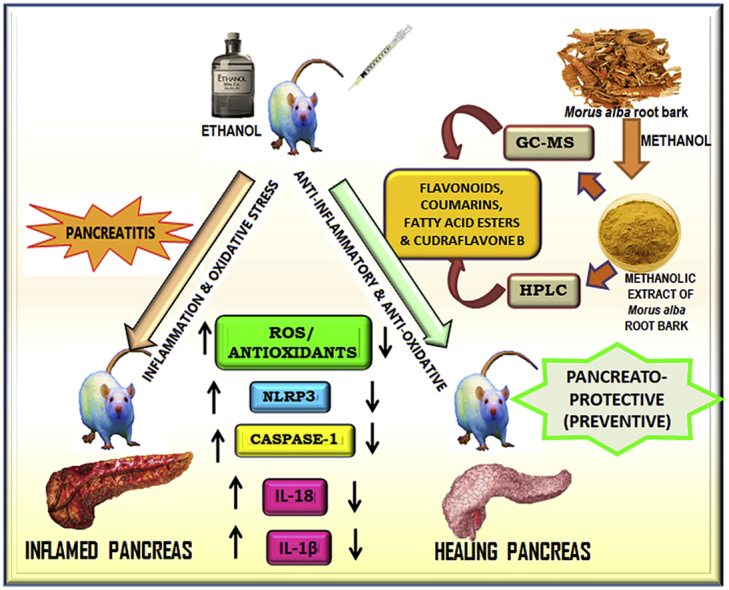
Abstract
Pancreatitis is characterized by highly morbid inflammation in the pancreas. Currently, there is no specific drug available for pancreatitis except supportive medicines. The present study assessed the pancreato-protective effect of Morus alba root bark extract by using alcohol and cerulein-induced model of pancreatitis. The study also investigated the phytochemical profile through GC-MS and HPLC. Methanolic extract of Morus alba root bark extract (MEMARB) was subjected to GC-MS and HPLC studies. Male albino Wistar rats were administered ethanol (0%–36%) and cerulein (20 μg/kg b.wt. i.p.) with or without MEMARB. Serum lipase, amylase, caspase-1, lipid peroxidation products, glutathione and enzymatic antioxidants were determined. Histological changes in the pancreas were assessed. Cudraflavone B in MEMARB was quantified by HPLC. Significant amount of Cudraflavone B was detected by quantitative HPLC. Marked increase in the levels of serum amylase, lipase, caspase-1, IL-18 and IL-1β were observed in ethanol and cerulein administered rats than in MEMARB co-administered rats. In MEMARB co-administered rats, the antioxidant status was restored to near normal levels. Histological examinations showed that MEMARB significantly reduced the inflammatory and fibrotic changes. The results reveal the potent pancreato-protective effects of Morus alba root bark. The anti-inflammatory effect of Morus alba root bark extract might be due to the presence of various phytonutrients including Cudraflavone B.
Keywords: Pancreatitis, NLRP3, GC-MS, HPLC, Cudraflavone B, Morus alba
Abbreviations: AP, acute pancreatitis; CP, chronic pancreatitis; TCP, tropical calcific pancreatitis; MCP 1, monocyte chemotactic protein 1; DAMPS, damage associated molecular patterns; PSC, pancreatic stellate cell; NF-kappa B, nuclear factor kappa-light-chain-enhancer of activated B cells; AP1, activator protein 1; MEMARB, methanolic extract of Morus alba root bark
Graphical abstract
1. Introduction
Pancreatitis is an inflammatory response initiated in the pancreatic parenchyma due to acinar cell injury. The underlying pathomechanism is the premature ectopic activation of the master proteolytic zymogen trypsinogen, to active trypsin, precipitating a cascade of zymogen activation and ‘auto digestion’ of the pancreas.1
Persistent, irreversible inflammation and fibrosis of the pancreas are characteristics of chronic pancreatitis (CP). Symptoms of CP include abdominal pain, frequent acute pancreatitis (AP) episodes, exocrine and endocrine insufficiency.2 Alcohol abuse causes 70% of CP while smoking, gene mutations, hyperparathyroidism and autoimmunity are other etiologies.3 Chronic pancreatitis has a worldwide prevalence of 50/100,000 persons. Tropical calcific pancreatitis (TCP), a rare form of CP, has a prevalence of 20–125/100,000 persons in southern India. 5% of CP patients and 40–50% of hereditary pancreatitis patients develop highly lethal pancreatic cancer.4
Current management guidelines recommend only supportive measures like hospitalization, intensive fluid resuscitation, bowel rest, parenteral nutrition, enzyme supplements and pain management through non-steroidal anti-inflammatory drugs (NSAIDs).
In both acute and chronic pancreatitis injured and dying pancreatic acinar cells are the primary drivers of inflammation and initiators of necroptosis and pyroptosis, key determinants of disease severity. Acinar cells elaborate immune responses by secreting cytokines (TNF-α, IL-1β, IL-6, and IL-10), chemokines (MCP-1) and endogenous damage associated molecular patterns (DAMPs).
NLRP3 inflammasome is a multiprotein intracellular innate immune sensor consisting of NLRP3, apoptosis-associated speck-like protein (ASC) and procaspase-1. It assembles in response to diverse stimuli and forms the scaffold for the activation of pro-inflammatory cytokines IL-1beta and IL-18 and induces the release of HMGB1 expression important in pancreatic inflammation, parenchymal cell injury and disease resolution. Pro-inflammatory cytokines IL-1 beta and IL-18 are triggers of pyroptosis, a highly lytic form of cell death, which appears to be predominant in pancreatitis.5., 6., 7.
In the face of a threat from rising pancreatitis cases and the absence of a specific licensed drug, inflammatory pathways have been under scrutiny to find suitable targets for potential anti-inflammatory drug molecules. Various anti-inflammatory effectors that have been tested include thalidomide (targets TNF-alpha), panhaematin (decreases leukocyte infiltration), IL-R antagonist montelukast, MCP 1 inhibitors, COX - 2 inhibitor flavocoxid, vitamin K3 (inhibits autophagy) and the broad anti-inflammatory effects of agents like quercetin, resveratrol and curcumin.1
Morus alba L. or white mulberry is native to northern China and has been naturalized and cultivated throughout Asia and Europe. It has long-standing ethno medicinal significance. Various parts of the plant have been used in traditional Asian medicine. Phytochemical analyses have identified alkaloids, flavonoids, flavones, flavanones, stilbenes, benzophenones, coumarin derivatives and terpenoids in the root bark of M. alba8. Morus is one of the few genera to contain prenylated flavonoids. Prenyl flavonoids are credited with enhanced biological effects attributed to the prenyl side-chains.9 But, their scientific validation should be taken care of.
The bioactive principles from Morus alba root bark are reported to have antibacterial, antiviral, antioxidant, hypoglycemic, neuroprotective, nephroprotective, antiulcer, analgesic and anti-inflammatory properties.10
The aim of the present investigation is to assess the therapeutic efficacy of phytonutrients of white mulberry roots with special reference to their influence on the level of cytokine production and their impact on acinar cell damage monitored in terms of serum and pancreatic marker enzymes and cellular antioxidants.
2. Materials and methods
2.1. Chemicals
Reference standards for HPLC - gallic acid, galangin, rutin, quercetin and thymoquinone - were obtained from LGC Promochem India Pvt. Ltd., (Bangalore, India). HPLC grade solvents were purchased from Merck India. ELISA kit for IL-Iβ was purchased from Abcam and Invitrogen ELISA kit for IL-18 was purchased from Thermo Fisher Scientific. Lipase and alpha-amylase assay kits were procured from Coral Clinical Systems, Goa, India. All other chemicals used were of analytical grade.
2.2. Plant material collection, identification and extraction
Fresh Morus alba roots were collected from the Plant Sciences department, University of Madras, Guindy Campus, Chennai, department of Sericulture, Vitchanthangal, Kancheepuram District, Tamil Nadu and a private mulberry farm at Purisai, Kancheepuram District, Tamil Nadu. The plant material was authenticated by Professor P. Jayaraman, Director, Plant Anatomy Research Centre, West Tambaram, Chennai. The herbarium specimen (PARC/2015/3144) was preserved for future reference in the Department of Biochemistry, Bharathi Women's College. The roots were pooled, cleaned under tap water and air-dried for 2–3 weeks. Completely dry roots were lightly scraped to reveal the yellowish layer underneath. The root bark or cortex was peeled off with the help of a knife, cut into small pieces and powdered in a blender. The crude powder was sieved to obtain a fine homogenous powder. M.alba root bark powder was soaked in methanol and left to agitate on a shaker for 24hrs. The extract was filtered. The residue was extracted again with fresh methanol to ensure complete extraction. The filtrates were air dried to a powder, sieved and stored in an air-tight container at 4 °C. For animal experimentation, a homogenous suspension of the bark powder was prepared with 0.1% DMSO.
2.3. Animals
Adult male albino Wistar rats (175–200g, seven-eight weeks old) used for the study were housed under hygienic conditions [22-24 °C] in polypropylene cages under 12 h light/12 h dark cycle. The animals were allowed free access to water and standard pelleted rat chow during the acclimatization period. Animal maintenance and experimentation protocols conformed to the guidelines of the Institutional Animal Ethics Committee constituted by the Committee for the Purpose of Control and Supervision of Experiments on Animal (CPCSEA), Government of India, [XVII/VELS/PCOL/02/2000/CPCSEA/IAEC/06.10.15].
2.4. Experimental protocols
2.4.1. Preliminary phytochemical screening by GC-MS
The methanolic extract of the root bark of white mulberry (M.alba) was subjected to GC-MS analysis. The analysis was carried out on Agilent 6890N gas chromatograph with HP-5ms column coupled to a mass spectrometer JEOL GC-MATE II in the electron ionization (EI) mode with ionization voltage set to 70eV. The mass spectral scan range of the mass analyser was set to 50–600 amu. Helium was used as a carrier gas at a constant flow of 1 mL/min. The front inlet temperature was 220 °C. The GC-oven was set for the following temperature profile: ramp rate from 50◦C-250 °C at 10 °C/min. NIST (National Institute of Standards and Technology) GC-MS mass spectral database was used to interpret the mass spectrum using the retention time.
2.4.2. HPLC for identification of flavonoids and quantification of Cudraflavone B
Accurately weighed quantities of the standards and the sample were transferred to separate volumetric flasks and dissolved in methanol and diluted to a specific concentration. A specific quantity of Morus alba root bark powder was refluxed with extraction solvent (methanol: water: hydrochloric acid) for 135 min to hydrolyse the flavonoid glycosides and contents were made up with methanol. An equal volume of the standard and sample (20 μL) was then used for the HPLC analysis on Shimadzu (Japan), HPLC Class VP series with a UV–vis detector. The samples were run on a C18 column (100 Å pore size, 3.5 μm, 4.6 mm × 250 mm) 40 min run time. The mobile phase used was a mixture of methanol, water and phosphoric acid mixed in the ratio (100:100:1). The elution was isocratic with the flow rate set at 1.5 mL/min. The flavonoids were monitored by the ultraviolet detector set at 270 nm. EZChrom Data System was used for data acquisition, processing and report generation. The resulting chromatograms were recorded and the areas under the major peaks measured. Flavonoids were identified by matching the retention time and their spectral characteristics against those of the standards.
2.4.3. Ethanol and cerulein-induced chronic pancreatitis
After one-week of acclimatization, the animals were randomly divided into four groups of six animals each. Group 1: received normal diet (standard rat chow) for 5 weeks; Group 2: received the normal diet and MEMARB (300 mg/kg body weight/day) orally for the last 3 weeks of the experimental period; Group 3: received an ethanol containing isocaloric diet, and 20 μg/kg body weight of cerulein intraperitoneally for the last 3 weeks Group 4: received an ethanol containing isocaloric diet, 20 μg/kg body weight of cerulein intraperitoneally and MEMARB (300 mg/kg body weight) orally, for the last 3 weeks.
At the end of the experimental period, rats were fasted overnight and anesthetized by intramuscular injection of ketamine hydrochloride [30 mg/kg body wt.] and killed by cervical decapitation. Blood was collected and the serum separated was stored appropriately until further analyses.
2.4.4. Tissue homogenate preparation
Pancreas was removed carefully, washed and homogenized in 0.1M Tris-HCl buffer pH 7.4 and centrifuged at low speed to remove all the cell debris. The supernatant was used for the determination of caspase-1, and antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx), estimation of reduced glutathione (GSH) and estimation of lipid peroxides.
2.4.5. Biochemical investigations
2.4.5.1. Assay of IL-1β
IL-1 beta rat sandwich ELISA Kit (AB100767) was purchased and the assay was performed conforming to manufacturer's instructions.
2.4.5.2. Assay of IL-18
The IL-18 Rat ELISA Kit (KRC2341) was used to quantify serum IL-18. Instructions provided in the kit manual were followed.
2.4.5.3. Assay of caspase-1
For the colorimetric assay of caspase-1 activity in serum and pancreatic homogenate, the method of Thornberry11 was adopted. The pancreas was homogenised in a lysis buffer (25 mM HEPES pH 7.5), 1 mM EDTA, 10 μg of aprotinin/mL, 10 μg of leupeptin/mL, 2 mM dithiothreitol at 5mL/100 mg of pancreas tissue. Extracts were centrifuged at 15,000 g for 30 min at 4 °C. The supernatant was recentrifuged at 200,000 g for 1 h at 4 °C and the supernatant used for the measurement of capase-1 activity. The assay is based on the spectrophotometric detection of the chromophore p-nitroanilide (pNA), released from the labelled substrate YVAD-pNA by caspase-1, at 400 or 405 nm. Activity was expressed as pg/mL serum and pM/mg protein.
2.4.5.4. Assay of myeloperoxidase
The supernatants of cell free extracts were assayed for MPO as described by Bradley et al.12 Briefly, the rate at which a coloured product formed during the MPO dependent reaction with o-dianisidine dihydrochloride (0.167 mg/mL) was measured kinetically at 460 nm and the activity was expressed as units/mg protein.
2.4.5.5. Assay of serum lipase and serum alpha-amylase
Serum lipase and serum alpha-amylase levels were determined by turbidimetry and colorimetry respectively, following instructions provided in the kit manual.
2.4.5.6. Estimation of lipid peroxides
Thiobarbituric acid reactive substances (TBARS) assay was performed to determine level of malondialdehyde in the pancreas according to the protocol of Draper and Hadley.13 Briefly, the reaction mix which consisted of 0.5 mL pancreatic homogenate, 10% PTA and TBA was incubated in a boiling water bath, cooled and centrifuged. Absorbance of the pink chromogen formed was measured at 540 nm to determine the level of malondialdehyde formation which was expressed as nM/100 mg tissue protein. Estimation of 4-HNE (4-hydroxynonenal) in the pancreas was done following the method of Kinter et al.14 where, 4-HNE in the sample was derivatized with dinitrophenyl hydrazine (DNPH). The product formed was extracted 3 times with hexane, evaporated to dryness and solubilized with methanol prior to measurement of absorbance at 350 nm. Levels of 4-HNE in the samples was expressed as μM/mg tissue protein.
2.4.5.7. Estimation of reduced glutathione and antioxidant enzymes
GSH level in the pancreas was measured following the protocol of Moron et al.15 DTNB was added to the pancreatic homogenate and the yellow complex formed was estimated spectrophotometrically at 412 nm and the level was expressed as mg/gm tissue protein. Glutathione peroxidase (GPx) was assayed according to the method of Flohé and Günzler16 where a specific volume of the enzyme preparation was allowed to react with H2O2 for a specified time period and the remaining GSH was estimated by Ellman's reaction. Activity expressed as nM of glutathione oxidized/min/mg protein. Superoxide dismutase (SOD) activity was measured according to the protocol of Kakker et al.17 The inhibition of reduction of nitroblue tetrazolium (NBT) to blue coloured formazan in the presence of phenazine methosulfate (PMS) and NADH was measured at 560 nm using n-butanol as blank. Following the method of Sinha et al.,18 catalase activity was measured as the amount of enzyme required to decompose hydrogen peroxide in the presence of dichromate and acetic acid. Absorbance of chromic acetate formed was measured at 620 nm. The enzyme activity was expressed as μM of H202 consumed/min/mg protein.
2.4.5.8. Estimation of protein
Bradford method19 was used to determine the protein concentration in the tissue homogenate. The protein level was used to calculate the enzyme activity in tissue homogenate.
2.4.6. Histological assessment
Freshly dissected pancreas was washed with ice-cold 0.9% saline and fixed in 10% formo-saline for 24 h. The tissue specimens were dehydrated with alcohol, cleaned with methyl benzoate and embedded in paraffin wax. Sections in cryostat from snap frozen tissues were cut into 5 μM thickness and stained with haematoxylin and eosin for microscopic evaluation.
2.4.7. Statistical analyses
The statistics software package (SPSS for Windows V.10) was used for the data analyses. The statistical significance of mean values between different groups was determined by applying one way ANOVA with post hoc Bonferroni test and the P value < 0.05 was considered as significant.
3. Results
3.1. Phytochemical screening by GC-MS
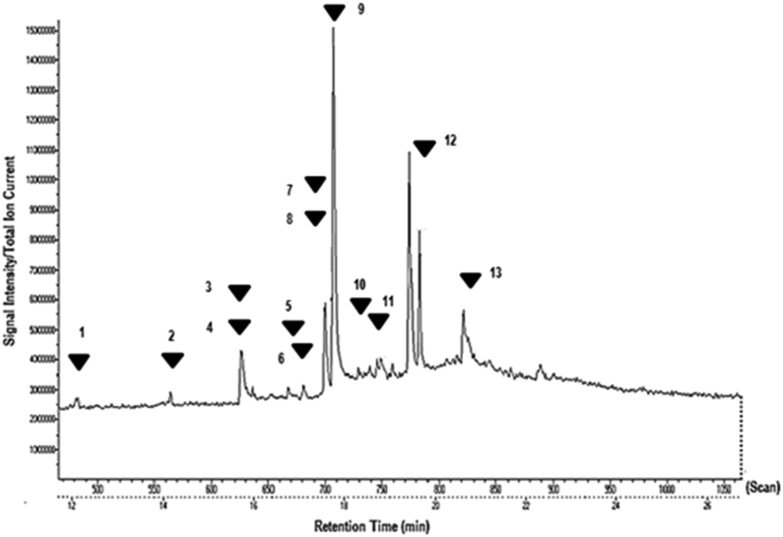
GC-MS analysis revealed the presence of the following compounds: 2H-1-Benzopyran-2-one (Coumarin), Eugenol, 5,7-dihydroxyflavone (Chrysin), 5,7-dihydroxyisoflavone (Mefenamic acid), 4′5,7-Trihydroxy isoflavone (Genistein), 4-H1-Benzopyran-4-one, 5,7-dihydroxy-2-(4-hydroxyphenyl)- (Apigenin), 4′-methoxy-5,7-dihydroxy isoflavone (Biochanin A), Psi-baptigenin, Rhein, Morin, 5,7-dimethoxy flavone, Vitamin E, 8-glycosyl apigenin (Vitexin). Table 1 lists out their retention times and bioactivities. Fig. 1 shows the GC-MS chromatogram of MEMARB.
Table 1.
List of compounds identified in the methanolic extract of Morus alba root bark by GC-MS.
| No. | RT (min) | Name of the compound | Molecular formula | Molecular Weight (g/mol) | Bioactivityab |
|---|---|---|---|---|---|
| 1 | 12.1 | 2H-1-Benzopyran-2-one (Coumarin) | C9H6O2 | 146.14 | Venotonic |
| 2 | 14.5 | Eugenol | C10H12O2 | 164.2 | Improves cell-mediated immunity, local antiseptic |
| 3 | 15.72 | Chrysin | C15H10O4 | 254.24 | Antiinflammatory, antioxidative, anti-hypercholesterolemic |
| 4 | 15.72 | 5,7-dihydroxyisoflavone (Mefenamic acid) | C15H10O4 | 254.23 | Anti-inflammatory, inhibitor of prostaglandin synthesis, analgesic,anti-pyretic |
| 5 | 16.75 | 4′,5,7-Trihydroxy isoflavone (Genistein) | C15H10O5 | 270.24 | Anti-angiogenic, vasculoprotective |
| 6 | 17.1 | Apigenin | C15H10O5 | 270.4 | Anti-inflammatory, anti-proliferative, anti-metastatic |
| 7 | 17.57 | Biochanin A | C16H12O5 | 284.27 | Anti-inflammatory,antidiabetic, anticarcinogenic, lipid metabolism regulator |
| 8 | 17.57 | Pseudobaptigenin | C16H10O5 | 282.24 | Nutrient isoflavone |
| 9 | 17.75 | Rhein (Cassic acid) | C15H8O6 | 284.22 | Anti-microbial, antibiotic, apoptotic, antiproliferative |
| 10 | 18.55 | Morin | C15H10O7 | 302.24 | Anti-inflammatory, antihypertensive, anti-angiogenic, hepatoprotective, neuroprotective |
| 11 | 18.8 | 5,7-dimethoxy flavone | C17H14O4 | 282.29 | Anti-proliferative, apoptotic |
| 12 | 19.7 | Vitamin E | C29H50O2 | 430.71 | Anti-oxidant |
| 13 | 20.63 | 1,6-Heptadiene-3,5 dione,1,7-bis(4-hydroxy-3-methoxy phenyl)-(Curcumin) | C21H20O6 | 368.38 | Anti-inflammatory, anti-oxidant, antimicrobial, anticancer |
| 14 | 20.67 | Vitexin | C21H20O10 | 432.38 | Anti-inflammatory, anti-cancer, neuroprotective |
Pubchem.
Human Metabolome Database (Ref).41
Fig. 1.
Total Ion Chromatogram generated from the GC-MS analysis of the methanolic extract of Morus alba root bark: 1) 2H-1-Benzopyran-2-one (Coumarin) 2) Eugenol 3) 5,7-dihydroxyflavone (Chrysin) 4) 5,7-dihydroxyisoflavone (Mefenamic acid) 5) 4′5,7-Trihydroxy isoflavone (Genistein) 6) 4-H1-Benzopyran-4-one, 5,7-dihydroxy-2-(4-hydroxyphenyl)- (Apigenin) 7) 4′-methoxy-5,7-dihydroxy isoflavone (Biochanin A) 8) Psi-baptigenin 9) Rhein 10) Morin 11) 5,7-dimethoxy flavone 12) Vitamin E 13) 8-glycosyl apigenin (Vitexin).
3.2. Phytochemical analysis of MEMARB by HPLC-UV analysis
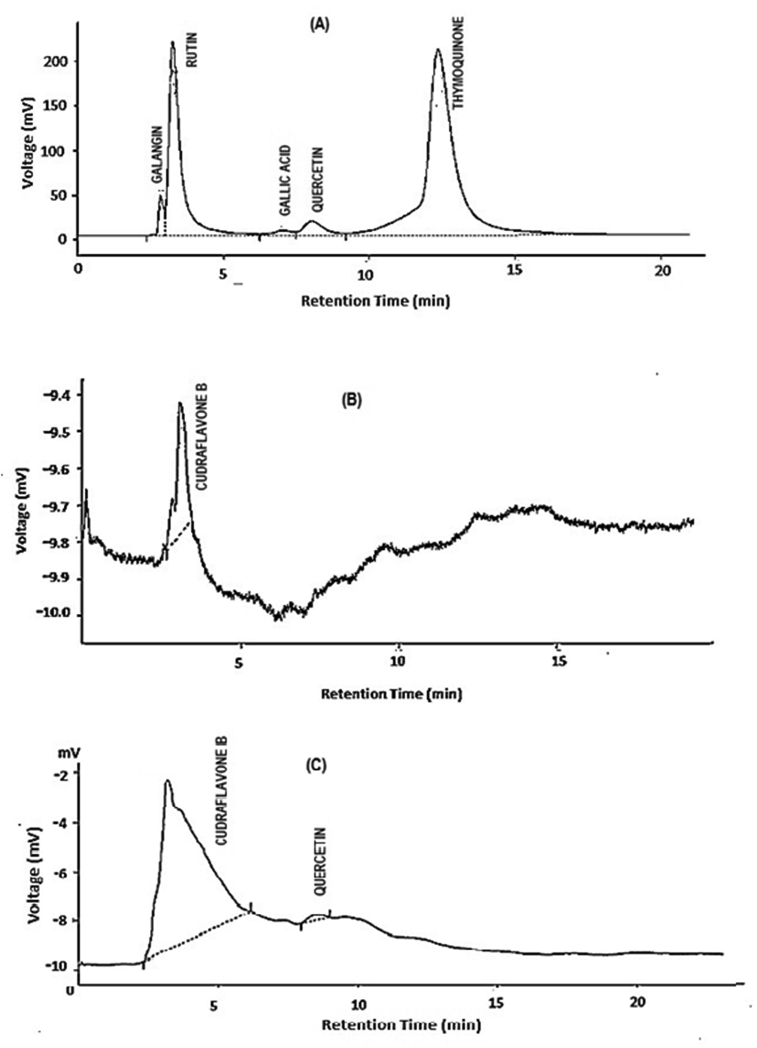
Fig. 2 displays the HPLC-UV chromatograms. HPLC-UV chromatogram of MEMARB generated two peaks at 270 nm with retention times 3.19 and 8.53 min (Fig. 2C). The peaks were identified to be Cudraflavone B (0.9 mg/gm; RT 3.19 min) and Quercetin (0.1 mg/gm; RT 8.53 min), by matching the retention times with those of the reference standards run under the same conditions (Fig. 2A and B).
Fig. 2.
High Performance Liquid Chromatography (HPLC) chromatograph of (A) reference standards; (B) Cudraflavone B standard; (C) MEMARB. Conditions: Mobile phase: methanol: acetic acid: water (100:100:1); flow rate: 1.5 ml/min; Run time: 40 min. Wavelength: 270 nm.
3.3. Effect of MEMARB on the levels of serum lipase and serum alpha-amylase
Table 2 shows the levels of serum lipase and serum alpha-amylase in the experimental groups. Rats administered ethanol and cerulein showed significant elevation in the levels of serum lipase and serum amylase when compared to the normal control animals (p < 0.05). Serum levels of these markers of pancreatic injury reversed towards normal values in MEMARB treated rats (p < 0.05). Rats fed normal diet and MEMARB showed normal levels of the enzymes.
Table 2.
Effect of MEMARB on the levels of serum lipase and serum alpha-amylase.
| Group | Lipase (IU/L) | Amylase (IU/L) |
|---|---|---|
| Normal Control | 182 ± 19 | 2732.21 ± 360.65 |
| MEMARB Control | 156 ± 16NS | 2614.02 ± 316.3NS |
| EtOH + Cerulein | 425 ± 50* | 4772.47 ± 629.97* |
| EtOH + Cerulein + MEMARB | 236 ± 31* | 3198.78 ± 422.24* |
Data were analysed by one-way ANOVA followed by post-hoc Bonferroni test. Values are expressed as mean ± S.D. of 6 rats in each group. Statistical significance was calculated by comparing normal control vs. MEMARB control; Control vs. EtOH + Cerulein; EtOH + Cerulein vs. EtOH + Cerulein + MEMARB. *p = 0.000; NS non-significant.
3.4. Effect of MEMARB on serum and tissue inflammatory markers
Table 3 charts out the activity levels of MPO and caspase-1 in the pancreas and the levels of caspase-1, IL-1beta, and IL-18 in the serum of the experimental animals. Notable increase in the levels of these inflammatory markers was observed in the ethanol and cerulein administered rats when compared to rats co-administered MEMARB. A non-significant reduction of the inflammatory markers was seen in the MEMARB control group.
Table 3.
Effect of MEMARB on serum and tissue inflammatory markers.
| Group | Caspase-1 |
MPO |
IL-1beta |
IL-18 |
|
|---|---|---|---|---|---|
| Serum (pg/ml) | Pancreas (pM/mg protein) | Pancreas (Units/mg protein) | Serum pg/ml | Serum pg/ml | |
| Normal Control | 12.6 ± 1.8 | 12.6 ± 1.5 | 2.16 ± 0.4 | 15.5 ± 2.6 | 200.5 ± 30.1 |
| MEMARB Control | 11.5 ± 1.3NS | 10.3 ± 1.2NS | 1.82 ± 0.2NS | 13.6 ± 2.1NS | 170 ± 21.6NS |
| EtOH + Cerulein | 21.5 ± 2.8* | 49.6 ± 5.2* | 3.98 ± 0.4* | 31.5 ± 4.5* | 360.5 ± 42* |
| EtOH + Cerulein + MEMARB | 14.3 ± 1.8* | 20.5 ± 2.8* | 2.56 ± 0.3* | 20.5 ± 3.1* | 240 ± 32* |
Data were analysed by one-way ANOVA followed by post-hoc Bonferroni test. Values are expressed as mean ± S.D. of 6 rats in each group. Statistical significance was calculated by comparing normal control vs. MEMARB control; Control vs. EtOH + Cerulein; EtOH + Cerulein vs. EtOH + Cerulein + MEMARB. *p = 0.000; NS non-significant.
3.5. Effect of MEMARB on the redox status – levels of TBARS, 4-HNE and reduced glutathione (GSH)
Table 4 displays the levels of lipid peroxidation products and the level of the antioxidant glutathione in the pancreatic tissue. Increase in the levels of lipid peroxidation products were found to be highly significant in the ethanol and cerulein administered experimental group while the level of the molecular antioxidant GSH was found to be decreased, when compared to the normal rats. MEMARB administration markedly reduced the levels of TBARS and 4-HNE and increased levels of GSH. Levels of TBARS, 4-HNE and GSH showed no significant change in MEMARB control rats.
Table 4.
Effect of MEMARB on the redox status – levels of TBARS, 4-HNE and reduced glutathione (GSH).
| Group | TBARS (nM/100 mg protein) |
4-HNE (μM/g protein) |
GSH (mg/g protein) |
|---|---|---|---|
| Pancreas | Pancreas | Pancreas | |
| Normal Control | 1776.00 ± 225.55 | 2.000 ± 0.24 | 96.015 ± 11.62 |
| MEMARB Control | 1782.40 ± 190.72NS | 2.13 ± 0.21NS | 98.82 ± 12.06NS |
| EtOH + Cerulein | 3408.12 ± 415.8* | 8.100 ± 0.96* | 49.600 ± 6.00* |
| EtOH + Cerulein + MEMARB | 1806.66 ± 229.44* | 5.062 ± 0.60* | 93.330 ± 11.29* |
Data were analysed by one-way ANOVA followed by post-hoc Bonferroni test. Values are expressed as mean ± S.D. of 6 rats in each group. Statistical significance was calculated by comparing normal control vs. MEMARB control; Control vs. EtOH + Cerulein; EtOH + Cerulein vs. EtOH + Cerulein + MEMARB. *p = 0.000; NS non-significant.
3.6. Effect of MEMARB on the redox status – activity levels of antioxidant enzymes
Activity levels of the antioxidant enzymes, GPx, SOD and catalase are shown in Table 5. In the ethanol and cerulein-induced pancreatitis group, levels of GPx, SOD and catalase were significantly depleted relative to the normal control group. No significant changes were observed in the MEMARB control animals. In the experimental group co-administered with MEMARB, the levels of the antioxidant enzymes were restored.
Table 5.
Effect of MEMARB on the redox status - Activity levels of antioxidant enzymes.
| Group | GPx (nM GSH consumed/min/mg protein) |
SOD (U/mg protein) |
Catalase (U/mg protein) |
|---|---|---|---|
| Pancreas | Pancreas | Pancreas | |
| Normal Control | 324.5 ± 42.5 | 14.2 ± 1.49 | 114.4 ± 15.90 |
| MEMARB Control | 339.2 ± 40.02NS | 14.5 ± 1.65NS | 112.1 ± 16.37NS |
| EtOH + Cerulein | 233.6 ± 29.43* | 8.2 ± 0.97* | 72.8 ± 7.79* |
| EtOH + Cerulein + MEMARB | 353.33 ± 46.29* | 11.7 ± 1.51* | 106.3 ± 12.65* |
Data were analysed by one-way ANOVA followed by post-hoc Bonferroni test. Values are expressed as mean ± S.D. of 6 rats in each group. Statistical significance was calculated by comparing normal control vs. MEMARB control; Control vs. EtOH + Cerulein; EtOH + Cerulein vs. EtOH + Cerulein + MEMARB. *p = 0.000; NS non-significant.
3.7. Effect of MEMARB on the histology of the pancreatic and liver tissue
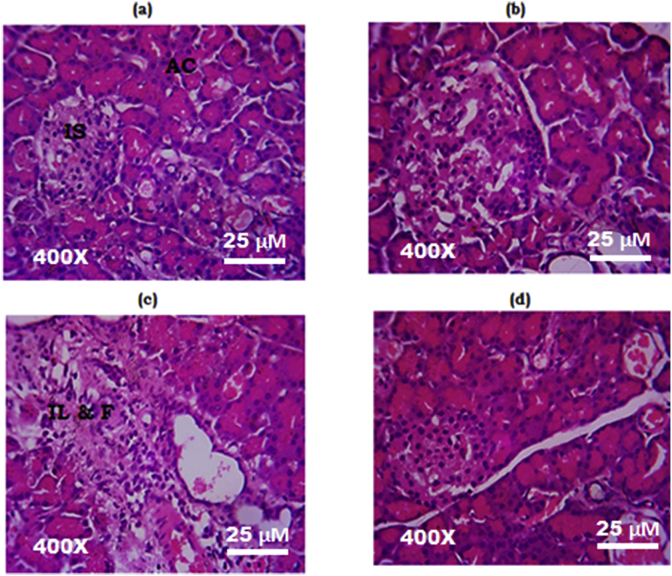
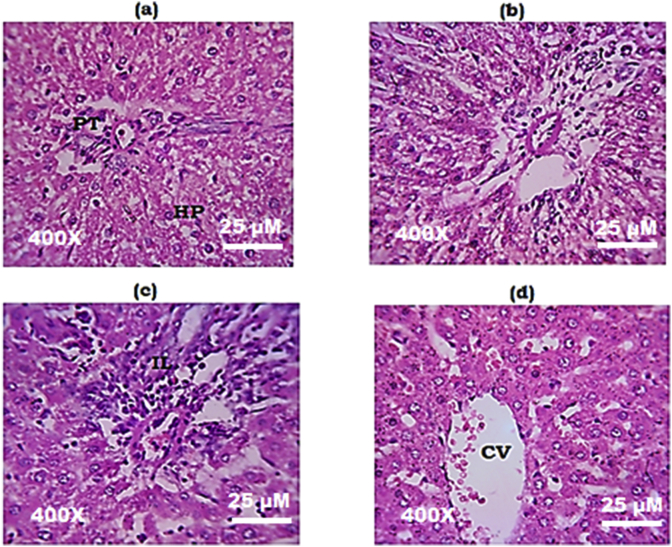
Fig. 3 and Fig. 4 show photomicrographs of hematoxylin and eosin stained sections (400X) of the pancreas and the liver respectively. Pancreatic and liver sections from normal control and MEMARB control rats showed normal tissue architecture (Fig. 3 (a, c); Fig. 4 (a, c)). Section of the pancreas from the ethanol and cerulein administered group showed neutrophil infiltration and fibrotic changes (Fig. 3 (c)) and the liver section from the same group showed tissue infiltration of neutrophils (Fig. 4c). Tissue architecture of the pancreas (Fig. 3d) and the liver (Fig. 4d) was found to be restored to normal in MEMARB treated experimental group.
Fig. 3.
Histology of the pancreas (H&E stain, 400X): (a) & (b) Photomicrograph of pancreas from normal control and MEMARB control rats with intact acini and islets. (c) Photomicrograph of pancreas from ethanol and cerulein administered rats showing neutrophil infiltration and fibrosis. (d) Photomicrograph of pancreas from MEMARB co-administered rats showed restored tissue architecture. H&E: Haematoxylin & Eosin; AC: acini; IS: islet; IL & F: inflammation and fibrosis.
Fig. 4.
Histology of the liver (H&E stain, 400X): (a) & (b) Photomicrograph of liver from normal control and MEMARB control rats with normal tissue structure (c) Photomicrograph of liver from ethanol and cerulein administered rats showing neutrophil infiltration (d) Photomicrograph of liver from MEMARB co-administered rats with reduced inflammatory cell infiltration. H&E: Haematoxylin & Eosin; PT: portal triad; HP: hepatocytes; CV: central vein; IL: inflammation.
4. Discussion
In this investigation, the anti-inflammatory and antioxidant capacity of MEMARB was evaluated in vivo in the best-characterised ethanol and cerulein-induced rat model of experimental pancreatitis.
Supramaximal doses of cerulein, an analogue of the physiological secretagogue cholecystokinin, stimulates protein-rich pancreatic secretions and disturbs the actin cytoskeleton and vesicular transport, thereby suppressing the export of digestive enzymes. This precipitates premature zymogen activation within the acinar cells with subsequent tissue injury and pathology similar to human pancreatitis. Ethanol when co-administered potentiates the effect of cerulein.20 Ethanol is the principal etiological factor for acute and chronic pancreatitis with multifarious effects on the pancreas. Pancreas metabolizes ethanol via oxidative and non-oxidative pathways generating the toxic by-products, acetaldehyde and fatty acid ethyl esters (FAEE), respectively. Acetaldehyde activates pancreatic stellate cells (PSCs) promoting fibrosis while both ethanol and acetaldehyde regulate transcription factors NF-kappaB and AP1. FAEEs promote accumulation of cholesteryl esters leading to lysosomal membrane fragility, destabilization of zymogen granule membranes and cause a sustained increase in intra acinar calcium levels. Recent research also indicates that ethanol influences cholinergic pathways and CFTR channels in the pancreatic ductal epithelium.21., 22., 23.
Presently, there being no specific and effective medication to treat highly morbid pancreatic inflammation, it becomes necessary to seek potential therapeutic agents. The diverse array of secondary metabolites present in plants has become the focus of research on inflammation. According to recent reports, apart from their antioxidant properties, polyphenols including flavonoids have been found to have far-reaching modulatory effects on key mitochondrial pathways thus protecting the cell from the toxic effects of xenobiotics and ROS.24
Root bark of Morus alba, a highly valued traditional Chinese medicine, is rich in Diels-Alder type adducts, stilbenes, alkaloids and flavonoids.25 Prenylated flavonoids, a sub-class of flavonoids abundant in Morus spp., have a lipophilic prenyl side-chain attached to the flavonoid skeleton. Prenylation is said to confer flavonoids with improved bioactivities.9 Morus alba root bark has been shown to have significant antioxidant, anti-inflammatory and anti-cancer properties.26 Cudraflavone B, a prenylated flavone from the root bark of Morus alba is credited with potent anti-inflammatory properties. Current literature indicates that Morus alba root bark is a rich source of this bioactive principle which could be a potential anti-inflammatory drug lead.27
GC-MS is the method of choice for the screening of plant bioactive constituents. GC-MS results showed the presence of a variety of phytonutrients (Fig. 1; Table 1). HPLC-UV methods are widely used to identify and quantify prenyl flavonoids in biological samples. Cudraflavone B is reported to be a major prenyl flavonoid bioactive in M.alba root bark, with impressive anti-inflammatory effects.28 HPLC-UV analysis of MEMARB (Fig. 2) confirmed the presence of a significant quantity of this compound in the sample and could be responsible for its anti-inflammatory properties. HPLC analysis also revealed the presence quercetin, a potent anti-inflammatory, anti-oxidant and immunomodulatory flavonoid.29,30
Significant increase in the serum levels of the digestive enzymes, lipase and pancreatic alpha-amylase is seen in pancreatic diseases.31 Serum lipase and pancreatic alpha-amylase levels were measured to assess the extent of injury to the pancreatic tissue caused by EtOH and cerulein administration and the pancreato-protective effect of MEMARB. Both serum lipase and pancreatic alpha-amylase levels were found to be increased in EtOH and cerulein administered animals. The amount of these enzymes was restored to levels close to normal physiological levels in MEMARB treated rats underlining the pancreato-protective potency of MEMARB.
Inflammatory pathways underpin the basic pathology of pancreatitis. Sterile inflammation in pancreatitis leads to the activation of NLRP3 inflammasome components caspase – 1 and its effectors- IL-1beta, and IL-18, the key determinants of the extent of injury.32,33 Serum levels of caspase-1 and the pro-inflammatory cytokines, IL-1beta and IL-18 are determined to evaluate the progress of inflammation. Levels of the pro-inflammatory cytokines and caspase-1 were found to be higher than normal in pancreatitis-induced rats. MEMARB co-administered rats showed near-normal levels of these markers of inflammation adding further evidence to the anti-inflammatory property of MEMARB. Oxyresveratrol, another major bioactive in white mulberry root bark, has been shown to modulate inflammatory responses by inhibiting MEK/ERK pathway that leads to the activation of pro-inflammatory cytokines.34 So, along with cudraflavone B, oxyresveratrol could synergistically contribute to the anti-inflammatory nature of Morus alba. Oxyresveratrol from Morus bombycis, a Japanese mulberry, has been experimentally proven to attenuate NLRP3 activation which could be linked to its anti-inflammatory potential35 and could account for the NLRP3 attenuating effect of MEMARB.
Neutrophil infiltration is a prominent feature of inflammation in any tissue. Acinar cell injury leads to the release of cytokines that recruits neutrophils. Myeloperoxidase is expressed primarily by the phagocytic neutrophil granulocytes. It generates the powerful oxidant HOCl from H2O2 and Cl− with bactericidal properties. Myeloperoxidase activity is measured to assess the extent of neutrophil infiltration in pancreatic tissue.36 Our results show significant elevation in MPO activity in the pancreas of EtOH and cerulein administered rats which could mirror pancreatic tissue inflammation induced by these molecules. This increase in inflammatory activity is countered in MEMARB co-administered animals as evidenced by a fall in MPO activity in the pancreas. MEMARB bio constituents and cudraflavone B could be implicated in this anti-inflammatory effect.
Reactive oxygen and reactive nitrogen species (ROS/RNS) play key roles in the pathogenesis of acute and chronic pancreatitis. Pancreatic acinar cells contain phase I cytochrome P450 (CYP 450) enzymes and phase II conjugation proteins for the metabolism of xenobiotics. Oxidative stress may result from xenobiotic overload, including alcohol, tobacco smoke, dietary toxins etc., and the increased activity of these enzymes, leading to overwhelming increase in ROS. Free radicals may deplete the endogenous antioxidant molecules (mainly GSH) and the major antioxidant enzymes, glutathione peroxidase (GPx), Superoxide dismutase (SOD) and catalase and may also lead to an increase in the peroxidation of cellular components, mainly unsaturated lipids. In this study, the redox status of pancreatic tissue in diseased rats versus the MEMARB-treated rats was determined by measuring the levels of GSH, GPx, SOD, catalase, TBARS and 4-HNE. Depletion of antioxidant enzymes GPx, SOD, and catalase was seen in the pancreatic tissue and blood of the EtOH and cerulein administered rats. The levels were restored in MEMARB co-administered rats. A significant drop in the molecular antioxidant GSH and an elevation in the lipid peroxidation products malondialdehye and 4-HNE were noted in the results obtained from the pancreatitis-induced group. With MEMARB treatment the level of GSH increased and the levels of malondialdehye and 4-HNE decreased to relatively normal levels. MEMARB with a large number of polyphenols like quercetin could be responsible for this antioxidant effect.37,38 Oxyresveratrol from Morus alba root cortex is a potent lipid peroxidation inhibitor evidenced by the inhibitory effect against FeSO4/H2O2-induced microsomal peroxidation. It has also been shown to scavenge DPPH radical in vitro.39 A present review indicates that the root barks of Morus plants contain diverse polyphenols such as Diels-Alder-type adducts, derivatives of benzofuran, stilbenes and polyhydroxylated alkaloids in addition to flavonoids.40 Generally polyphenols are hydroxyl group containing polar compounds which can donate the protons to neutralize the toxic free radicals. Hence, the antioxidant potential of the test compound could be attributed to the presence of polyphenols.
Histopathological observations of pancreas and liver sections from the respective experimental groups reinforce findings from the biochemical investigations. When compared to the normal control and the drug control groups, the liver and pancreas sections from the diseased group show inflammatory infiltrates and the pancreas shows fibrotic changes. Sections of liver and pancreas from the MEMARB treated group show restored tissue architecture with a significant reduction in leukocyte infiltration and only mild fibrosis in the pancreas proving the pancreato-protective effect of MEMARB.
5. Conclusion
This is the pilot study which authenticates the anti-inflammatory activity of phytonutrients present in the root bark extract of Morus alba. The anti-inflammatory property observed in this study might be due to the effect of flavonoids which could intervene in the formation of cytokines. This study represents the preventive effect of Morus alba on the development of pancreatitis. However, as a future perspective, the same study can be applied on animals with fully developed pancreatitis which would prove its curative effect.
Conflicts of interest
The authors declare no competing interests.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Acknowledgements
We are grateful for the generous gift of Cudraflavone B from Dr. Karel Smejkal, Associate Professor, Department of Natural Drugs, Faculty of Pharmacy, University of Veterinary and Pharmaceutical Sciences, Brno, Czech Republic.
The authors thank the Sophisticated Analytical Instruments Facility, Indian Institute of Technology-Madras, Chennai, India, for providing the infrastructure and technical support for the GC-MS analysis.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Sah R.P., Saluja A.K. Molecular mechanisms of pancreatic injury. Curr Opin Gastroenterol. 2012;28(5):507–515. doi: 10.1097/MOG.0b013e328349e346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Machicado J.D., Rebours V., Yadav D. Epidemiology of Chronic Pancreatitis. Pancreapedia. 2016;1:1–15. American Pancreatic Association. [Google Scholar]
- 3.Ammann R.W. Diagnosis and management of chronic pancreatitis: current knowledge. Swiss Med Wkly. 2006;136:166–174. doi: 10.4414/smw.2006.11182. [DOI] [PubMed] [Google Scholar]
- 4.Yadav D., Lowenfels A.B. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144(6):1252–1261. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu H., Werner J., Bergmann F., Whitcomb D.C., Buchler M.W., Fortunato F. Necro-inflammatory response of pancreatic acinar cells in the pathogenesis of acute alcoholic pancreatitis. Cell Death Dis. 2013;4 doi: 10.1038/cddis.2013.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sendler M., Mayerle J., Lerch M.M. Necroptosis, Apoptosis, Necroptosis, Pyroptosis: it matters how cells die during pancreatitis. Cell Mol Gastroenterol Hepatol. 2016;2:407–408. doi: 10.1016/j.jcmgh.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoque R., Mehal W.Z. Inflammasomes in pancreatic physiology and disease. Am J Physiol Gastrointest Liver Physiol. 2015;308(8):G643–G651. doi: 10.1152/ajpgi.00388.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner H., Bauer R., Melchart D., Pei-Gen X., Staudinger A. Springer Science & Business Media; 2011. Chromatographic Fingerprint Analysis of Herbal Medicines: Thin-layer and High Performance Liquid Chromatography of Chinese Drugs; pp. 535–548. [Google Scholar]
- 9.Yang X., Jiang Y., Yang J. Prenylated flavonoids, promising nutraceuticals with impressive biological activities. Trends Food Sci Technol. 2015;44(1):93–104. [Google Scholar]
- 10.Chan E.W., Phui-Yan L.Y., Siu-Kuin W.O. Phytochemistry, pharmacology, and clinical trials of Morus alba. Chin J Nat Med. 2016;14(1):17–30. doi: 10.3724/SP.J.1009.2016.00017. [DOI] [PubMed] [Google Scholar]
- 11.Thornberry N.A. Interleukin – 1β converting enzyme. Methods Enzymol. 1974;244:615–631. doi: 10.1016/0076-6879(94)44045-x. [DOI] [PubMed] [Google Scholar]
- 12.Bradley P.P., Christensen R.D., Rothstein G. Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood. 1982;60(3):618–622. [PubMed] [Google Scholar]
- 13.Draper H.H., Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- 14.Kinter M., Grimminger L.C., Gillies P.J., Shimshick E.J., Ayers C. Whole blood and plasma concentrations of 4-Hydroxy-2-nonenal in watanabe heritable hyperlipidemic versus New Zealand white rabbits. Biochem Biophys Res Commun. 1994;199(2):671–675. doi: 10.1006/bbrc.1994.1280. [DOI] [PubMed] [Google Scholar]
- 15.Moron M.S., De Pierre J.W., Vik B.M. Levels of glutathione, glutathione reductase and glutathione - S - transferase activities in rat, lung and liver. Biochim Biophys Acta. 1979;582:3170–3185. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 16.Flohé L., Günzler W.A. Meth Enzymol. 1984;105(1):114–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 17.Kakkar P., Das B., Viswanathan P.N. A modified spectrophotometric assay of superoxide dismutase (1984) Indian J Biochem Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- 18.Sinha A.K. Colorimetric assay of catalase. Anal Biochem. 1972;47(2):389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 19.Bradford M.M. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 20.Aghdassi A.A., Mayerle J., Christochowitz S., Weiss F.U., Sendler M., Lerch M.M. Animal models for investigating chronic pancreatitis. Fibrogenesis Tissue Repair. 2011;4(1):26. doi: 10.1186/1755-1536-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vonlaufen A., Wilson J.S., Pirola R.C., Apte M.V. Role of alcohol metabolism in chronic pancreatitis. Alcohol Res Health. 2007;30(1):48–54. [PMC free article] [PubMed] [Google Scholar]
- 22.Lugea A., Gong J., Nguyen J., Nieto J., French S.W., Pandol S.J. Cholinergic mediation of alcohol-induced experimental pancreatitis. Alcohol Clin Exp Res. 2010;34(10):1768–1781. doi: 10.1111/j.1530-0277.2010.01264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maléth J., Balazs A., Pallagi P. Alcohol disrupts levels and function of the cystic fibrosis transmembrane conductance regulator to promote development of pancreatitis (2015) Gastroenterology. 2015;148(2):427–439. doi: 10.1053/j.gastro.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandoval-Acuña C., Ferreira J., Speiski H. Polyphenols and Mitochondria: An Update on their increasingly emerging ROS-scavenging independent actions. Arch Biochem Biophys. 2014;559:75–90. doi: 10.1016/j.abb.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y.N., Liu M.F., Hou W.Z. Bioactive benzofuran derivatives from cortex mori radicis, and their neuroprotective and analgesic activities mediated by mGluR1. Molecules. 2017;22(2):236. doi: 10.3390/molecules22020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eo H.J., Park J.H., Park G.H. Anti-inflammatory and anti-cancer activity of mulberry (Morus alba L.) root bark. BMC Compl Alternative Med. 2014;14(1):200. doi: 10.1186/1472-6882-14-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hošek J., Bartos M., Chudík S. Natural compound cudraflavone B shows promising anti-inflammatory properties in vitro. J Nat Prod. 2011;74(4):614–619. doi: 10.1021/np100638h. [DOI] [PubMed] [Google Scholar]
- 28.Kollar P., Bárta T., Hošek J. Prenylated flavonoids from Morus alba L. cause inhibition of G1/S transition in THP-1 human leukemia cells and prevent the lipopolysaccharide-induced inflammatory response. Evid base Compl Alternative Med. 2013;13 doi: 10.1155/2013/350519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu F., Nie X., Shi J. Quercetin inhibits LPS-induced inflammation and ox-LDL-induced lipid deposition. J Chromatogr Sci. 2004;42:378–382. doi: 10.3389/fphar.2017.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin X., Lin C.H., Zhao T. Quercetin protects against heat stroke-induced myocardial injury in male rats: antioxidative and anti-inflammatory mechanisms. Chem Biol Interact. 2017;265:47–54. doi: 10.1016/j.cbi.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Frulloni L., Patrizi F., Bernardoni L., Cavallini G. Pancreatic hyperenzymemia: clinical significance and diagnostic approach. JOP. 2005;6(6):536–541. [PubMed] [Google Scholar]
- 32.Ramadani M., Gansauge F., Schlosser S., Yang Y., Beger H.G., Gansauge S. Overexpression of caspase-1 in pancreatic disorders: implications for a function besides apoptosis. J Gastrointest Surg. 2001;5(4):352–358. doi: 10.1016/s1091-255x(01)80061-5. [DOI] [PubMed] [Google Scholar]
- 33.Hoque R., Malik A., Gorelick F., Mehal W. The sterile inflammatory response in Acute Pancreatitis. Pancreas. 2012;41(3):353–357. doi: 10.1097/MPA.0b013e3182321500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y.C., Tien Y.J., Chen C.H. Morus alba and active compound oxyresveratrol exert anti-inflammatory activity via inhibition of leukocyte migration involving MEK/ERK signalling. BMC Compl Alternative Med. 2013;13(1):45. doi: 10.1186/1472-6882-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh N.H., Han J.W., Shim D.W. Anti-inflammatory properties of Morus bombycis Koidzumi via inhibiting IFN-β signaling and NLRP3 inflammasome activation. J Ethnopharmacol. 2015;24(176):424–428. doi: 10.1016/j.jep.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 36.Sandoval D., Gukovskaya A., Reavey P. The role of neutrophils and platelet-activating factor in mediating experimental pancreatitis. Gastroenterology. 1996;111:1081–1091. doi: 10.1016/s0016-5085(96)70077-x. [DOI] [PubMed] [Google Scholar]
- 37.Shahedi K., Pandol S.J., Hu R. Oxidative stress and alcoholic pancreatitis. J Gastroenterol Hepatol Res. 2013;2(1):335–342. [Google Scholar]
- 38.Bhardwaj P., Yadav R.K. Chronic pancreatitis: role of oxidative stress and antioxidants. Free Radic Res. 2013;47(11):941–949. doi: 10.3109/10715762.2013.804624. [DOI] [PubMed] [Google Scholar]
- 39.Chung K.O., Kim B.Y., Lee M.H. In-vitro and in-vivo anti-inflammatory effect of oxyresveratrol from Morus alba L. J Pharm Pharmacol. 2003;55(12):1695–1700. doi: 10.1211/0022357022313. [DOI] [PubMed] [Google Scholar]
- 40.Wei H., Zhu J.J., Liu X.Q., Feng W.H., Wang Z.M., Yan L.H. Review of bioactive compounds from root barks of Morus plants (Sang-Bai-Pi) and their pharmacological effects. Cogent Chem. 2016;2(1) [Google Scholar]
- 41.Wishart D.S., Jewison T., Guo A.C. HMDB 3.0-the human Metabolome database in 2013. Nucleic Acids Res. 2013;41(D1):D801–D807. doi: 10.1093/nar/gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]