Abstract
In the present review article, the phytochemical, antioxidant and pharmacological studies are congregated and summarized concerning the current knowledge of the phenolic compounds of a traditional medical plant Acacia confusa in Taiwan. This plant is native to Taiwan and South-East Asia. It possesses major pharmacological activities, including antioxidant and radical scavenging activity, hepatoprotective effect, xanthine oxidase inhibition, semicarbazide-sensitive amine oxidase inhibition, angiotensin I converting enzyme inhibition, antihyperuricemic effect and anti-inflammatory activity. Phenolic compounds, especially flavonoids, flavonol glycoside and phenolic acid derivatives, are the main phytochemical compounds isolated from different plant parts of A. confusa. Recent interest in this species has focused on pharmacological investigations of the phytochemicals which exhibit potent antioxidant activity based on the multiple phenolic functionalities. The consequence of this review will further extend the potential applications of this plant and offer persuasive support to its future use in the fields of clinical medicine and health functional food.
Keywords: Antioxidant, Flavonoids, Pharmacological properties, Acacia confusa, Leguminosae, Functional food
Graphical abstract
1. Introduction
Acacia confusa Merr. (Leguminosae) is an endemic species of Taiwan and is one of the most widespread plants. A. confusa is a very adaptable and fast-growing plant suited to living in extreme conditions.1 It can be found along the coast and river, slightly in from the high tide mark; up to the temperate forests of the higher mountains, but well below the freeze line. However, it usually tends to be more common below 1000 m elevation and temperatures in the range of 20–30 °C. In the wild, it can grow in the soils with poor nutritional value, such as hard clay, silt, dirt and rocky plain and hills. In southern Taiwan, where the winter months can be totally without rain and the summer months can have very heavy rain and typhoons, this plant is able to withstand and grow quite comfortably in a range of climates.2 A. confusa, unlike many plant species of the Leguminosae family, forms a symbiotic association with rhizobia in which its root plays host to them. Many of the associations fix nitrogen from the atmosphere and eventually make it available to the plant and fertile to the surrounding soil.3
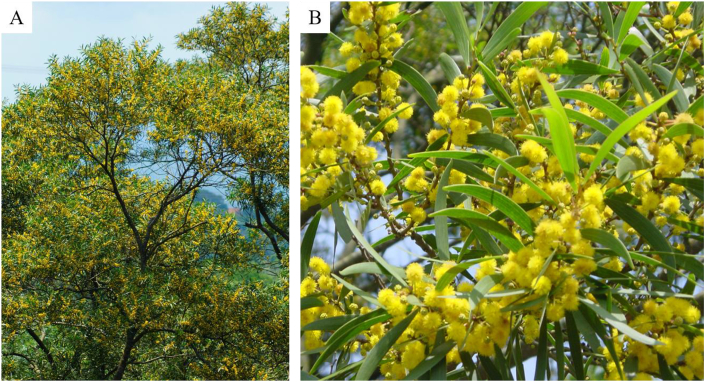
A. confusa is an evergreen plant (Fig. 1A). The stems and roots are incredibly hard and extremely strong. The sapwood is pale yellow and the heartwood is chocolate brown from the tannins. The barks are rough without ridge and spines. The leaves only appear on seedlings and young plants, and the phyllodes grow on mature plants (Fig. 1B). The phyllodes are dull green, 8–10 cm long and 10–15 mm wide, alternate, narrowly curved-shaped, slightly thickened, hairless, with 3–5 slim parallel veins from the base. The flower clusters of bright yellow balls roughly in 6–20 mm diameter emerge from the twigs (Fig. 1B). In Taiwan, flowering season is usually the coming summer, but, sometimes, it may occur year round. The fruits (pods) are narrow and flat, 5–10 cm long, 8–10 mm wide, dark brown, and split open. The seeds are beanlike, around 5 mm long, 4–8 pre pod, elliptical, dark brown, slightly flattened and shiny.2
Fig. 1.
Plants (A), flowers and phyllodes (B) of Acacia confusa.
In Taiwan, A. confusa was used as a traditional medicine. The aqueous extract of A. confusa leaves was applied to cure wounds and antiblood stasis.1 The commercial and industrial uses of A. confusa were fire wood, charcoal-making, railroad tie, mining construction and mushroom cultivation.4 For water and soil conservation, this plant is planted in the wilderness for a long time because its root system is extremely strong and can grow extensively and deeply into the ground. Recently, this plant has shown great potential for air pollution prevention because of its remarkable carbon dioxide sequestration ability and foliar dust retention.5 The bark and wood of A. confusa, like many other Acacia species, are rich in tannins which are used to dye and stain clothes and tan leather. Due to the high content of tannins and phenolic compounds, many studies have focused on the phytochemistry of A. confusa extract in recent years.6 The aim of this contribution is to review the current literatures on the bioactivities and active compounds of A. confusa.
2. Phytochemistry
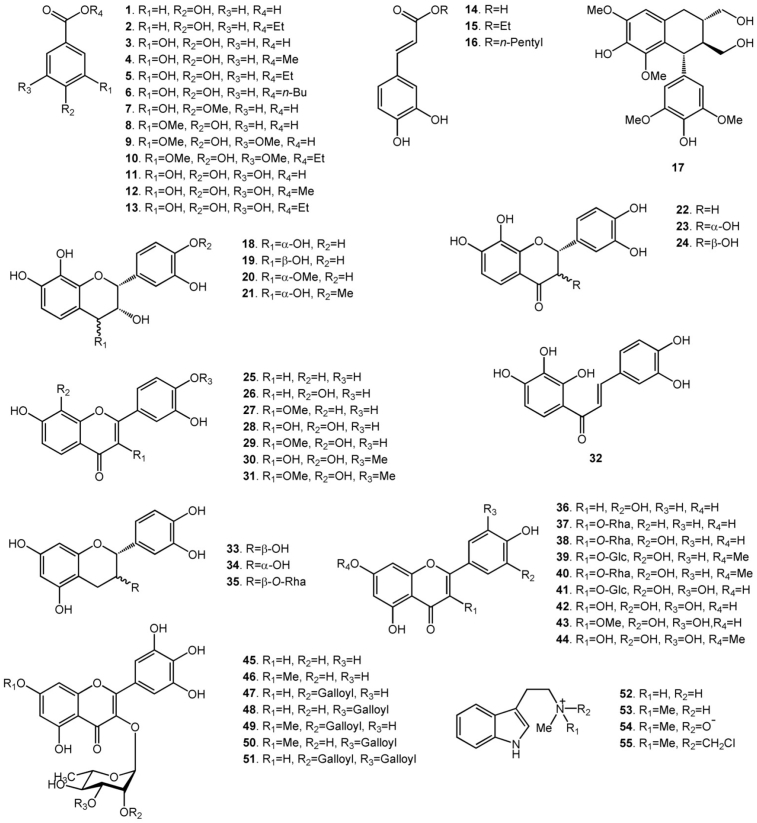
Secondary metabolite researches have been carried out on A. confusa and have led to the isolation of phenolic acid derivatives and flavonoids from its different plant parts. There have been 55 compounds, including 13 phenolic acid derivatives, 3 caffeic acid derivatives, 21 flavonoids, 13 flavonol glycosides, 1 lignan and 4 alkaloids, isolated from the extracts of different plant parts of A. confusa. The heartwood extract and the root extract contain flavonoids and phenolic acid derivatives. The bark extract is almost phenolic acid derivatives and caffeic acid derivatives. The extracts of the leaves, branches, twigs, flowers and buds contain flavonol glycosides, some flavonoids and phenolic acid derivatives. The flavonoids in the heartwood and the root extract contain 7,8-dihydroxyflavonoids rather than the usual 5,7-dihydroxyflavonoids in other parts. The following section collected the chemical names of the isolated compounds, and their chemical structures are shown in Fig. 2.
Fig. 2.
Compounds isolated from Acacia confusa.
2.1. Constituents in the wood
Ethanol (70%) was used to extract the heartwood. For phenolic acid derivatives, like 3,4-dihydroxybenzoic acid (3), 3,4-dihydroxybenzoic acid methyl ester (4), 3,4-dihydroxybenzoic acid ethyl ester (5) and 3-hydroxy-4-methoxybenzoic acid (7) were isolated.7,8
Thirteen flavonoids, including 3 flavanols, 1 flavanone, 1 flavanonol, 2 flavones, 5 flavonols and 1 chalcone, were isolated.7, 8, 9 Three flavanols are melacacidin (18), 4-O-methylmelacacidin (20) and 4′-O-methylmelacacidin (21). One flavanone is 7,8,3′,4′-tetrahydroxyflavanone (22). One flavanonol is trans-3,7,8,3′,4′-pentahydroxyflavanone (24). Two flavones are 7,3′,4′-trihydroxyflavone (25) and 7,8,3′,4′-tetrahydroxyflavone (26). Five flavonols are 7,3′,4′-trihydroxy-3-O-methylflavonol (27), melanoxetin (28), transilitin (29), 4′-O-methylmelanoxetin (30) and 4′-O-methyltransilitin (31). One chalcone is okanin (32).
2.2. Constituents of the root
Fourteen compounds, including 1 phenolic acid, 11 flavonoids and 2 alkaloids were isolated from 95% ethanolic root extract. These compounds are 3,4-dihydroxybenzoic acid (3), melacacidin (18), isomelacacidin (19), 4-O-methylmelacacidin (20), 4′-O-methylmelacacidin (21), cis-3,7,8,3′,4′-pentahydroxyflavanone (23), trans-3,7,8,3′,4′-pentahydroxyflavanone (24), melanoxetin (28), transilitin (29), okanin (32), (+)-catechin (33), (-)-epicatechin (34), N-methyltryptamine (52) and N,N-dimethyltryptamine (53).10,11 Flavonoids are the main constituents of the heartwood and the root, however, the constituents of the root contain alkaloids not found in heartwood.
2.3. Constituents of the bark
Phenolic acid derivatives are the most abundant constituents in the 70% ethanolic bark extract. Fifteen phenolic acid derivatives, including 4-hydroxybenzoic acid (1), 4-hydroxybenzoic acid ethyl ester (2), 3,4-dihydroxybenzoic acid (3), 3,4-dihydroxybenzoic acid methyl ester (4), 3,4-dihydroxybenzoic acid ethyl ester (5), 3,4-dihydroxybenzoic acid butyl ester (6), 3-hydroxy-4-methoxybenzoic acid (7), 4-hydroxy-3-methoxybenzoic acid (8), 4-hydroxy-3,5-dimethoxybenzoic acid (9), 4-hydroxy-3,5-dimethoxybenzoic acid ethyl ester (10), gallic acid (11), gallic acid ethyl ester (13), 3,4-dihydroxy-trans-cinnamic acid (14), 3,4-dihydroxy-trans-cinnamic acid ethyl ester (15), 3,4-dihydroxy-trans-cinnamic acid pentyl ester (16) and one lignan, (-)-lyoniresinol (17), were isolated.12,13
The bark of A. confusa is a good source of condensed tannins (proanthocyanidins). The stem bark extract and the root bark extract comprised 247.76 ± 10.93 and 280.70 ± 11.75 mg/g of extractable condensed tannins. According to the results of MALDI-TOF MS, the degree of polymerization (DP) for both extracts can reach 12 and 11, respectively, and the structures of the identified condensed tannins show almost B-type bonding.6
2.4. Constituents of the branches and twigs
The branches and the twigs were extracted respectively in 95% ethanol. (+)-Catechin (33), (-)-epicatechin (34), catechin-3-O-α-rhamnopyranoside (35) and quercitrin (quercetin-3-O-α-rhamnopyranoside) (38) were isolated from the branches extract and luteolin (36), isomyricetin (myricetin-3-O-β-glucopyranoside) (41), myricitrin (myricetin-3-O-α-rhamnopyranoside) (45) and myricetin-3-O-(2″-O-galloyl)-α-rhamnopyranoside (47) were isolated from the twigs.14 Chemical composition analysis revealed the total flavonoids content (TFC) is in the order of twigs (7.7 ± 0.3 mg of quercetin equivalent (QE)/g), branches (2.1 ± 0.0 mg of QE/g) and branch bark (0.9 ± 0.0 mg of QE/g). On the contrary, the total proanthocyanidins content (TPAC) is in the order of the branch bark (128.4 ± 7.6 mg of catechin equivalent (CE)/g), branches (30.4 ± 0.4 mg of CE/g) and twigs (non-detected). Hence, the chemical composition analysis is inconsistent with the compounds isolated from the branch and twig extracts. According to the flavonoids synthesis mechanisms, flavones and flavonols are the up-stream products and flavanols are the down-stream products.15 Since flavanols are the monomers of condensed tannins, it is postulated when the twigs gradually grow into branches, the condensed tannin content is also augmented. Condensed tannins have been closely associated with plant defense mechanisms against mammalian herbivores, birds, insects and fungi.16, 17, 18 Therefore, variations of flavonoids in different plant parts of A. confusa may be involved in plant protective effects, which agrees with previous reports.19,20
According to recent studies, the constituents of the branches and twigs are 5,7-dihydroxyl flavonoids which have the same flavonoids synthesis mechanism as reported in the literature.15 When the stem keeps growing and the heartwood part is formed, 5,7-dihydroxyl flavonoids are transformed to 7,8-dihydroxyl flavonoids and its sugar moiety is removed. Last, 7,8-dihydroxyl flavonoids are accumulated in the heartwood part.
2.5. Constituents of the leaves
Methanol and hot water were used to extract the leaves of A. confusa. Flavonol glycosides, like quercitrin (38), isomyricetin (41), myricitrin (45), 7-O-methylmyricitrin (myricetin-3-O-α-rhamnopyranoside-7-O-methyl ether) (46), myricetin-3-O-(2″-O-galloyl)-α-rhamnopyranoside (47), myricetin-3-O-(3″-O-galloyl)-α-rhamnopyranoside (48), myricetin-3-O-(2″-O-galloyl)-α-rhamnopyranoside-7-methyl ether (49), myricetin-3-O-(3″-O-galloyl)-α-rhamnopyranoside-7-methyl ether (50) and myricetin-3-O-(2″,3″-di-O-galloyl)-α-rhamnopyranoside (51), are major constituents of the leaf extracts.21, 22, 23 Besides, flavonoids and phenolic acid derivatives, like (+)-catechin (33), (-)-epicatechin (34), luteolin (36), myricetin (42), 3-O-methylmyricetin (43), europetin (7-O-methylmyricetin) (44), gallic acid (11) and gallic acid methyl ester (12) were also found in the leaf extracts.21,22 The leaf extracts also contained condensed tannins and the content of the extractable condensed tannins was 64.17 ± 1.44 mg/g.6
2.6. Constituents of the flowers and buds
Gallic acid (11), afzelin (kaempferol-3-O-α-rhamnopyranoside) (37), quercitrin (38), rhamnetin-3-O-α-glucopyranoside (quercetin-3-O-α-glucopyranoside-7-O-methyl ether) (39), rhamnitrin (quercetin-3-O-α-rhamnopyranoside-7-O-methyl ether) (40), myricitrin (45), 7-O-methylmyricitrin (46) were found to be the major secondary metabolites in the ethanolic extract of A. confusa flowers and buds.24,25
2.7. Alkaloids of the shrubs
The alkaloids study on the methanolic extract of A. confusa shrubs has led to the isolation of four tryptamine compounds, and they are N-methyltryptamine (52), N,N-dimethyltryptamine (53), N,N-dimethyltryptamine-N-oxide (54) and N-chloromethyl-N,N-dimethyltryptamine (55).26
3. Biological activities
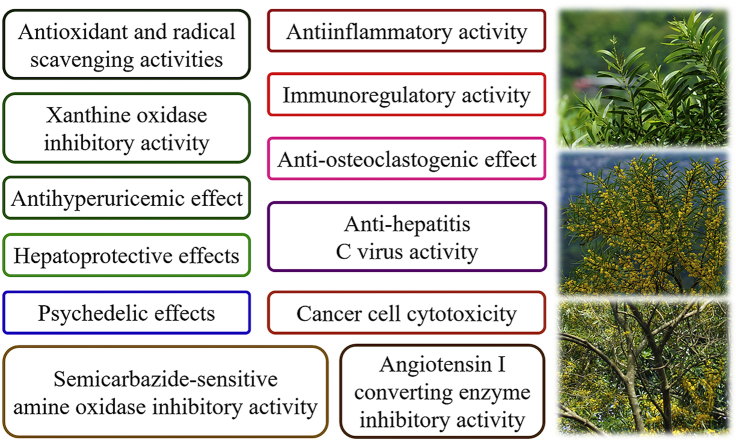
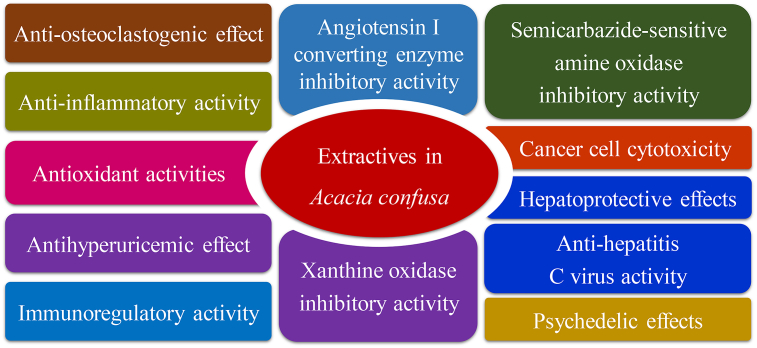
The biological activities of the extractives from A. confusa have been investigated and found that they possess various activities in antioxidant, scavenging radical, anti-inflammatory, anti-virus, inducing hallucination, etc (Fig. 3 and Table 1). The following section introduces their effective constituents and related bioactivities.
Fig. 3.
Summary of the main medical properties of Acacia confusa.
Table 1.
Medical benefits and pharmacologic properties of the major constituents in Acacia confusa.
| Pharmacologic properties | Major constituentsa | References |
|---|---|---|
| Antioxidant and radical scavenging activities | 11, 28, 32, 47 | 10, 12, 13, 22 |
| Hepatoprotective effects | 11 | 32 |
| Xanthine oxidase inhibitory activity | 26, 28, 32, 36 | 9, 14, 41 |
| Semicarbazide-sensitive amine oxidase inhibitory activity | 47, 48, 49, 50, 51 | 47 |
| Angiotensin I converting enzyme inhibitory activity | 49, 50, 51 | 47 |
| Antihyperuricemic effect | 18, 21, 28, 29, 32 | 9 |
| Anti-inflammatory activity | 28 | 7 |
| Anti-hepatitis C virus activity | Ceramides | 57 |
| Immunoregulatory activity | 28, 47b | 68, 73b |
| Anti-osteoclastogenic effect | 25b, 26b | 74b, 75b |
| Cancer cell cytotoxicity | 47, 48, 49, 50 | 23 |
| Psychedelic effects | 53b | 79b , 80b , 81b , 82b , 83b , 84b , 85b , 86b |
The chemical structures of the major constituents are referred to Fig. 2.
Pharmacologic properties of the constituents were determined from other plant species or commercial suppliers.
3.1. Antioxidant and radical scavenging activities
Evaluation of the antioxidant potency of the extracts from different plant parts using Folin-Ciocalteu's method shows the root extract has the highest total phenolic contents (TPC) (652.2 mg gallic acid equivalence (GAE)/g),10 followed by the heartwood extract (529.7 mg GAE/g),27 the bark extract (470.6 mg GAE/g),27 the branches extract (348.2 mg GAE/g),14 the leaves extract (190.2 mg GAE/g),28 the buds extract (173.3 mg GAE/g),24 the twigs extract (121.4 mg GAE/g)14 and the flowers extract (105.1 mg GAE/g).24 The isolated compounds show strong antioxidant activity and radical scavenging activity in vitro mainly because their phenolic groups form catechol group and pyrogallol group in their structures. Flavonoids isolated from the heartwood and the root extracts have unique 7,8-dihydroxyl structures which lead to an increment in their antioxidant activity. Further, okanin (32) and melanoxetin (28) are the strongest antioxidant flavonoids which show the lowest IC50 values of radical scavenging ability against DPPH (2,2-diphenyl-1-picrylhydrazyl) (3.1 and 3.1 μM) and superoxide (2.2 and 2.5 μM) radicals and highest trolox equivalent antioxidant capacity (TEAC, 5.4 and 4.4 mmol of Trolox equivalence (TE)/mmol) and reducing power (5.3 and 5.0 mmol of TE/mmol).10 Gallic acid (11) found in bark, leaves, flowers and buds extracts shows remarkable DPPH and superoxide radical scavenging activity, with IC50 values of 8.2 and 12.4 μM, and TEAC of 5.2 mmol of TE/mmol, which is the best antioxidant for phenolic acid derivatives.12,13 Myricetin-3-O-(2″-O-galloyl)-α-rhamnopyranoside (47) isolated from the twigs and the leaves extracts is the most active flavonol glycoside, exhibiting IC50 values of 3.9 and 8.7 μM for inhibiting DPPH and superoxide radicals and TEAC of 6.9 TE/mmol.22 Therefore, extracts from different plant parts of A. confusa contain abundant amounts and various types of phenolic compounds and possess excellent antioxidant activity in vitro. The in vivo antioxidant efficacies of the extract in A. confusa twig had been preliminarily evaluated by the model organism, Caenorhabditis elegans.29 Since twig extract could effectively remove the reactive oxygen species (ROS) in C. elegans, the oxidative stress resistance of C. elegans was enhanced following pretreatment with the extract.
ROS has harmful effects on DNA damage, lipid peroxidation and oxidative deactivation of specific enzymes and proteins on the cells, which have been implicated in the pathogenesis of many human diseases, such as Alzheimer's disease, amyotrophic, anxiety, atherosclerosis, asthma, cancer, degenerative eye diseases, depression, diabetes, epilepsy, Huntington's disease lateral sclerosis, inflammatory joint disease, multiple sclerosis, Niemann-pick diseases, Parkinson's disease, schizophrenia, senile dementia, etc.30,31 Use of natural phenolic compounds and flavonoids as an antioxidant has been advised as a common treatment to deal with these disorders. Accordingly, the antioxidant potential of different plant parts of A. confusa and their isolated compounds can be further discovered in ameliorating the oxidative stress related disorders.
3.2. Hepatoprotective effects
The ethyl acetate fraction of A. confusa bark extract and its active compounds, gallic acid (11), demonstrated excellent protective effect against carbon tetrachloride (CCl4)-induced chronic liver injury in rats.32 The pathological histology result for the rats liver showed dietary supplementation with gallic acid (11) at a dose of 50 mg/kg inhibited big vacuole formation and reduced small vacuole formation by 78%, and decreased inflammation by 57%. It can significantly reduce the plasma levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) by 94% and 100%, respectively, which have been considered effective indicators of hepatic injury. Gallic acid (11) can elevate the activities of antioxidant enzymes, such as superoxide dismutase (SOD), glutathione reductase (GRD), glutathione peroxidase (GPX) and catalase (CAT). For the erythrocytes, the activities of SOD, GPX, CAT and GRD strongly increased from 19, 2.3 and 852 U/mg and 2.1 U/g to 97, 4.9 and 1939 U/mg and 5.1 U/g following treatment with gallic acid (11). For liver tissues, the activities of GPX, CAT and GRD increased from 0.21, 48 and 0.49 U/g to 0.32, 79 and 0.54 U/g, and the activities of SOD decreased from 6.9 U/g to 5.6 U/g. Gallic acid (11) treatments can obviously reduce the lipid peroxidation level and oxidative stress in plasma and also in liver tissues. By using the thiobarbituric acid reactive substances (TBARS) method, the lipid peroxidation level decreased from 6.2 μM to 3.3 μM and 57 nmol/g liver to 39 nmol/g liver for the plasma and the liver tissues, respectively. The oxidative stress was defined as a reciprocal of the ratio of glutathione (GSH) and oxidized glutathione (GSSG). The GSH/GSSG ratio strongly increased from 27 to 107 and 15 to 93 for the erythrocytes and the liver tissues. Molecular biology study illustrated the expression of hepatic CYP2E1, the major isozyme involved in CCl4 bioactivation and subsequent free radical production, was significantly decreased by 45% by gallic acid (11). In sum, the hepatoprotective effects of gallic acid (11) may be due to regulating the activities of antioxidant enzymes and suppressing lipid peroxidation and CYP2E1 activation.
According to the World Health Organization, chronic liver diseases derived from alcoholic liver disease, fatty liver disease and especially viral hepatitis, etc., remain one of the major threats to public health and are a worldwide problem, causing up to 1.45 million deaths worldwide annually.33,34 Although modem medicine provides great advances in liver disease treatment, no effective drug is available that protects the liver from damage, stimulates liver function or helps regenerate hepatic cells.35,36 Phytochemicals from traditional medical plants have been evaluated for their hepatoprotective effects involved with free radical scavenging activities, antioxidant properties and adoptogenic effects.37, 38, 39 The treatment of liver diseases using natural remedies and their derivatives has a long history and are still used all over the world. Liver protective plants have all manner of chemical constituents, like phenolic compounds, flavonoids, coumarins, monoterpenes, glycosides, alkaloids and xanthenes.40 Tung et al.32 reported the hepatoprotective effects of gallic acid (11) isolated from A. confusa bark extract show exciting progress in the discovery of effective liver protective agents, especially at present, with the urgent need for innovative drugs.
3.3. Xanthine oxidase (XOD) inhibitory activity
A. confusa heartwood extract exhibited remarkable inhibitory activity against XOD in vitro. Tung and Chang41 reported okanin (32) showed the strongest XOD inhibitory effect with an IC50 value of 0.076 μM, followed by melanoxetin (28, 0.274 μM), allopurinol (positive control, 4.784 μM), and 7,8,3′,4′-tetrahydroxyflavone (26, 10.488 μM). The results of the kinetics and molecular mechanisms demonstrated melanoxetin (28) and 7,8,3′,4′-tetrahydroxyflavone (26) were in a competitive mode, as well as allopurinol, and okanin (32) was in a noncompetitive mode. Further studies41,42 demonstrated melanoxetin (28) is a better XOD inhibitor than the commercial drug, allopurinol for two reasons. First, at the same dosage of inhibitor, melanoxetin (28) showed lower heat release than allopurinol in exothermic XOD and xanthine reaction. Second, the Michaelis constants (Km) of XOD and xanthine reaction were 34.6 and 24.5 μM for melanoxetin (28) and allopurinol, respectively.9 The molecular docking studies showed the melanoxetin (28) molecule occupies the same binding site as allopurinol, and its carbonyl and multihydroxyl functions may contribute to a higher binding affinity to XOD than allopurinol.9 Besides, luteolin (36) isolated from the twigs extract showed excellent XOD inhibitory activity with an IC50 value of 11.6 μM.14
XOD plays an important role in the catabolic sequence of the purine nucleotide metabolism in some species, including humans.42 It not only catalyzes the serial oxidation processes in the transformation of xanthine from hypoxanthine and further from xanthine to uric acid, but also generates ROS, like hydrogen peroxide and superoxide anion radicals, accompanying the catalyzed reaction. Consequently, XOD inhibitors are employed to interrupt the synthesis of uric acid in the final step, decreasing the ROS level in the human body, and promoting the production of anti-inflammatory agents to relieve the symptoms from the disease.43, 44, 45, 46 It is surprising okanin (32) and melanoxetin (28) exhibit much better XOD inhibitory activity than allopurinol, which is one of the clinical drugs for treating hyperuricemia and acute gout, and shows great potential in the development of new drugs.
3.4. Semicarbazide-sensitive amine oxidase (SSAO) inhibitory activity
Lee et al.47 reported five myricetin galloglycosides were isolated from the leaves of A. confusa and showed inhibitory activity against SSAO (EC 1.4.3.6). Among them, myricetin-3-O-(3″-O-galloyl)-α-rhamnopyranoside-7-methyl ether (50) and myricetin-3-O-(2″,3″-di-O-galloyl)-α-rhamnopyranoside (51) showed the highest inhibitory activity with IC50 values of 36.16 μM and 39.35 μM, respectively, followed by myricetin-3-O-(2″-O-galloyl)-α-rhamnopyranoside-7-methyl ether (49), myricetin-3-O-(3″-O-galloyl)-α-rhamnopyranoside (48), myricetin-3-O-(2″-O-galloyl)-α-rhamnopyranoside (47). Lee et al.47 also found these five compounds showed an identical order for inhibitory activity against SSAO and DPPH radical scavenging activity. SSAO is present in plants, microorganisms, and in many organs of mammals.48 It converts primary amines into the corresponding aldehydes, generating hydrogen peroxide and ammonia. It has been proven plasma SSAO is increased in diabetes mellitus and heart failure and SSAO is implicated in atherosclerosis, endothelial damage, and glucose transport into adipocytes. Therefore, myricetin galloglycosides isolated from the leaf extract of A. confusa show benefits to human health.
3.5. Angiotensin I converting enzyme (ACE) inhibitory activity
Myricetin-3-O-(2″,3″-di-O-galloyl)-α-rhamnopyranoside (51), myricetin-3-O-(3″-O-galloyl)-α-rhamnopyranoside-7-methyl ether (50) and myricetin-3-O-(2″-O-galloyl)-α-rhamnopyranoside-7-methyl ether (49) isolated from the leaf extract of A. confusa showed inhibitory activities against ACE (EC 3.4.15.1), with IC50 values of 19.82, 60.32 and 151.90 μM.47 Lee et al.47 suggested ACE inhibitory activity is referring to the gallic acid groups. ACE, a dipeptide-liberating exopeptidase, is involved in the blood pressure regulation around the renin-angiotensin system.49 ACE inhibitor is the preferred class of antihypertensive drugs, due to its low adverse side-effects.50
3.6. Antihyperuricemic effect
Tung et al.9 reported A. confusa heartwood extract can significantly suppress serum uric acid levels in oxonate-induced mice, and lead to the isolation of five active compounds. At an equimolar dose (100 μmol/kg), animals treated with melacacidin (18), 4′-O-methylmelacacidin (21), melanoxetin (28), transilitin (29), okanin (32) and allopurinol (positive control) showed significant reductions in uric acid to 66, 72, 75, 65, 69 and 79%, respectively, relative to the untreated group. Hyperuricemia may lead to the pathogenesis of many serious complications, including: gouty arthritis, gout, stroke, ischemic heart disease, kidney dysfunction, uremia, urolithiasis, etc. Wang et al.51 reported cinnamaldehyde, the major compound of Cinnamomum osmophloeum leaves, significantly reduced the serum uric acid level by 60% at a dosage of 150 mg/kg (1135 μmol/kg). Zhu et al.52 also found treating with quercetin and rutin at a dosage of 150 mg/kg (496 μmol/kg for quercetin and 245 μmol/kg for rutin) markedly reduced the serum uric acid levels by 36 and 32%, respectively. Therefore, the study of Tung et al.9 indicates the heartwood extract of A. confusa and its isolated five flavonoids as a potential candidate for reducing serum uric acid levels and gout treatment.
3.7. Anti-inflammatory activity
A. confusa heartwood extract showed moderate anti-inflammatory activity in the inhibition of nitric oxide (NO) generation in lipopolysaccharide (LPS)-stimulated RAW 264.7 cells.7 Melanoxetin (28) is the most active compound which strongly inhibited NO production with an IC50 value of 6.9 μM and reduced PGE2 accumulation by 60% at a dosage of 100 μM. In addition, at a dosage of 50 μM, melanoxetin (28) completely suppressed the iNOS mRNA expression and reduced the cyclooxygenase-2 (COX-2) mRNA expression by 76%. New anti-inflammatory agent discoveries alleviating the symptoms of acute inflammation always attract the attention of pharmacological scientists because they aim to prevent the adverse effect of clinical drugs. On the other hand, chronic inflammation has been gradually proven to be a possible cause of cardiovascular disease, Alzheimer's disease, cancer, allergies, autoimmune diseases and metabolic disorders.53, 54, 55, 56 A. confusa heartwood extract contains abundant amounts of phenolic compounds and flavonoids and shows anti-inflammatory activities, thus, it may have the potential to become a natural source of anti-inflammatory food supplements and drugs.
3.8. Anti-hepatitis C virus activity
Lee et al.57 showed the glycosyl lipidoids, ceramides, in A. confusa stems have inhibition efficacy on anti-hepatitis C virus (HCV). HCV is an enveloped, positive-sense and single-stranded RNA virus belonging to the family Flaviviridae that causes chronic hepatitis, cirrhosis and hepatocellular carcinoma in humans.58,59 The only treatment to cure for HCV infection is using pegylated interferon-α in combination with the nucleoside analog ribavirin which has unfavorable side-effects such as flu-like symptoms, hemolytic anemia and depression.60 Virus-induced hepatic diseases cause chronic inflammation or proliferation of hepatoma cells involving activation of nuclear factor-kappaB (NF-κB).61 HCV produces at least 10 individual proteins, 4 structural proteins (core, E1, E2 and p7) and 6 nonstructural proteins (NS2, NS3, NS4A, NS4B, NS5A and NAS5B), and some of them (core, E2, NS3, NS5A) induce hepatocellular carcinoma via promotion of NF-κB mediated through COX-2 expression.57,62, 63, 64, 65 Lee et al.57 demonstrated the effective constituents in the stems of A. confusa can inhibit the HCV RNA replication by suppressing COX-2 expression and NF-κB activation and providing antiviral synergy in combination with INF-α.
3.9. Immunoregulatory activity
Dendritic cells (DCs), one type of professional antigen-presenting cells, present in lymphoid and nonlymphoid tissues, are responsible for regulating immune responses.66,67 It has been demonstrated the activation of DCs leads to the maturation and expression of pro-inflammatory cytokines such as TNF-α, IL-6 and IL-12.68, 69, 70 Ho et al.68 reported melanoxetin (28), the major constituent in A. confusa heartwood extract, can effectively enhance immune regulation by inhibiting the production of pro-inflammatory cytokines in LPS-stimulated dendritic cells (DCs) at a concentration of 12.5 μM. Besides, LPS also generates ROS which can activate DCs and make immature DCs become mature.71 Ho et al.68 found LPS-induced DCs maturation was inhibited by treatment with melanoxetin. Since melanoxetin is a good antioxidant, it is believed that the melanoxetin can enhance immune-regulation by suppressing LPS-generated ROS. Besides, human peripheral blood mononuclear cells (PBMCs) are the important hinge of the immune responses.72 The activation and proliferation of PBMCs would promote the immune responses.72 Kuo et al.73 reported myricetin-3-O-(2″-O-galloyl)-α-rhamnopyranoside (47) has an antiproliferative effect on PBMCs, with an IC50 value of 11.9 μM.
3.10. Anti-osteoclastogenic effect
Kang et al.74,75 demonstrated 7,3′,4′-trihydroxyflavone (25) and 7,8,3′,4′-tetrahydroxyflavone (26) have the potential to treat bone-lytic diseases owing to their anti-osteoclastogenic effect. Osteoclastogenesis causes the immature bone-resorbing osteoclast to mature which is activated by the essential key cytokines, macrophage colony stimulating factor and receptor activator of NF-κB ligand (RANKL).76,77 Osteoclast is accountable for the primary bone remodeling process via resorption and resorbing of the bone; however, the over activation of osteoclasts would induce bone-related diseases such as postmenopausal osteoporosis, inflammatory arthritis, osteolytic bone metastasis and Paget's bone disease.78 Kang et al.74,75 showed 7,3′,4′-trihydroxyflavone (25) and 7,8,3′,4′-tetrahydroxyflavone (26) can inhibit RANKL-induced osteoclast differentiation by reducing both the expression levels of nuclear factor of activated T cells c1 (a key transcription factor of osteoclast differentiation) and the mRNA of osteoclast marker genes.
3.11. Cancer cell cytotoxicity
Brine shrimp (Artemia salina) lethality assay is a rapid, inexpensive, in-house and general bioassay and is usually used for evaluating the cancer cell cytotoxicity of organic compounds, especially for natural products. Lee et al.23 reported flavonol galloglycosides were the major compounds for A. confusa leaf extracts and their anti-hatch activity against brine shrimp were evaluated. Myricetin-3-O-(2″-O-galloyl)-α-rhamnopyranoside (47), myricetin-3-O-(3″-O-galloyl)-α-rhamnopyranoside (48), myricetin-3-O-(2″-O-galloyl)-α-rhamnopyranoside-7-methyl ether (49), myricetin-3-O-(3″-O-galloyl)-α-rhamnopyranoside-7-methyl ether (50) exhibited moderate brine shrimp lethality, with IC50 values of 75, 64, 89 and 50 μg/mL.
3.12. Psychedelic effects
It is well known ingesting N,N-dimethyltryptamine (53) at high concentration causes transient and intermittently visual hallucinations.79, 80, 81, 82 Carbonaro and Gatch83 reviewed the psychedelic pharmacological mechanisms of N,N-dimethyltryptamine (53) involved in the interactions of various receptors (i.e. serotonin receptors, trace amine-associated receptors, sigma-1 receptor and ionotropic and metabotropic glutamate receptors) and neurotransmitters (dopamine, acetylcholine and glutamate) in brain, concurrently, N,N-dimethyltryptamine (53) can be a model of psychiatric disorders in investigations of schizophrenia, depression and anxiety. Besides, since the use of N,N-dimethyltryptamine (53) is not as harmful as other synthetic hallucinogenic compounds, such as 25I-NBOMe, N,N-dimethyltryptamine (53) is also considered a powerful media for self-discovery and understanding consciousness.83, 84, 85, 86
4. Conclusions
Traditional medical plants are potent sources in the drug discovery process and the development of health functional food because their active phenolic compounds are in charge of various antioxidant and pharmacological activities. A. confusa is a medical plant endemic in Taiwan and is one of the most widespread plants. To date, this report is the most comprehensive review of the phytochemistry, antioxidant and pharmacological properties of A. confusa. Flavonoids, flavonol glycosides and phenolic acid derivatives are the major phytochemical constituents isolated from different plant parts which contain multiple phenolic functionalities exhibiting impressive antioxidant ability. Further, these compounds exhibited remarkable inhibitory activities against XOD, AASO and ACE. Knowledge of the chemical constituents of A. confusa is necessary, not only for the discovery of new medical agents, but because such information may be valuable to those interested in the actual worth of folklore drugs. Only a few studies have been done on the biological activities and plausible functional food and medicinal applications of these compounds. Therefore, extensive investigation and development work is needed to exploit their therapeutic utility against diseases and the feasibility of being healthy food supplements.
Conflicts of interest
All authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
Huan-You Lin, Email: killer-gundam@yahoo.com.tw.
Tzu-Cheng Chang, Email: d00625001@ntu.edu.tw.
Shang-Tzen Chang, Email: peter@ntu.edu.tw.
References
- 1.Kan W.S. Leguminosae. In: Kan W.S., editor. vol. 2. National Research Institute of Chinese Medicine; Taipei: 1978. pp. 239–240. (Manual of Medicinal Plants in Taiwan). [Google Scholar]
- 2.Huang T.C., Ohashi H. Leguminosae. In: Huang T.C., editor. second ed. vol. 3. National Taiwan University; Taipei: 1994. pp. 160–162. (Flora of Taiwan). [Google Scholar]
- 3.Chen Y.S.G., Wong S.M., Whitton B.A. Effects of landfill leachate on growth and nitrogen fixation of two Leguminous trees (Acacia confusa, Leucaena leucocephala) Water Air Soil Pollut. 1999;111:29–40. [Google Scholar]
- 4.Wang D.M., Wu S.H., Su C.H., Peng J.T., Shih Y.H., Chen L.C. Ganoderma multipileum, the correct name for ‘G. lucidum’ in tropical Asia. Bot Stud. 2009;50:451–458. [Google Scholar]
- 5.Wang Y.C. Carbon sequestration and foliar dust retention by woody plants in the greenbelts along two major Taiwan highways. Ann Appl Biol. 2011;159:244–251. [Google Scholar]
- 6.Wei S.D., Zhou H.C., Lin Y.M., Liao M.M., Chai W.M. MALDI-TOF MS analysis of condensed tannins with potent antioxidant activity from the leaf, stem bark and root bark of Acacia confusa. Molecules. 2010;15:4369–4381. doi: 10.3390/molecules15064369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J.H., Tung Y.T., Chien S.C. Effect of phytocompounds from the heartwood of Acacia confusa on inflammatory mediator production. J Agric Food Chem. 2008;56:1567–1573. doi: 10.1021/jf072922s. [DOI] [PubMed] [Google Scholar]
- 8.Wu J.H., Tung Y.T., Wang S.Y., Shyur L.F., Kuo Y.H., Chang S.T. Phenolic antioxidants from the heartwood of Acacia confusa. J Agric Food Chem. 2005;53:5917–5921. doi: 10.1021/jf050550m. [DOI] [PubMed] [Google Scholar]
- 9.Tung Y.T., Hsu C.A., Chen C.S., Yang S.C., Huang C.C., Chang S.T. Phytochemicals from Acacia confusa heartwood extracts reduce serum uric acid levels in oxonate-induced mice: their potential use as xanthine oxidase inhibitors. J Agric Food Chem. 2010;58:9936–9941. doi: 10.1021/jf102689k. [DOI] [PubMed] [Google Scholar]
- 10.Lin H.Y., Chang S.T. Antioxidant potency of phenolic phytochemicals from the root extract of Acacia confusa. Ind Crop Prod. 2013;49:871–878. [Google Scholar]
- 11.Lee T.H., Chou C.H. Flavonoid aglycones and indole alkaloids from the roots of Acacia confusa. J Chin Chem Soc. 2000;47:1287–1290. [Google Scholar]
- 12.Tung Y.T., Wu J.H., Huang C.Y., Kuo Y.H., Chang S.T. Antioxidant activities and phytochemical characteristics of extracts from Acacia confusa bark. Bioresour Technol. 2009;100:509–514. doi: 10.1016/j.biortech.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Tung Y.T., Wu J.H., Kuo Y.H., Chang S.T. Antioxidant activities of natural phenolic compounds from Acacia confusa bark. Bioresour Technol. 2007;98:1120–1123. doi: 10.1016/j.biortech.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh C.Y., Chang S.T. Antioxidant activities and xanthine oxidase inhibitory effects of phenolic phytochemicals from Acacia confusa twigs and branches. J Agric Food Chem. 2010;58:1578–1583. doi: 10.1021/jf903569k. [DOI] [PubMed] [Google Scholar]
- 15.Dewick P.M. 3th ed. John Wiley & Sons Ltd; New York: 2009. Medicinal Natural Products: a Biosynthetic Approach. [Google Scholar]
- 16.Hassanpour S., Sadaghian M., Maheri-Sis N., Eshratkhah B., Chaichi-Semsari M. Effect of condensed tannin on controlling faecal protein excretion in nematode-infected sheep: in vivo study. J Am Sci. 2011;7:896–900. [Google Scholar]
- 17.Zucker W.V. Tannins: does structure determine function? An ecological perspective. Am Nat. 1983;121:335–365. [Google Scholar]
- 18.Hagerman A.E., Buttler L.G. The specificity of proanthocyanidin-protein interactions. J Biol Chem. 1981;256:4494–4497. [PubMed] [Google Scholar]
- 19.Hassanpour S., Maheri-Sis N., Eshratkhah B., Mehmandar F.B. Plants and secondary metabolites (tannins): a review. Int J For Soil Eros (IJFSE) 2011;1:47–53. [Google Scholar]
- 20.Haslam E. Cambridge University Press; Cambridge: 1989. Plant Polyphenols Vegetable Tannins Revisited. [Google Scholar]
- 21.Tung Y.T., Wu J.H., Hsieh C.Y., Chen P.S., Chang S.T. Free radical-scavenging phytochemicals of hot water extracts of Acacia confusa leaves detected by an on-line screening method. Food Chem. 2009;115:1019–1024. [Google Scholar]
- 22.Lin S.S., Shiau I.L., Chang S.T. Antioxidant activity of constituents from the methanolic extract of Acacia confusa leaves. Taiwan J For Sci. 2009;24:61–68. [Google Scholar]
- 23.Lee T.H., Qiu F., Waller G.R., Chou C.H. Three new flavonol galloylglycosides from leaves of Acacia confusa. J Nat Prod. 2000;63:710–712. doi: 10.1021/np990482w. [DOI] [PubMed] [Google Scholar]
- 24.Tung Y.T., Chang W.C., Chen P.S., Chang T.C., Chang S.T. Ultrasound-assisted extraction of phenolic antioxidants from Acacia confusa flowers and buds. J Separ Sci. 2011;34:844–851. doi: 10.1002/jssc.201000820. [DOI] [PubMed] [Google Scholar]
- 25.Wu J.H., Huang C.Y., Tung Y.T., Chang S.T. Online RP-HPLC-DPPH screening method for detection of radical-scavenging phytochemicals from flowers of Acacia confusa. J Agric Food Chem. 2008;56:328–332. doi: 10.1021/jf072314c. [DOI] [PubMed] [Google Scholar]
- 26.Buchanan M.S., Carroll A.R., Pass D., Quinn R.J. NMR spectral assignments of a new chlorotryptamine alkaloid and its analogues from Acacia confusa. Magn Reson Chem. 2007;45:359–361. doi: 10.1002/mrc.1959. [DOI] [PubMed] [Google Scholar]
- 27.Chang S.T., Wu J.H., Wang S.Y., Kang P.L., Yang N.S., Shyur L.F. Antioxidant activity of extracts from Acacia confusa bark and heartwood. J Agric Food Chem. 2001;49:3420–3424. doi: 10.1021/jf0100907. [DOI] [PubMed] [Google Scholar]
- 28.Ho S.T., Tung Y.T., Chen Y.L., Zhao Y.Y., Chung M.J., Wu J.H. Antioxidant activities and phytochemical study of leaf extracts from 18 indigenous tree species in Taiwan. Evid-based Complement Altern Med. 2012 doi: 10.1155/2012/215959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang C.H., Li W.H., Shi Y.C., Huang C.W., Chang S.T., Liao V.H.C. Caenorhabditis elegans as model organisms to study the antioxidant potency of Acacia confusa twig extract. Quart J Chin Forest. 2012;45:503–514. [Google Scholar]
- 30.Adibhatla R.M., Hatcher J.F. Altered lipid metabolism in brain injury and disorders. Subcell Biochem. 2008;49:241–268. doi: 10.1007/978-1-4020-8831-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Florence T.M. The role of free radicals in disease. Aust N Z J Ophthalmol. 1995;23:3–7. doi: 10.1111/j.1442-9071.1995.tb01638.x. [DOI] [PubMed] [Google Scholar]
- 32.Tung Y.T., Wu J.H., Huang C.C. Protective effect of Acacia confusa bark extract and its active compound gallic acid against carbon tetrachloride-induced chronic liver injury in rats. Food Chem Toxicol. 2009;47:1385–1392. doi: 10.1016/j.fct.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 33.http://www.who.int/mediacentre/news/releases/2016/world-hepatitis-day/en/. Accessed 20 July 2016.
- 34.Thilakchand K.R., Mathai R.T., Simon P., Ravi R.T., Baliga-Rao M.P., Baliga M.S. Hepatoprotective properties of the Indian gooseberry (Emblica officinalis Gaertn): a review. Food Funct. 2013;4:1431–1441. doi: 10.1039/c3fo60237k. [DOI] [PubMed] [Google Scholar]
- 35.Adewusi E.A., Afolayan A.J. A review of natural products with hepatoprotective activity. J Med Plants Res. 2010;4:1318–1334. [Google Scholar]
- 36.Chattopadhyay R.R. Possible mechanism of hepatoprotective activity of Azadirachta indica leaf extract: part II. J Ethnopharmacol. 2003;89:217–219. doi: 10.1016/j.jep.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Jain S.K., Rajvaidy S., Desai P., Singh G.K., Nagori B.P. Herbal extract as hepatoprotective-a review. J Pharmacogn Phytochem. 2013;2:170–175. [Google Scholar]
- 38.Kumar A. A review on hepatoprotective herbal drugs. Int J Res Pharm Chem. 2012;2:96–102. [Google Scholar]
- 39.Kumar S.V., Sanjeev T., Ajay S., Kumar S.P., Anil S. A review on hepatoprotective activity of medicinal plants. Int J Pharm Biosci. 2012;2:31–38. [Google Scholar]
- 40.Bhawna S., Kumar S.U. Hepatoprotective activity of some indigenous plants. Int J Pharmtech Res. 2009;4:1330–1334. [Google Scholar]
- 41.Tung Y.T., Chang S.T. Inhibition of xanthine oxidase by Acacia confusa extracts and their phytochemicals. J Agric Food Chem. 2010;58:781–786. doi: 10.1021/jf901498q. [DOI] [PubMed] [Google Scholar]
- 42.Harris M.D., Siegel L.B., Alloway J.A. Gout and hyperuricemia. Am Fam Physician. 1999;59:925–934. [PubMed] [Google Scholar]
- 43.Rasmussen J.T., Rasmussen M.S., Petersen T.E. Cysteines involved in the interconversion between dehydrogenase and oxidase forms of bovine xanthine oxidoreductase. J Dairy Sci. 2000;83:499–506. doi: 10.3168/jds.S0022-0302(00)74909-5. [DOI] [PubMed] [Google Scholar]
- 44.Wortmann R.L. Management of hyperuricemia. In: McCarthy D.J., Koopman W.J., editors. Arthritis and Allied Conditions. twelfth ed. Lea and Febiger; Philadelphia: 1993. pp. 1807–1818. [Google Scholar]
- 45.Star V.L., Hochberg M. Prevention and management of gout. Drugs. 1993;45:212–222. doi: 10.2165/00003495-199345020-00004. [DOI] [PubMed] [Google Scholar]
- 46.Kelley W.N. Antihyperuricemic drugs. In: Kelley W.N., Harris E.D., Ruddy S., Sledge C.B., editors. Textbook of Rheumatology. W. B. Saunders Co; Philadelphia: 1991. pp. 862–877. [Google Scholar]
- 47.Lee T.H., Liu D.Z., Hsu F.L., Wu W.C., Hou W.C. Structure-activity relationships of five myricetin galloylglycosides from leaves of Acacia confusa. Bot Stud. 2006;47:37–43. [Google Scholar]
- 48.Boomsma F., van Dijk J., Bhaggoe U.M., Bouhuizen A.M.B., van den Meiracker A.H. Variation in semicarbazide-sensitive amine oxidase activity in plasma and tissues of mammals. Comp Biochem Physiol, C. 2000;126:69–78. doi: 10.1016/s0742-8413(00)00101-8. [DOI] [PubMed] [Google Scholar]
- 49.Mullally M.M., Meisel H., FitzGerald R.J. Synthetic peptides corresponding to α-lactalbumin and β-lactoglobulin sequences with angiotensin-I-converting enzyme inhibitory activity. Biol Chem. 1996;377:259–260. doi: 10.1515/bchm3.1996.377.4.259. [DOI] [PubMed] [Google Scholar]
- 50.Fotherby M.D., Panayiotou B. Antihypertensive therapy in the prevention of stroke: what, when, and for whom? Drugs. 1999;58:663–674. doi: 10.2165/00003495-199958040-00006. [DOI] [PubMed] [Google Scholar]
- 51.Wang S.Y., Yang C.W., Liao J.W., Zhen W.W., Chu F.H., Chang S.T. Essential oil from leaves of Cinnamomum osmophloeum acts as a xanthine oxidase inhibitor and reduces the serum uric acid levels in oxonate-induced mice. Phytomedicine. 2008;15:940–945. doi: 10.1016/j.phymed.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Zhu J.X., Wang Y., Kong L.D., Yang C., Zhang X. Effects of Biota orientalis extract and its flavonoid constituents, quercetin and rutin on serum uric acid levels in oxonate-induced mice and xanthine dehydrogenase and xanthine oxidase activities in mouse liver. J Ethnopharmacol. 2004;93:133–140. doi: 10.1016/j.jep.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 53.de Heredia F.P., Gomez-Martinez S., Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. 2012;71:332–338. doi: 10.1017/S0029665112000092. [DOI] [PubMed] [Google Scholar]
- 54.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 55.Sastre M., Klockgether T., Heneka M.T. Contribution of inflammatory processes to Alzheimer's disease: molecular mechanisms. Int J Dev Neurosci. 2006;24:167–176. doi: 10.1016/j.ijdevneu.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 56.Gorman C., Park A., Dell K. Health: the fires within. Time. 2004;163:38–45. 23 February, USA edition. [Google Scholar]
- 57.Lee J.C., Chen W.C., Wu S.F. Anti-hepatitis C virus activity of Acacia confusa extract via suppressing cyclooxygenase-2. Antivir Res. 2011;89:35–42. doi: 10.1016/j.antiviral.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 58.Alter M.J. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13:2436–2441. doi: 10.3748/wjg.v13.i17.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levrero M. Viral hepatitis and liver cancer: the case of hepatitis C. Oncogene. 2006;25:3834–3847. doi: 10.1038/sj.onc.1209562. [DOI] [PubMed] [Google Scholar]
- 60.Schaefer M., Mauss S. Hepatitis C treatment in patients with drug addiction: clinical management of interferon-alpha-associated psychiatric side effects. Curr Drug Abuse Rev. 2008;1:177–187. doi: 10.2174/1874473710801020177. [DOI] [PubMed] [Google Scholar]
- 61.Sun B., Karin M. NF-kappaB signaling, liver disease and hepatoprotective agents. Oncogene. 2008;27:6228–6244. doi: 10.1038/onc.2008.300. [DOI] [PubMed] [Google Scholar]
- 62.Lu L., Wei L., Peng G. NS3 protein of hepatitis C virus regulates cyclooxygenase-2 expression through multiple signaling pathways. Virology. 2008;371:61–70. doi: 10.1016/j.virol.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waris G., Siddiqui A. Hepatitis C virus stimulates the expression of cyclooxygenase-2 via oxidative stress: role of prostaglandin E2 in RNA replication. J Virol. 2005;79:9725–9734. doi: 10.1128/JVI.79.15.9725-9734.2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Núñez O., Fernández-Martinez A., Majano P.L. Increased intrahepatic cyclooxygenase 2, matrix metalloproteinase 2, and matrix metalloproteinase 9 expression is associated with progressive liver disease in chronic hepatitis C virus infection: role of viral core and NS5A proteins. Gut. 2004;53:1665–1672. doi: 10.1136/gut.2003.038364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Penin F., Dubuisson J., Rey F.A., Moradpour D., Pawlotsky J.M. Structural biology of hepatitis C virus. Hepatology. 2004;39:5–19. doi: 10.1002/hep.20032. [DOI] [PubMed] [Google Scholar]
- 66.Flaherty D.K. Mosby. Elsevier Inc; St. Louis: 2012. Immunology for Pharmacy. [Google Scholar]
- 67.LeBlanc D.M., Barousse M.M., Fidel P.L., Jr. Role for dendritic cells in immunoregulation during experimental vaginal candidiasis. Infect Immun. 2006;74:3213–3221. doi: 10.1128/IAI.01824-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ho S.T., Tung Y.T., Wu Y.J., Lin C.C., Wu J.H. Immune-regulatory activity of methanolic extract of Acacia confusa heartwood and melanoxetin isolated from the extract. Holzforschung. 2015;69:645–652. [Google Scholar]
- 69.Dalod M., Chelbi R., Malissen B., Lawrence T. Dendritic cell maturation: functional specialization through signaling specificity and transcriptional programming. EMBO J. 2014;33:1104–1116. doi: 10.1002/embj.201488027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trucci V.M., Salum F.G., Figueiredo M.A., Cherubini K. Interrelationship of dendritic cells, type 1 interferon system, regulatory T cells and toll-like receptors and their role in Lichen planus and Lupus erythematosus - a literature review. Arch Oral Biol. 2013;58:1532–1540. doi: 10.1016/j.archoralbio.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 71.Yamada H., Arai T., Endo N. LPS-induced ROS generation and changes in glutathione level and their relation to the maturation of human monocyte-derived dendritic cells. Life Sci. 2006;78:926–933. doi: 10.1016/j.lfs.2005.05.106. [DOI] [PubMed] [Google Scholar]
- 72.Sen P., Kemppainen E., Orešič M. Perspectives on systems modeling of human peripheral blood mononuclear cells. Front. Mol. Biosci. 2018 doi: 10.3389/fmolb.2017.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuo Y.C., Yang L.M., Lin L.C. Isolation and immunomodulatory effect of flavonoids from Syzygium samarangense. Planta Med. 2004;70:1237–1239. doi: 10.1055/s-2004-835859. [DOI] [PubMed] [Google Scholar]
- 74.Kang J.H., Lee J., Moon M., Yim M. 3’4’7-Trihydroxyflavone inhibits RANKL-induced osteoclast formation via NFATc1. Pharmazie. 2015;70:661–667. [PubMed] [Google Scholar]
- 75.Kang J.H., Jung H., Yim M. 3',4',7,8-Tetrahydroxyflavone inhibits RANKL-induced osteoclast formation and bone resorption. Pharmazie. 2017;72:161–166. doi: 10.1691/ph.2017.6845. [DOI] [PubMed] [Google Scholar]
- 76.Yavropoulou M.P., Yovos J.G. Osteoclastogenesis - current knowledge and future perspectives. J Musculoskelet Neuronal Interact. 2008;8:204–216. [PubMed] [Google Scholar]
- 77.Takayanagi H., Kim S., Koga T. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 78.Novack D.V., Teitelbaum S.L. The osteoclast: friend or foe? Ann Rev Pathol. 2008;3:457–484. doi: 10.1146/annurev.pathmechdis.3.121806.151431. [DOI] [PubMed] [Google Scholar]
- 79.Shulgin A., Shulgin A. Transform Press; Berkeley: 1997. TIHKAL: the Continuation. [Google Scholar]
- 80.Strassman R.J., Qualls C.R. Dose-response study of N,N-dimethyltryptamine humans: I. neuroendocrine autonomic, and cardiovascular effects. Arch Gen Psychiatr. 1994;51:85–97. doi: 10.1001/archpsyc.1994.03950020009001. [DOI] [PubMed] [Google Scholar]
- 81.Strassman R.J., Qualls C.R., Uhlenhuth E.H., Kellner R. Dose-response study of N,N-dimethyltryptamine in humans: II. subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatr. 1994;51:98–108. doi: 10.1001/archpsyc.1994.03950020022002. [DOI] [PubMed] [Google Scholar]
- 82.Stoff D.M., Moja E.A., Gillin J.C., Wyatt R.J. Dose response and time course effects of N,N-dimethyltryptamine on disruption of rat shuttle box avoidance. Biol Psychiatr. 1977;12:339–346. [PubMed] [Google Scholar]
- 83.Carbonaro T.M., Gatch M.B. Neuropharmacology of N,N-dimethyltryptamine. Brain Res Bull. 2016;126:74–88. doi: 10.1016/j.brainresbull.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lowe L.M., Peterson B.L., Couper F.J. A case review of the first analytically confirmed 25I-NBOMe-related death in Washington State. J Anal Toxicol. 2015;39:668–671. doi: 10.1093/jat/bkv092. [DOI] [PubMed] [Google Scholar]
- 85.Hill S.L., Doris T., Gurung S. Severe clinical toxicity associated with analytically confirmed recreational use of 25I-NBOMe: case series. Clin Toxicol. 2013;51:487–492. doi: 10.3109/15563650.2013.802795. [DOI] [PubMed] [Google Scholar]
- 86.Strassman R.J. Park Street Press; Rochester: 2001. DMT: the Spirit Molecule. A Doctor's Revolutionary Research into the Biology of Near-death and Mystical Experiences. [Google Scholar]