. [Google Scholar]
- 6.Bylka W., Kowalewski Z. Flavonoids in Chenopodium album and Chenopodium opulifolium L. (Chenopodiaceae) Herba Pol. 1997;XLIII:208–213. [Google Scholar]
- 7.Dellagreca M., Previtera L., Zarrelli A. A new xyloside from Chenopodium album. Nat Prod Res. 2005;19:87–90. doi: 10.1080/14786410410001686391. [DOI] [PubMed] [Google Scholar]
- 8.Arora S.K., Itankar P.R., Verma P.K., Bharne A.P., Kokare D.M. Involvement of NF kappa B in the antirheumatic potential of Chenopodium album aerial parts extracts. J Ethnopharmacol. 2014;155:222–229. doi: 10.1016/j.jep.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 9.Stahl E. 2nd ed. vol. 86. Springer; New York: 1969. (Thin Layer Chromatography: A Laboratory Hand Book). [Google Scholar]
- 10.Stanojevic L., Stankovic M., Nikolic V., et al. Antioxidant activity and total phenolic and flavonoid contents of Hieracium pilosella L. Extr Sensors (Basel, Switzerland) 2009;9:5702–5714. doi: 10.3390/s90705702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franz B., Abraham W., Martin S. Natural product isolation - how to get from biological material to pure compounds. Nat Prod Rep. 2013;30:525–545. doi: 10.1039/c3np20106f. [DOI] [PubMed] [Google Scholar]
- 12.Weber P., Hamburger M., Schafroth N., Potterat O. Flash chromatography on cartridges for the separation of plant extracts: rules for the selection of chromatographic conditions and comparison with medium pressure liquid chromatography. Fitoterapia. 2011;82:155–161. doi: 10.1016/j.fitote.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Sebastian S., Guido J., Helen S., Jörg H. Phloroglucinol derivatives from Hypericum empetrifolium with antiproliferative activity on endothelial cells. Phytochemistry. 2012;77:218–225. doi: 10.1016/j.phytochem.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Uckoo R.M., Jayaprakasha G.K., Patil B.S. Chromatographic techniques for the separation of polymethoxyflavones from citrus. Am Chem Soc. 2012;1093:3–15. [Google Scholar]
- 15.Tsimogiannis D., Samiotaki M., Panayotou G., Oreopoulou V. Characterization of flavonoid subgroups and hydroxy substitution by HPLC-MS/MS. Molecules. 2007;12:593–606. doi: 10.3390/12030593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khalifa T.I., Muhtadi Farid J., Hassan M.A. PMR assay of rutin in drug plants and pharmaceuticals. Pharmazie. 1983;122(8):809–813. [Google Scholar]
- 17.Ma Y.L., Li Q.M., Van den Heuvel H., Claeys M. Characterization of flavone and flavonol aglycones by collision-induced dissociation tandem mass spectrometry. Rapid Commun Mass Spectrom. 1997;11:1357–1364. [Google Scholar]