Abstract
Herbal medicines are known to mitigate radical induced cell damage. Hence identification and scientific validation of herbal medicines contribute to better use in Ayurvedic/Unani research. In the present study, we investigated antioxidant and anti-apoptotic properties of Convolvulus pluricaulis (C. pluricaulis). C. pluricaulis exhibited antioxidant potential evident by free radical scavenging activities. C. pluricaulis pretreatment inhibited H2O2 induced macromolecule damage such as plasmid DNA damage and AAPH induced oxidation of bovine serum albumin and lipid peroxidation of rat hepatic tissues. Further to identify the neuroprotective properties of C. pluricaulis, SHSY5Y cells were treated with H2O2 with or without pretreatment of C. pluricaulis. The C. pluricaulis pretreatment at 50 μg/ml dose exhibited 50% cell survival against 100 μM H2O2 challenge for 24 h and it also decreased the lactate dehydrogenase leakage. Further C. pluricaulis pretreatment restored and regulated the antioxidant and apoptosis markers such as SOD, CAT, p53, and caspase-3 and inhibited, reactive oxygen species generation and depolarization of the mitochondrial membrane. C. pluricaulis possess a high content of flavonoids and polyphenols and GC-MS and FTIR analysis showed a wide variety of compounds which may contribute to the observed effects.
Keywords: Convolvulus pluricaulis, GC-MS, FTIR, Antioxidant, SH-SY5Y, Neuroprotection
Graphical abstract
1. Introduction
The brain is an amazing, extremely complex organ in the human body that contains millions of mitochondria which are vulnerable to attack from free radicals. The imbalance between oxidants and antioxidants within the cells leads to oxidative stress and further causes cellular damage. Oxidative radicals such as hydroxyl radical (OH), superoxide anion (O−2) and hydrogen peroxide (H2O2) which are collectively called reactive oxygen species (ROS) are constantly produced in cells through normal metabolic processes.1 Where the oxidants within the cell exceed than the levels of antioxidants present, causes macromolecules such as DNA, protein and lipid damage.2, 3, 4 These macromolecules damage is observed in several diseases such as diabetes, ischemia/reperfusion and neurodegenerative diseases such as Parkinson's, Huntington's and Alzheimer's disease, hypertension.5, 6, 7, 8 Antioxidant supplementation protects against reactive oxygen species-mediated cell damage. Several medicinal plants are used to treat various diseases that are also a rich source of natural antioxidants such as flavonoids, polyphenols, terpenoids.9, 10, 11
Convolvulus pluricaulis (C. pluricaulis) also called Shankhpushpi in India commonly known as bindweed, is a perennial herb. It has been widely used in Ayurvedic and Unani medicine which include bioactive components such as cinnamic acid, pentanoic acid, ascorbic acid, vitamin E, phthalic acid, squalene, silane, decanoic acid, linoleic acid, β-sitosterol, tropane alkaloids, kaempferol etc.12, 13, 14 Previous studies showed that active compound of C. pluricaulis exhibited hepatoprotective, anxiolytic, antimicrobial activities.15 Scopoletin exhibited antioxidant effects and also regulated tumor necrosis factor (TNF-α) and prostaglandin E2 (PGE2) levels.16 C. pluricaulis also possess hypotensive, anti-anxiety, hepatoprotective properties and also used to treat hypertension.17, 18, 19 C. pluricaulis has been found to be used as a nerve tonic and also it augments both memory enhancing and cognitive effects as observed by many behavioral studies.20, 21 It has been long recognized that oxidative stress may cause neurodegenerative diseases such as Alzheimer's disease (AD), Parkinson's disease (PD) and also induce apoptosis.
C. pluricaulis (CP) has been already reported as a neuroprotective plant in vitro and in vivo models. It shows the protective effects of aluminum induced neurotoxicity in rat brain and scopolamine-induced neurotoxicity in the cerebral cortex of male Wistar rats.21, 22 Further Dhuna et al.23 reported the neuroprotective effect of methanolic extract of C. pluricaulis on hydrogen peroxide-induced oxidative stress in human IMR32 neuroblastoma cell line. Although literature pertaining to antioxidant status, macromolecule damage protection and neuroprotective activity of C. pluricaulis are scanty, further no data available on the oxidative stress-mediated apoptosis mechanism. Hence the present study was carried out to investigate the antioxidant potential, macromolecule damage protective effect and neuroprotective activity of C. pluricaulis against H2O2 induced neuronal cell damage. Along with this we also analyzed the chemical composition and functional groups of C. pluricaulis by GC-MS and FTIR.
2. Materials and methods
2.1. Chemicals and reagents
DMEM-F12 from HIMEDIA (Bangalore, India), MTT (3-(4, 5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide), DCFH2DA, rhodamine 123, were obtained from Sigma (St Louis, MO, USA) while H2O2 was procured from Merck (Bangalore, India). AAPH (2,2′- Azo bis isobutyramidinium chloride), DPPH (2,2-diphenyl-1-picrylhydrazyl) gallic acid and quercetin were purchased from Sigma, Bangalore, India. FC reagent was procured from Merck, Bangalore, India. Whereas TPTZ (2,4,6-Tris (2-pyridyl)-s-triazine), was procured from Himedia, Bangalore, India and the other chemicals used were high-quality grade and were procured from SRL, Bangalore, India.
2.2. Plant material
C. pluricaulis plant was collected from Chandravana Ayurvedic plant nursery Mysore, India. The plant was identified by Dr. K. Madhava Chetty, Botanist, Department of Botany, Sri Venkateswara University, Tirupati, India. A voucher specimen (Herbarium Accession Number 2021) was deposited in the herbarium, Department of Botany, Sri Venkateswara University, Tirupati, India.
2.3. Preparation of plant extract of C. pluricaulis (CP)
Plant leaves were separated from the herb and washed thoroughly and dried in shade and powdered. The leaves powder was macerated with 70% ethanol in a shaker for 2 days. The extract was filtered and concentrated using flash evaporator and lyophilized to remove the residual water. The yield of extract was recorded as 9%.
2.4. GC–MS analysis of 70% ethanolic fraction of C. pluricaulis
The GC–MS analysis was performed in EI mode (70 eV) with an Agilent 7890 GC system, equipped with model 5975 mass selective detector (Agilent Technologies, USA). SGE BPX5 fused silica capillary columns (30 m × 0.32 mm id., 0.25 μm film thickness) were employed for separation, the column oven temperature was raised linearly from 80 °C (hold for 2 min) to 280 °C (hold for 5 min) at 20 °C/min. Helium was used as carrier gas at a constant flow of 1.2 ml/min. The samples were analyzed in splitless mode at an injection temperature of 250 °C, EI source temperature 230 °C and quadrupole analyzer at 150 °C, ionization current at 235 eV.
2.5. Fourier transformed infrared spectroscopy
The functional groups of active compounds of C. pluricaulis were analyzed using FTIR (Nicolet 380 Thermo) based on the peaks values in the region of IR radiation. The plant extract was passed into the FTIR, the functional groups of the compounds were separated based on its peaks ratio. The FTIR spectra were recorded in the absorption range between 4000 and 500 cm−1.
2.6. Polyphenol content
The total polyphenols were determined by Folin-Ciocalteu method.24 An aliquot of each extract was mixed with 0.5 ml Folin-Ciocalteu reagent (previously diluted with water 1:1 v/v) and incubated in boiling water bath for 10 min, to this mixture 2 ml of (7%) sodium carbonate was added. Absorbance was measured at 650 nm and gallic acid was used as a standard.
2.7. Flavonoid content
Plant extracts of different concentration were mixed with ethanol, 75 μl of sodium nitrite and 150 μl of aluminum chloride was added to test solution and incubated at room temperature for 5 min 1 N sodium hydroxide was added to all the samples and the final volume of the solution was made up to 1 ml using distilled water. The absorbance of the reaction mixture was measured at 510 nm using a spectrophotometer. Quercetin was used as a standard antioxidant.25
2.8. Free radical scavenging activity
Free radicals scavenging activity was determined by DPPH method. To different concentrations of plant extract, 500 μM DPPH solution was added and incubated in dark for 45 min at room temperature. BHA was used as standard and the absorbance was recorded at 515 nm.26 The scavenging activity was determined by calculating IC50 values using the equation.
DPPH_scavenging effect (%) = (ODcontrol − ODsamples/ODcontrol × 100).
2.9. Metal chelating activity
The chelating activity of C. pluricaulis was determined by the method of Dinis et al. (27). Different concentrations of plant extracts were mixed with 2 mM FeCl2 and the reaction was followed by the addition of 5 mM ferrozine and incubated at room temperature for 10 min. The absorbance was measured at 562 nm. EDTA was used as a standard. The percentage inhibition of ferrozine Fe2+ complex formation was calculated as:
% of chelating activity = (Acontrol − Asample)/Acontrol × 100
2.10. Ferric reducing antioxidant power (FRAP) activity
Total FRAP activity was determined using FRAP reagent. Different concentrations of test solution was prepared and to this 4.75 ml of FRAP reagent [2.5 ml of a 10 mM TPTZ solution in 40 mM HCl, 2.5 ml of 20 mM FeCl3·6H2O and 25 ml of 300 mM acetate buffer (pH 3.6)] was added and final volume was made up to 5 ml using distilled water. The absorbance was measured at 593 nm using a spectrophotometer. FeSO4 was used as a standard.28
2.11. Total antioxidant activity
The total antioxidant capacity was evaluated using ammonium molybdate reagent (H2SO4 0.6 M, ammonium molybdate 4 mM, sodium phosphate monobasic 28 mM). To different concentrations of plant extracts, ammonium molybdate reagent was added. The reaction mixture was boiled at 95 °C for 90 min. After cooling, the absorbance was measured at 695 nm. Gallic acid was used as a standard.29
2.12. Plasmid DNA nick assay
pUC19 Plasmid DNA was treated with AAPH to induce DNA damage and the DNA damage inhibitory activity of C. pluricaulis was analyzed by agarose gel electrophoresis. Plasmid DNA (200 ng) was incubated with 2.5, 5 and 10 μg of C. pluricaulis for 30 min. AAPH (10 mM) was added and incubated for 1 h. The DNA samples were analyzed on a 1% agarose gel in TBE buffer pH8.30
2.13. Protein oxidation assay
Protein oxidation was performed based on the method reported by Mayo et al. (31). Bovine serum albumin (BSA) was used as a source of protein and was challenged with H2O2 which decomposes the oxygen and generates peroxyl radicals. BSA (5 μg) was dissolved in water and incubated in the presence or absence of C. pluricaulis for 15 min followed by 1 h treatment with 100 μM H2O2. After incubations, the protein samples were subjected to SDS-PAGE electrophoresis. The gels were stained with 0.15% Coomassie brilliant blue R-250 and the amount of protein damage was quantified by measuring the density of each band using NIH Image J software.
2.14. Lipid peroxidation activity
Lipid peroxidation was quantitatively estimated according to the method followed by Wright et al. (32). Liver tissues were collected from male Wistar rats (3 to 4-month-old 120–130 g) and were homogenized. Liver homogenates were challenged with AAPH with or without C. pluricaulis and the final volume was made up to 0.5 ml using PBS and incubated for 2 h. After incubation, TCA and TBA were added and the contents were boiled at 95 °C for 20 min. The reaction mixtures were centrifuged at 2500xg. The supernatants were collected and the absorbance was measured at 535 nm using a spectrophotometer.
2.14.1. Cell culture and treatments
The human neuroblastoma cell line SH-SY5Y was obtained from NCCS (National Centre for Cell Sciences), Pune, India. The cells were cultured in Petri plates, flasks or dishes and maintained in DMEM/F-12 mixture supplemented with 10% FBS (Thermoscientific, Bangalore, India), and penicillin and streptomycin solution (at 10 ml/l, Sigma, Bangalore, India) in a humid atmosphere of 5% CO2 and 95% air at 37 °C. The media was changed on alternate days and 100 μM of H2O2 was added to the cells with or without pretreatment of C. pluricaulis for 2 h before any experiment.
2.14.2. Cell viability assay
The mitochondrial metabolic status of SH-SY5Y cells was assessed by 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The principle of MTT assay is based on the formation of formazan by mitochondrial succinate reductase on the cleavage of tetrazolium salts in viable cells.33 The cells were cultured at a density of 1 × 104 cell/ml in 96-well plates and incubated for 24 h before treatments. The cells were then subjected to the treatments of interest. After 24 h MTT (0.5 mg/ml) was added to the cells. Further, the cells were incubated for 2 h at 37 °C. The insoluble formazan crystals were dissolved by addition of DMSO. The absorbance was measured at 540 nm using a VERSA max Hidex plate chameleon TMV (Turku, Finland) and the cell viability was expressed as the percent of control.
2.14.3. Lactate dehydrogenase (LDH) leakage assay
The Plasma membrane damage of SHSY5Ycells was quantified by LDH-estimation kit (Agappe-11407002, Mysore, India) following the manufacturers' instructions. Increase in the cytosolic LDH leads to plasma membrane damage. The LDH leakage was measured through the oxidation of lactate to pyruvate with simultaneous reduction of nicotinamide adenine dinucleotide (NAD+) at a wavelength of 340 nm. The rate of increase in enzyme activity due to the formation of reduced nicotinamide adenine dinucleotide (NADH) is directly proportional to the LDH activity in the sample. The SH-SY5Y cells were plated at a density of 5 × 104 cells/well on 24-well plates and after 24 h of adherence, the cells were subjected to the treatments of interest. After the treatment period, 10 μl of cell lysis solution (2% Triton X-100) was added to the untreated cells, which were selected as the total LDH activity. The cells were separated by centrifugation at 2500xg for 5 min at 4 °C and the supernatant was measured for LDH activity.
2.14.4. Estimation of superoxide dismutase (SOD) and catalase (CAT) activities
The SOD antioxidant enzyme estimation was carried out by kit supplier protocol (Randox, Cat no. SD. 125, Canada) and the CAT was estimated by the method of Cohen et al(34). which is based on the decay of 6 mM H2O2 solution at 240 nm, measured by the spectrophotometric degradation method. The cells (1 × 104 cells/ml) were cultured in 75 cm2 flasks and treated as described earlier. After treatments, the cells were collected by trypsinization and lysed (50 mM potassium phosphate buffer, pH 7.4, 2 mM EDTA and 0.1% Triton X-100) by sonication and the cell debris was removed by centrifugation at 13,000xg for 10 min at 4 °C. The protein content in the supernatants was measured by Bradford method with BSA as a standard.
2.14.5. Estimation of intracellular ROS
The intracellular ROS was measured by oxidation-sensitive dye DCFH2DA.35 SH-SY5Y cells were seeded in 24-well plates (1 × 104 cells/ml) and treated as mentioned earlier. After treatments, DCFH2DA (5 mg/ml) was added to the cells and incubated for 30 min. After incubation cells were washed twice with PBS and the fluorescence was read at an excitation wavelength of 485 nm and an emission wavelength of 535 nm using Hidex plate chameleon ™ V (Finland). The cells were cultured on coverslips that were coated with poly-l-lysine for imaging. After treatments, the cells were incubated with DCFH2DA dye and washed with PBS to eliminate the excess of dye. The imaging was carried out using fluorescence microscope (Olympus) equipped with Cool SNAP® Pro color digital camera.
2.14.6. Measurement of mitochondrial membrane potential (MMP)
The mitochondrial membrane damage was measured using rhodamine 123 fluorescent probe. SH-SY5Y cells were seeded in 24-well plates (1 × 104 cells/ml) and treated as mentioned earlier. After treatments, rhodamine 123 dye (10 μg/ml) was added to the cells and incubated for 60 min at 37 °C. The cells were washed twice with PBS and the fluorescence was read at an excitation wavelength of 485 nm and an emission wavelength of 535 nm using Hidex plate chameleon ™ V (Finland). For imaging, the cells were cultured on coverslips that were coated with poly-l-lysine. After the experiment, the cells were incubated with rhodamine 123 dye and washed excess of dye with PBS. The cells were imaged using fluorescence microscope (Olympus) equipped with Cool SNAP® Pro color digital camera.
2.14.7. Single cell gel electrophoresis
SHSY5Y cells were treated with C. pluricaulis at different concentrations for 1 h followed by H2O2 treatment for 1 h and were mixed with 0.7% (w/v) low melting agarose. This mixture was loaded to the frosted slides pre-coated with 1.0% (w/v) normal melting agarose (NMA). Once the agarose set, the slides were covered with another of 0.7% (w/v) NMA. Slides were immersed in freshly prepared cold lysis buffer for 90 min. Later, the slides were transferred to an electrophoresis tank, and incubated in alkali buffer followed by electrophoresis with an electric current of 25 V/300 mA for 20 min. Further, the slides were washed twice with neutralizing buffer for 10 min and treated with ethanol for another 5 min followed by staining with 40 μl of ethidium bromide (20 μg/ml). Finally the DNA damage was evaluated with fluorescence microscope (Olympus, Japan) by measuring the percentage of fluorescence in tail using RS Image Pro® plus software and the results were expressed as percent inhibition of tail length.36
2.14.8. Immunoblotting
To perform immunoblotting the SHSY5Y cells seeded in 75 cm2 flasks and treated as mentioned earlier. After treatments cells were collected, washed with PBS and lysed with ice-cold RIPA buffer with protease and phosphatase inhibitor cocktail. The cell lysates were centrifuged at 12,000 rpm for 10 min at 4 °C, and the protein contents were estimated by Lowry et al.37 method. The protein separation was done by 10% SDS-PAGE followed by transfer to nitrocellulose membranes. The membranes were blocked with nonfat dry milk and incubated with primary antibodies namely SOD (sc-8637), CAT (sc-34280), p53 (sc-55476) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and caspase-3 (C8487, Sigma, St. Louis,MO,USA) at 1:1000 dilution for 3 h with shaking. The membranes were washed with TBST after incubation and the membranes were incubated in dark for 2 h with horseradish peroxidase conjugated rabbit anti-goat, goat anti-mouse and goat anti-rabbit secondary antibodies (DAKO, Denmark) at 1:10,000 dilutions. The membranes were developed using the enhanced chemiluminescence peroxidase substrate kit (CPS-160, Sigma, St. Louis, MO, USA).
2.14.9. Statistical analysis
The results were represented as the mean ± SD. Statistical significance was analyzed with one-way analysis of variance followed by a Tukey's HSD-post hoc test. Differences with a P value less than 0.05 were considered statistically significant.
3. Results
3.1. Metabolite analysis of C. pluricaulis by GC–MS
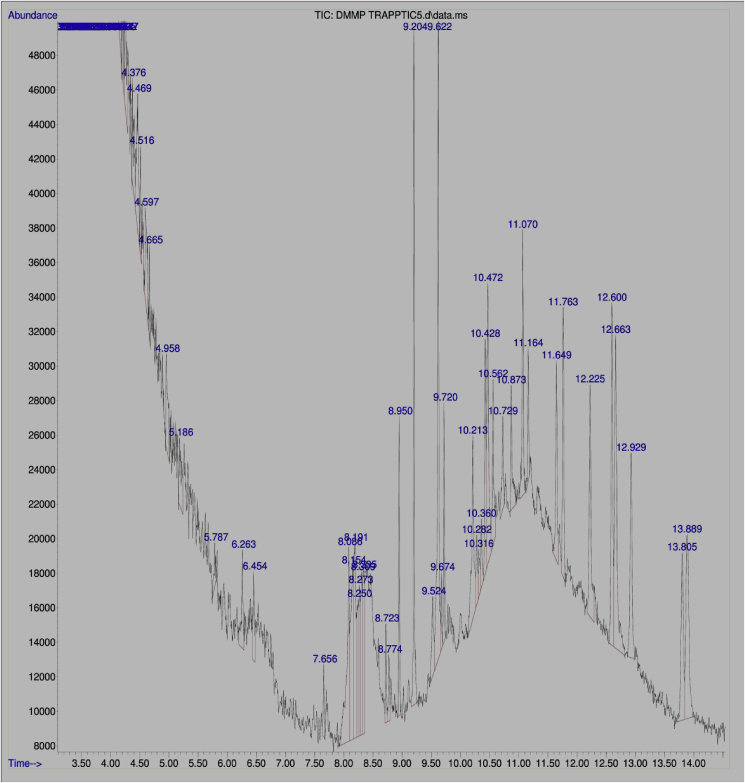
GC-MS is an analytical technique to identify the volatile compounds, alcohols, branched hydrocarbons, esters and other group of compounds. The GC-MS analysis of C. pluricaulis revealed the presence of 22 metabolites that could contain medicinal quality. The identification of metabolites was confirmed based on retention time, molecular weight and molecular formula. The GC–MS chromatogram (Fig. 1) analysis of C. pluricaulis showed a number of active compounds listed in Table 1.
Fig. 1.
GC–MS chromatogram of Convolvulus pluricaulis 70% ethanolic extract.
Table 1.
GC–MS analysis of phytochemical constituents of Convolvulus pluricaulis 70% ethanolic extract.
| S.No | compound | RT | Molecular weight | Molecular formula |
|---|---|---|---|---|
| 1 | 2-Butanone | 4.958 | 72.10572 | C4H8O |
| 2 | Pentanoic acid | 6.457 | 102.1317 | C5H10O2 |
| 3 | Cinnamic acid | 6.836 | 148.1586 | C9H8O2 |
| 4 | Silane | 6.928 | 32.117 | H4Si |
| 5 | Decanoic acid | 6.985 | 172.26 | C10H20O2 |
| 6 | 2-Pentanol | 8.191 | 88.148 | C5H12O |
| 7 | Ascorbic acid | 8.209 | 176.12 | C6H8O6 |
| 8 | 10-Bromodecanoic acid | 8.341 | 251.16 | C10H19BrO2 |
| 9 | tridecane | 8.774 | 184.36 | C13H28 |
| 10 | Phthalic acid | 9.204 | 166.14 | C6H4(COOH)2 |
| 11 | eicosane | 10.555 | 282.5475 | C20H42 |
| 12 | Octatriacontyl pentafluoropropionate | 10.560 | 697.0409 | C41H77F5O2 |
| 13 | 1-Octadecanesulphonyl chloride | 11.647 | 353.003 | C18H37ClO2S |
| 14 | Squalene | 11.791 | 410.718 | C30H50 |
| 15 | pyrimidine | 11.866 | 80.088 | C4H4N2 |
| 16 | Heneicosane | 12.225 | 296.57406 | C21H44 |
| 17 | 1,2-Benzenedicarboxylic acid | 12.602 | 166.1308 | C8H6O4 |
| 18 | Cyclononasiloxane, octadecamethyl | 12.665 | 667.3855 | C18H54O9Si9 |
| 19 | Nonacosane | 12.821 | 408.6 | C29H60 |
| 20 | Sulfurous acid pentadecyl 2-propyl ester | 12.929 | 334.55752 | C18H38O3S |
| 21 | Vitamin E | 13.010 | 430.7061 | C29H50O2 |
| 22 | Cyclononasiloxane | 13.890 | 370.7697 | C10H30O5Si5 |
3.2. Functional group analysis of C. pluricaulis by FTIR
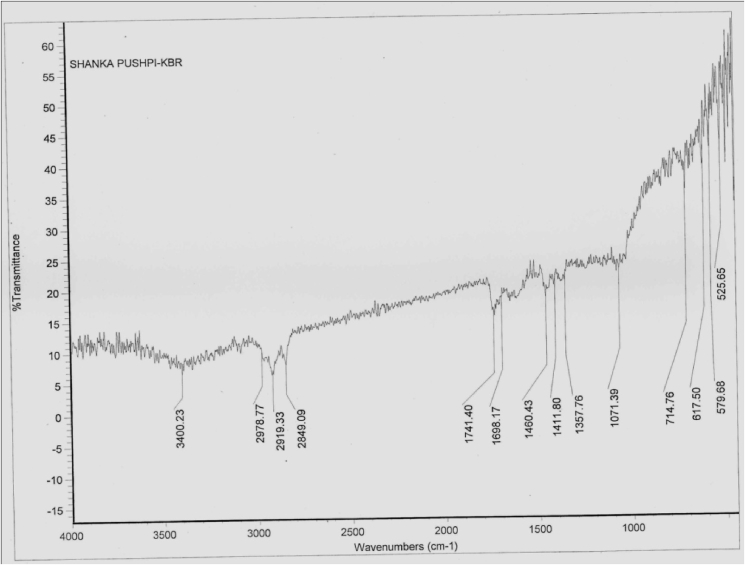
The FTIR spectrum identified functional groups of the active components based on peak value in the region of IR radiation. The results revealed the presence of alcohols, carboxylic acids, acid anhydrides, alkanes, aldehydes, phenols, alkanes, alkynes, alkyl halides, aldehydes, aromatics, halogens, esters. (Fig. 2 and Table 2).
Fig. 2.
FTIR spectrum of Convolvulus pluricaulis 70% ethanolic extract.
Table 2.
FTIR Peak Values and Functional groups of Convolvulus pluricaulis 70% ethanolic extract.
| Sl. No | Peak values | Functional groups |
|---|---|---|
| 1 | 3400.23 | Alcohol |
| 2 | 2978.77 | Alkanes |
| 3 | 2919.33 | Alkanes |
| 4 | 2849.09 | Carboxylic acids |
| 5 | 1741.40 | Acid Anhydrides |
| 6 | 1698.17 | Aldehydes |
| 7 | 1460.43 | Alkenes |
| 8 | 1411.80 | Aromatics |
| 9 | 1357.76 | Alkanes |
| 10 | 1071.39 | Ethers |
| 11 | 714.76 | Aromatic compounds |
| 12 | 617.50 | Halogen |
| 13 | 579.68 | Halogen |
| 14 | 525.65 | Alkyl halides |
3.3. Total polyphenols and flavonoids
The total polyphenol content of C. pluricaulis is expressed as gallic acid equivalents and it is found to be 123 ± 3.4 μg GAE/mg (Gallic acid) and the flavonoid content is expressed as quercetin equivalents and it is found to be 164 ± 2.0 μg QE/mg (Quercetin) (Table 3).
Table 3.
Antioxidant and free radical scavenging activities of Convolvulus pluricaulis. Each value represents the mean ± SD of three determinations.
| Assay | 70% ethanolic extract (CP) |
|---|---|
| Total polyphenolic content | 123 ± 3.4 GAE/mg |
| Total flavonoids | 164 ± 2.0 QE/mg |
| Total antioxidant activity | 92 ± 2.3 μg GAE/mg |
| FRAP | 55.6 ± 3.2 IC50 (μg/ml) |
| DPPH | 34.46 ± 3.4IC50 (μg/ml) |
| Metal chelating | 40.5 ± 4.8IC50 (μg/ml) |
| Anti-Lipid peroxidation | 45 ± 3.8 IC50 (μg/ml) |
3.4. Free radical scavenging activity
DPPH method was used to measure the hydrogen atom or electron donor capacity of the extracts and it measures the capacity of scavenged free radicals in solution.38 The reduction capability of DPPH radicals was determined by the decrease in the absorbance of plant extracts. Hence, DPPH was used to evaluate the antioxidant activity. The DPPH radical scavenging ability of C. pluricaulis was found to be 34.46 ± 3.4 IC50 (Table 3).
3.5. Ferric reducing antioxidant power (FRAP) assay
The principle of FRAP assay is based on the reduction of the colorless FeIII-TPTZ complex to blue colored FeII-TPTZ complex, by the action of electron donating antioxidants in biological samples.39 In this study, the capacity of the extract to reduce iron (III) to iron (II) was determined and compared to FeSO4, which is known for its strong reducing properties (Table 3). Ferric reducing antioxidant power was found to be 55.6 ± 3.2 IC50 (μg/ml).
3.6. Metal chelating activity
The chelating of ferrous ions by C. pluricaulis was estimated by the method of Dinis et al.27 Ferrozine quantitatively forms complexes with Fe2+. In the presence of other chelating agents, the complex formation is disrupted and with the result, the red color of the complex is decreased. Measurement of the rate of color reduction, therefore, allows estimation of the chelating activity of the coexisting chelator. In this assay the extracts of C. pluricaulis and standard compounds interfered with the formation of ferrous and ferrozine complex, suggesting that it has chelating activity and captures ferrous ion before ferrozine. The metal chelating activity was found to be 40.5 ± 4.8 IC50 (μg/ml) (Table 3).
3.7. Total antioxidant capacity
Total antioxidant capacity of C. pluricaulis expressed as the number of equivalents of gallic acid. The phosphomolybdenum method was based on the reduction of Mo (VI) to Mo (V) by the antioxidant compound and the formation of a green phosphate/Mo (V) complex with a maximal absorption at 695 nm.40 The total antioxidant capacity of C. pluricaulis is expressed as gallic acid equivalents and is found to be 92 ± 2.3 μg GAE/mg (Table 3).
3.8. Plasmid DNA nick assay
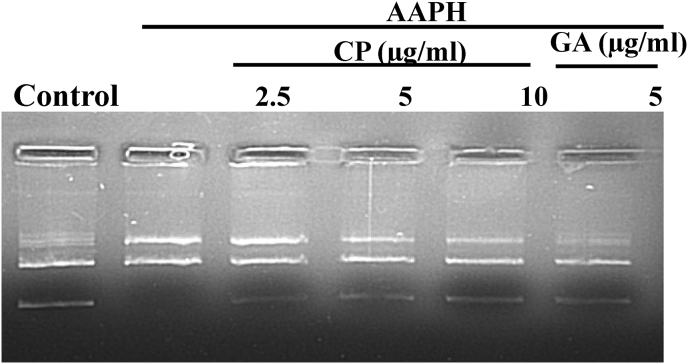
DNA is one of the major targets of free radicals that cause DNA damage. Under normal physiological conditions, the endogenous production of free radicals may lead to a minimal damage in DNA. Hydroxyl radical generated by Fenton's reaction, attacks on nitrogenous bases of DNA and sugar moiety of supercoiled pUC19 plasmid DNA, resulting in nicked circular form due to breakage of sugar-phosphate backbone of nucleic acid. The damage was effectively minimized by treatment with C. pluricaulis extract (2.5, 5 and10 μg) as shown in Fig. 3.
Fig. 3.
Protective effect of C. pluricaulis against AAPH induced pUC19 DNA damage.
3.9. Protein oxidation assay
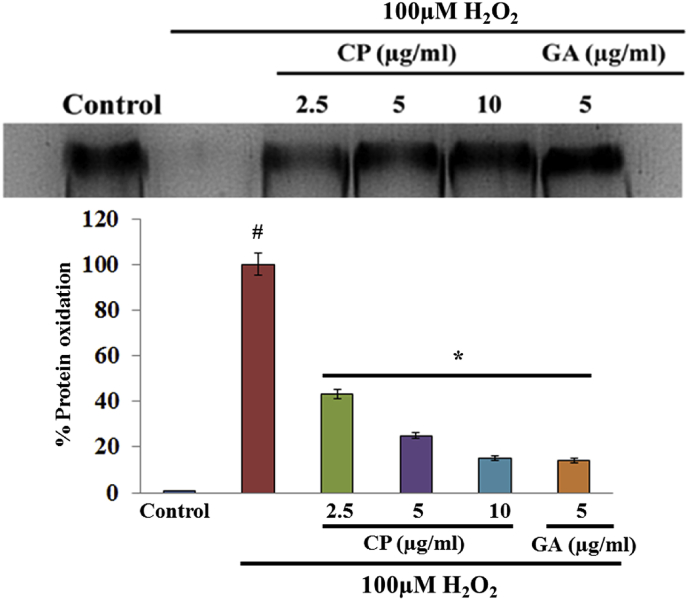
The oxidative protein damages caused by free radicals were initiated by radical-mediated electron leakage. The protection of the hydroxyl-mediated oxidation of BSA takes place by reducing the H2O2 concentration, a fundamental component in Fenton-type reaction, by chelating iron or by scavenging the hydroxyl radical formed on the immediate side of the target protein during oxidation. This may suggest that C. pluricaulis is able to scavenge hydroxyl radical or chelate iron. BSA is pretreated with 2.5, 5 and 10 μg of C. pluricaulis and it completely prevented this oxidative degradation of BSA (Fig. 4).
Fig. 4.
Protective effect of C. pluricaulis against H2O2 induced Protein oxidation.
3.10. Lipid peroxidation activity
Lipid peroxidation initiates by attack on a fatty acid fatty acyl side chain of any chemical species that as sufficient reactivity to abstracts hydrogen atom to methylene carbon in the side chain. Lipid peroxidation takes place by ferrous sulfate either through hydroxyl radical generation or ferryl–perferryl complex. It was estimated by the levels of malondialdehyde measured using the thiobarbituric acid reactive substances (TBARS). Increase in TBARS level causes the lipid peroxidation. In our present study, C. pluricaulis extract showed a decrease in TBARS level with an IC50 of 45 ± 3.8 (μg/ml) that indicates the lipid peroxidation inhibitory activity of C. pluricaulis (Table 3).
3.11. Protective effect of C. pluricaulis against H2O2 induced cytotoxicity
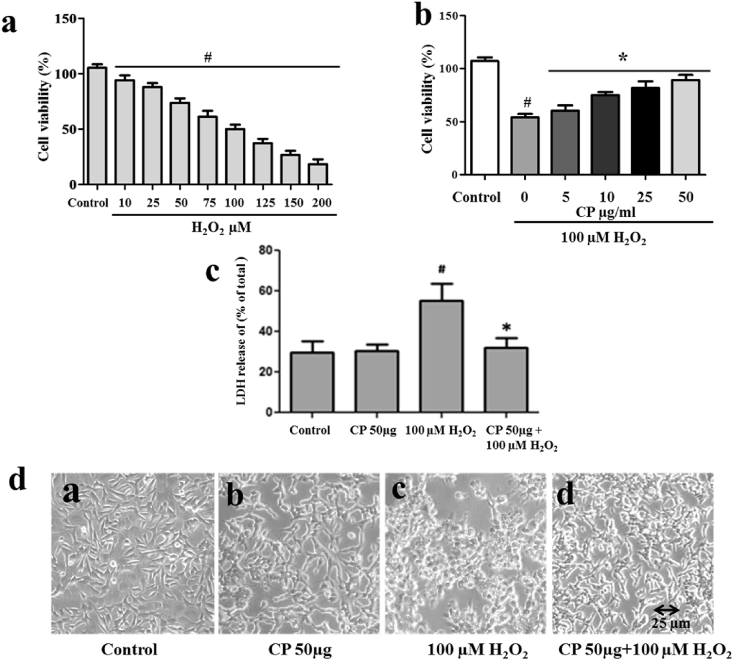
In the present study, we have evaluated the protective effect of C. pluricaulis against H2O2 challenge by MTT reduction assay in cultured SH-SY5Y cells. The assay is based on the principle that mitochondrial dehydrogenase reduces the MTT dye to formazan. The H2O2 (10–200 μM) treatment decreased the cell proliferation in a dose-dependent manner, and the cell viability was found to be 50% with 100 μM H2O2 treatment which was used for further assays (Fig. 5a). However, the cells pretreated with different concentrations of C. pluricaulis (5–50 μg) for 2 h before 100 μM H2O2 treatment (24 h) showed significant improvement in cell survival up to 50% with 50 μg of C. pluricaulis (Fig. 5b).
Fig. 5.
a Cytotoxic effects H2O2 on SHSY5Ycells.b Dose dependent protective effect of treatment with CP on H2O2 induced cytotoxicity in SHSY5Y cells, the cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. c Protective effect of C. pluricaulis pretreatment on H2O2 induced cytotoxicity by LDH leakage assay. The data are represented as mean ± SD of three independent experiments. #P < 0.01 versus control group,*P < 0.01 versus 100 μM H2O2 treated group. d Effects of CP pretreatment and 100 μM H2O2 induced morphological alterations in SH-SY5Y neurons observed by phase contrast microscopy.
3.12. Lactate dehydrogenase (LDH) leakage assay
The cytotoxicity of H2O2 and the protective activity of C. pluricaulis were further evaluated by LDH assay, which is based on the principle that the leakage of cytosolic LDH increases as the number of dead cells increases. SH-SY5Y cells were pretreated with 50 μg/ml of C. pluricaulis for 2 h, before treatment with 100 μM H2O2 for 24 h (Fig. 5c). The results show that the release of LDH is 57% of the total enzyme with 100 μM H2O2 which indicates that H2O2 induces cytotoxicity in the SH-SY5Y cells. In contrast, C. pluricaulis pretreatment lowered the LDH release up to 25% as compared with 100 μM H2O2 treated cells. In morphological observation, H2O2 treated cell appeared shrinkage which was protected with C. pluricaulis (Fig. 5d). The protective effect of C. pluricaulis was also confirmed by bright field microscope.
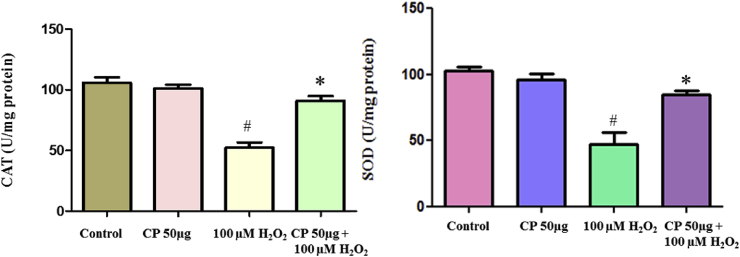
3.13. Estimation of superoxide dismutase (SOD) and catalase (CAT) activities
In the present study, we measured the SOD and CAT enzyme activities by spectrophotometric degradation method, which shows that the oxidative damage generated by H2O2 leads to the decreased enzyme activity of SOD and CAT. The pretreatment of C. pluricaulis significantly restored the antioxidant enzyme levels (Fig. 6).
Fig. 6.
Pre-treatment of C. pluricaulis for the restoration of SOD and catalase enzyme activities in SH-SY5Y cells challenged with 100 μM H2O2. The data are represented as mean ± SD of three independent experiments. #P < 0.01 versus control group, *P < 0.01 versus 100 μM H2O2 treated group.
3.14. Estimation of intracellular ROS
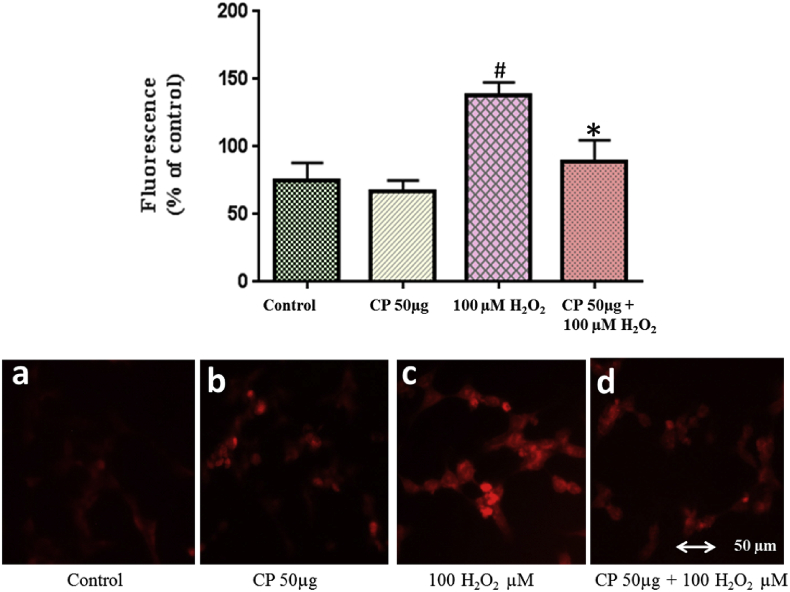
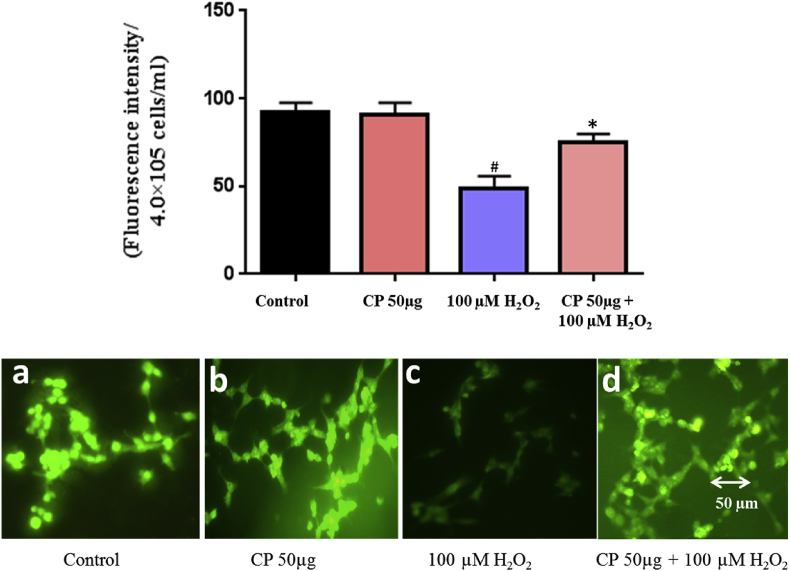
The H2O2 induced ROS generation and protective effects of C. pluricaulis were measured using fluorescent probe DCFH2DA. The fluorescence intensity of neuronal cells was 140% with 100 μM H2O2 challenge as compared with the control group. In cells pre-treated with C. pluricaulis followed by 100 μM H2O2 treatment, the fluorescence intensity was decreased up to 70% that was further confirmed by fluorescence imaging (Fig. 7).
Fig. 7.
Estimation of intracellular ROS production using DCFH2DA in SHSY5Y cells pre-treated with C. pluricaulis on 100 μM H2O2 challenge by spectrofluorimeter. The ROS generation was monitored by fluorescent microscope. The data are represented as mean ± SD of three independent experiments. #P < 0.01 versus control group,*P < 0.01 versus 100 μM H2O2 treated group.
3.15. Measurement of mitochondrial membrane potential (MMP)
In the present study, the mitochondrial damage was estimated by measuring, the accumulation of fluorescent probe, rhodamine 123 by MMP assay. Here, we observed 49% decrease in MMP with 100 μM H2O2 challenge which indicates the depolarization of the mitochondrial membrane. However, the cells pre-treated with C. pluricaulis prior to the addition of 100 μM H2O2, showed a significant regain in the fluorescence intensity to an extent of 80% of control (Fig. 8) that was further confirmed by fluorescence imaging.
Fig. 8.
Estimation of mitochondrial membrane potential in SHSY5Y cells with pre-treatment of C. pluricaulis on 100 μM H2O2. The fluorescence intensity was determined using a spectrofluorimeter. The membrane potential was monitored by fluorescent microscope. The data are represented as mean ± SD of three independent experiments. #P < 0.01 versus control group,*P < 0.01 versus 100 μM H2O2 treated group.
3.16. Single cell gel electrophoresis
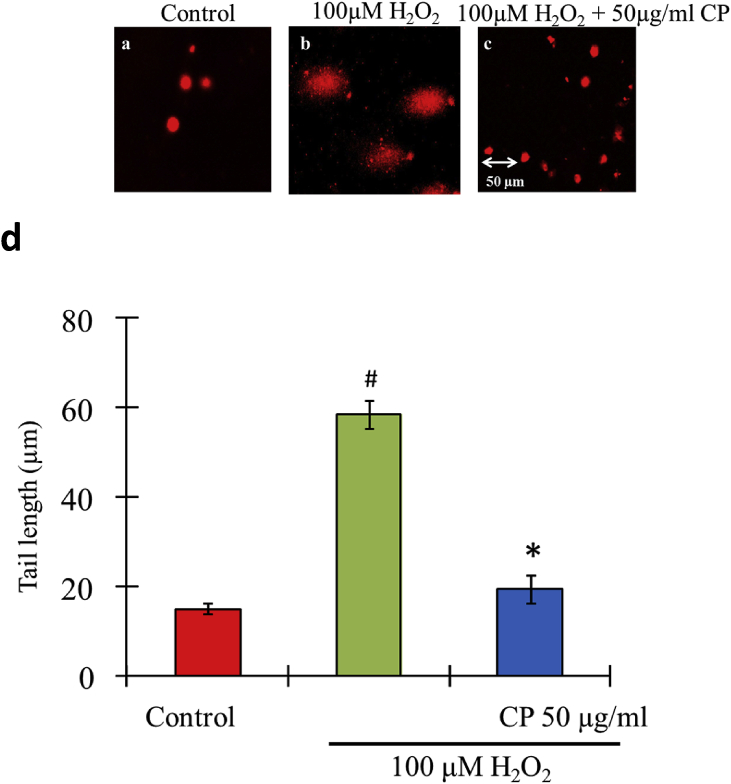
The oxidative DNA damage of SH-SY5Y cells induced by H2O2 and its protective efficacy of C. pluricaulis is measured by single cell gel electrophoresis. Here the quantification of DNA damage is done by measuring: (a) tail length (TL), (b) head length (HL) relative to the total amount of DNA damage. These parameters are employed by the Image-Pro plus software to determine the level of DNA damage. SHSY5Y cells exhibited a tail length of ∼15 μm and the cells treated with 100 μM H2O2 exhibited the DNA damage with an increase in tail length up to ∼62 μm. The cells pretreated with C. pluricaulis followed by H2O2 treatment showed decreased comet tail length to ∼18 μm that exhibited significant DNA damage inhibitory effect up to ∼84% against H2O2 mediated DNA damage (Fig. 9).
Fig. 9.
Protective effect of C. pluricaulis on DNA damage induced by 100 μM H2O2 in SHSY5Y cells. (a) Control cells without any treatment (b) Cells with 100 μM H2O2 treatment (c) Cells were pre-treated with C. pluricaulis for 2 h at 50 μg/assay and treated with 100 μM H2O2 (duration: 24 h) Tail length (50 μm) Bars. #P < 0.05 versus control group, *P < 0.05 versus H2O2 treated group.
3.17. Protective effects of C. pluricaulis on oxidative stress biomarkers
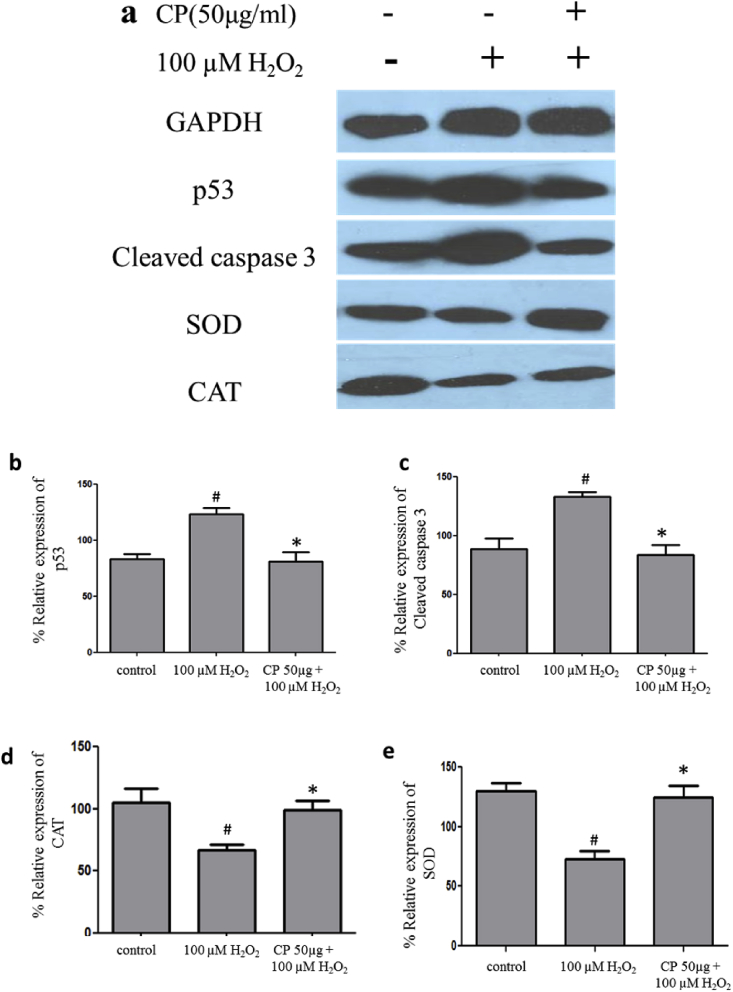
To investigate the antioxidant effects of C. pluricaulis against H2O2 mediated oxidative stress, antioxidant enzyme activities of SOD and CAT were measured in SH-SY5Y cells. In our study, SOD and CAT enzyme activities were decreased with H2O2 treatment. However, C. pluricaulis pretreatment significantly restored the level of the antioxidant enzymes. Similarly, the expression of antioxidant biomarker proteins down-regulated with H2O2 treatment, which was significantly restored with C. pluricaulis pretreated cells, which demonstrate plausible antioxidant defense potential of C. pluricaulis (Fig. 10).
Fig. 10.
The protective effect of pre-treatment of C. pluricaulis on H2O2 induced expression of oxidative stress marker proteins SOD, CAT and apoptosis marker proteins caspase 3, p53 analyzed by Western blotting. (b,c,d,e) The band intensity is calculated by the Image-J software. The data are represented as mean ± SD of three independent experiments. #P < 0.05 versus control group, *P < 0.05 versus H2O2 treated group.
3.18. Protective effect of C. pluricaulis on apoptotic biomarkers
Caspase-3 and p53 play a role in cell death, and we evaluated the expression of these apoptotic marker proteins. In the current study, we observed increased expression of caspase-3 and p53 with H2O2 treatment. However, C. pluricaulis pretreatment significantly reduced the expression of these apoptotic marker proteins (Fig. 10).
4. Discussion
Several studies reported that herbal antioxidants are exogenous or endogenous molecules, those act against any form of oxidative stress and they neutralize ROS and other kinds of free radicals. The metabolites of C. pluricaulis was analyzed by GC-MS and here 22 metabolites were detected which consists of saturated and unsaturated fatty acids, alkaloid, sterols, vitamins and inorganic compounds.14 The metabolites such as cinnamic acid, vitamin E, ascorbic acid are used to treat obesity, cardiovascular disease, Alzheimer disease and blood pressure. Phthalic acid is used as antimicrobial agent.41, 42, 43, 44 Vitamin supplementation proves the neuroprotective effect and to treat Parkinson's.45, 46, 47, 48 The FTIR spectrum was used to identify the functional group of the active components based on the peak value in the region of infrared radiation. In the FTIR analysis alcohol, alkanes, carboxylic acids, acid anhydrides, aldehydes, aromatic compounds, and halogens were identified. FTIR spectroscopy is proved to be a reliable and sensitive method for detection of the biomolecular composition. This is the first study to analyze the metabolites of C. pluricaulis by GC-MS and FTIR. The peak at 2978, 2919, 2849, 1460, 1411, 1357, 714 observed C-H binding vibration. 1698, 617, 579, 525 observed C=C binding vibration, 1741 showed C=O stretch, 1071 as C-O binding vibration and 3400 which indicates OH stretch. Some of the reports confirmed these peak value and functional groups from other plant extracts such as Acorus calamus, Warburgia ugandensis, Boerhaavia diffusa etc.49, 50, 51, 52
The phytochemical compounds identified by GC-MS analysis of C. pluricaulis have been also reported to possess various biological activities. Cinnamic acid and its derivatives significantly inhibited the formation of advanced glycation end products and showed strongest inhibitory activity against the formation of AGEs and also prevented oxidative protein damages, including effects on protein carbonyl formation and thiol oxidation of BSA.53 Cinnamic acid decreased DNA damage induced by H2O2 in human lymphocytes.54 Squalene is a triterpene and it exhibited an antioxidant effect in a model of lipid peroxidation of liposomes with an IC50 value of 0.023 mg/ml.55 In an earlier study, Behl et al.56 showed that Vitamin E protects nerve cells from amyloid β protein induced toxicity. Ascorbic acid has been reported for its diverse neuroprotective activities, such as improvement of cognition57; to treat epilepsy,58 and also inhibits seizures.59 Thus it can be postulated that the identified bioactive compounds of C. pluricaulis may account for the observed antioxidant and neuroprotective effects against H2O2 induced oxidative stress in SH-SY5Y cells.
C. pluricaulis contains rich phenolics and flavonoids and is used as a therapeutic agent against neurodegenerative diseases, cancer, diabetes, cardiovascular dysfunctions, inflammatory diseases and also aging. The phenolics have the free radical scavenging, chelation of redox-active metal ions, modulation of gene expression and interaction with the cell signaling pathways.60, 61, 62, 63 The plasmid DNA pUC19 which consists of 3 forms single-stranded relaxed nicked DNA (R Form) and double-stranded nicked and linear DNA (L-Form). Plasmid DNA damage caused by hydroxyl (•OH) radicals generates Fenton reaction which makes DNA strands to yield its open circular or relaxed forms. Hydroxyl radicals react with nitrogenous bases of DNA producing base radicals and sugar radicals. The base radicals, in turn, react with the sugar moiety causing breakage of sugar phosphate backbone of nucleic acid, resulting in strand break.64
Herbal extracts can restore the DNA when it in the Fenton state and protect the DNA damage from the radicals.65, 66 The observed results corroborate with our recent study which demonstrated that Picrorhiza kurroa inhibits AAPH induced DNA damage.65 Major molecular mechanisms, leading to structural changes in proteins are free-radical mediated protein oxidation characterized by carbonyl formation. The protection of oxidized protein mediated by scavenging the peroxyl radical from the antioxidants. Hence the C. pluricaulis inhibited the free radicals produced by H2O2 because which contains polyphenols and flavonoids. Lipid peroxidation was estimated by measuring thiobarbituric acid-reactive substances. Lipid peroxidation is an oxidative alteration of polyunsaturated fatty acids in the cell membranes that generates a number of degradation products. MDA, one of the products of lipid peroxidation, has been studied widely as an index of lipid peroxidation and as a marker of oxidative stress. This oxidation of protein and lipid peroxidation can be protected by several herbal plants.65, 67 Treatment with C. pluricaulis also showed the protective effect induced by H2O2 and which is compared to control it was significantly decreased the oxidative damage.
Several herbal extracts have been shown to inhibit the H2O2 induced neuronal cell damage.68, 69 In the present study, we have evaluated the protective effect of C. pluricaulis against H2O2 challenge by MTT reduction assay in cultured SH-SY5Ycells. The assay is based on the principle that mitochondrial dehydrogenase reduces the MTT dye to formazan. The cytotoxicity of H2O2 and the protective activity of C. pluricaulis were further evaluated by LDH assay, which is based on the principle that the leakage of cytosolic LDH increases as the number of dead cells increases. Antioxidant enzymes such as SOD and catalase play a vital role in detoxification of free radicals generated due to oxidative damage of the cell. The decrease in antioxidant enzymes level has been reported in chemical/oxidative stress challenge and which leads to several diseases. Supplementation of a diet rich of antioxidant enhances the defense system to detoxify the oxidative molecules.70, 71 In the present study we measured the SOD and CAT activity by spectrophotometric degradation method which shows that the oxidative damage generated by H2O2 leads to the decreased enzyme activity of SOD and CAT.
The pretreatment of C. pluricaulis significantly restored the antioxidant enzyme levels. The oxidative damage of neuronal cells induced by H2O2 has been found to increase ROS generation and mitochondrial membrane potential. The ROS generation and mitochondrial health were determined by fluorescent probe DCFH2DA and rhodamine 123. DCFH2DA is a nonionic, nonpolar dye that crosses cell membranes and is enzymatically hydrolyzed by intracellular esterases to nonfluorescent DCFH that is oxidized to highly fluorescent dichlorofluorescein (DCF) in the presence of ROS. Hence, total ROS was quantified by estimation of the intracellular DCF fluorescence, where the emitted fluorescence is directly proportional to the concentration of ROS. The rhodamine 123 lipophilic cationic dye partitions into mitochondria and interacts with the negative charges on the inner membrane of mitochondria. Whereas the radical-induced damage of mitochondria partitions the dye to the cytosol. Hence, mitochondrial accumulation of the dye is proportional to mitochondrial health and membrane potential. Earlier studies also reported that herbal extracts decrease intracellular levels of ROS and also showed a reduction in the MMP induced by oxidative stress.70, 71, 72
Hence the present study also followed the previous report and C. pluricaulis showed the significant inhibition property induced by H2O2.72, 73, 74 The Comet Assay, or single cell gel electrophoresis assay provides a simple and effective method for evaluating DNA damage in the cells. Under the electric field denatured cleaved DNA moved faster (the “comet tail”) than undamaged DNA, it remains within the confines of the cell membrane (the “comet head”) when a current is applied. The tail length of the comet is measured as an index of DNA damage in the cell. The fragmented DNA in the form of tail dispersion increased with H2O2 challenged with neuronal cell lines. There are several reports showed that stress-induced ROS generation has been implicated to cause DNA damage that can be regulated by antioxidant supplementation.75, 76, 77 In the present study also C. pluricaulis regulates the tail length which significantly protects the DNA damage. The results clearly indicate that H2O2 induced SHSY5Y cells damage was successfully overcome by the active compounds present in C. pluricaulis.
Antioxidant biomarkers such as SOD and CAT plays a crucial role in cellular defense mechanism. Prehn et al.78 and Lee et al.79 showed that oxidative stress seems to be more in neuronal stress, specifically in Parkinson's and Alzheimer's diseases. Therefore we made an attempt to confirm the effect of C. pluricaulis against H2O2 induced stress by immunoblotting. C. pluricaulis pretreatment successfully restored the protein expression of SOD and CAT which was challenged with H2O2. All the protein levels were found consistent with their enzymatic activity this may be associated with inhibition of ROS production by the enhanced antioxidant enzyme activities by C. pluricaulis pretreatment. Earlier Dhuna et al.23 has also demonstrated the neuroprotective role of C. pluricaulis against H2O2. Caspase-3 activated p53 enzymes play an essential role in the certain model of neuronal apoptosis according to Cregan et al.80 Activation of these enzymes leads to several neurodegenerative diseases.81, 82, 83 Hence study was conducted to evaluate the protein expression of caspase-3 and p53 by C. pluricaulis pretreatment using western blotting. According to Migliore and Coppede73 fundamental oxidative challenge to living organisms eventually, have a causal role in neurodegenerative diseases. The H2O2 oxidant may activate multiple signaling pathways hence influences cytotoxicity.84 Our results revealed a significant inhibition of caspase-3 and p53 by the C. pluricaulis pretreatment in SH-SY5Y cells challenged with H2O2. This is the first report that demonstrated that C. pluricaulis pretreatment inhibits apoptosis markers induced by the H2O2 challenge. Recent elegant studies were also reported by Kwon et al.84 Azmi et al.85 Law et al.86 Jiang et al.87 on amelioration of H2O2 induced neurotoxicity by herbal extracts and phenol/flavonoids.
5. Conclusion
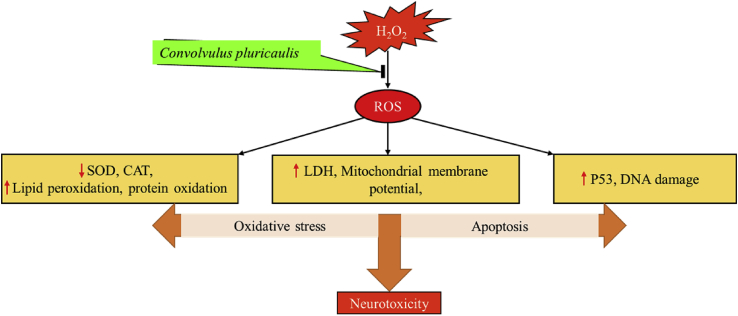
The results of our present study suggest that C. pluricaulis exhibits rich antioxidant status evident by free radical scavenging activities. C. pluricaulis attenuates macromolecule damage induced by AAPH. Further pretreatment of C. pluricaulis diminishes neuronal damage induced by H2O2 by decreasing oxidative stress and ameliorating apoptosis. Further GC-MS and FTIR analysis show a wide variety of bioactive compounds which were reported for the antioxidant effects. These data suggest that C. pluricaulis may be employed to treat stress-induced neurodegeneration (Fig. 11). Further investigation with animal models and clinical trials are needed to validate the use of C. pluricaulis has a therapeutic agent.
Fig. 11.
Neuroprotective effects of Convolvulus pluricaulis are mediated via its anti-oxidant and anti-apoptotic activity against H2O2 induced neurotoxicity in SH-SY5Y human neuronal cells.
Acknowledgements
The authors are highly thankful to Ex Director Dr. HV Batra and Current Director Dr. R K Sharma, DFRL, Mysuru for constant encouragement throughout the study. Thanks to chaya university of Mysuru for FTIR analysis.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Halliwell B. Role of free radicals in the neurodegenerative diseases. Drugs Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- 2.Sharma P., Jha A.B., Dubey R.S., Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. 2012;24:2012. [Google Scholar]
- 3.Fox J.T., Sakamuru S., Huang R. High-throughput genotoxicity assay identifies antioxidants as inducers of DNA damage response and cell death. Proc Natl Acad Sci Unit States Am. 2012;109:5423–5428. doi: 10.1073/pnas.1114278109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pande D., Negi R., Khanna R.S., Khanna H.D. Protein damage and antioxidant status alterations caused by oxidative injury in chronic myeloid leukemia. Einstein J Biol Medicine. 2016;27:55–58. [Google Scholar]
- 5.Selvaraju V., Joshi M., Suresh S., Sanchez J.A., Maulik N., Maulik G. Diabetes, oxidative stress, molecular mechanism, and cardiovascular disease–an overview. Toxicol Mech Meth. 2012;22:330–335. doi: 10.3109/15376516.2012.666648. [DOI] [PubMed] [Google Scholar]
- 6.Sanders L.H., Greenamyre J.T. Oxidative damage to macromolecules in human Parkinson disease and the rotenone model. Free Radic Biol Med. 2013;62:111–120. doi: 10.1016/j.freeradbiomed.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verri M., Pastoris O., Dossena M. Mitochondrial alterations, oxidative stress and neuroinflammation in Alzheimer's disease. Int J Immunopathol Pharmacol. 2012;25:345–353. doi: 10.1177/039463201202500204. [DOI] [PubMed] [Google Scholar]
- 8.Chen C.M., Wu Y.R., Cheng M.L. Increased oxidative damage and mitochondrial abnormalities in the peripheral blood of Huntington's disease patients. Biochem Biophys Res Commun. 2007;359:335–340. doi: 10.1016/j.bbrc.2007.05.093. [DOI] [PubMed] [Google Scholar]
- 9.Van Dam R.M., Naidoo N., Landberg R. Dietary flavonoids and the development of type 2 diabetes and cardiovascular diseases: review of recent findings. Curr Opin Lipidol. 2013;24:25–33. doi: 10.1097/MOL.0b013e32835bcdff. [DOI] [PubMed] [Google Scholar]
- 10.Williams R.J., Spencer J.P. Flavonoids, cognition, and dementia: actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic Biol Med. 2012;52:35–45. doi: 10.1016/j.freeradbiomed.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Ciz M., Denev P., Kratchanova M., Vasicek O., Ambrozova G., Lojek A. Flavonoids inhibit the respiratory burst of neutrophils in mammals. Oxidative Medicine Cell Longev. 2012;2012(23) doi: 10.1155/2012/181295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satish Bhalerao A., Deepa Verma R., Nikhil Teli C., Ashwin A., Trikannad Ethnobotany, phytochemistry and pharmacology of Convolvulus pluricaulis, choisy. Res J Pharmaceut Biol Chem Sci. 2014;5(3):629–636. [Google Scholar]
- 13.Chandel Urvashi, Kharoliwa Shivali. A review on traditional Indian herbs Convolvulus pluricaulis Linn and its medicinal importance. Int j pure appl biosci bioscience. 2014;2:326–329. [Google Scholar]
- 14.Agarwa P., Sharma B., Fatima A., Jain S.K. An update on Ayurvedic herb Convolvulus pluricaulis Choisy. Asian Pac j trop biomed. 2014;4:245–252. doi: 10.1016/S2221-1691(14)60240-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma P. Cinnamic acid derivatives: a new chapter of various pharmacological activities. J Chem Pharmaceut Res. 2011;3:403–423. [Google Scholar]
- 16.Kidd B.L., Photiou A., Inglis J.J. Novartis Foundation Symposium. 2004. The role of inflammatory mediators on nociception and pain in arthritis; pp. 122–138. [PubMed] [Google Scholar]
- 17.Nahata A., Patil U.K., Dixit V.K. Anxiolytic activity of Evolvulus alsinoides and Convulvulus pluricaulis in rodents. Pharmaceut Biol. 2009;47:444–451. [Google Scholar]
- 18.Dubey GP, Agrawal A, Dubey N, et al, Inventors. Novel Herbal Formulation for the Modulation of Immune System of Hiv Infected Patients and a Process of Preparation Thereof. United States Patent Application US 13/465. 2012:656.
- 19.Ravichandra V.D., Ramesh C., Sridhar K.A. Hepatoprotective potentials of aqueous extract of Convolvulus pluricaulis against thioacetamide induced liver damage in rats. Biomed Aging Pathol. 2013;3:131–135. [Google Scholar]
- 20.Mishra S.H., Sethiya N.K. Review on ethnomedicinal uses and phytopharmacology of memory boosting herb 'Convolvulus pluricaulis' Choisy. Aust J Med Herbal. 2010;22:19. [Google Scholar]
- 21.Bihaqi S.W., Sharma M., Singh A.P., Tiwari M. Neuroprotective role of Convolvulus pluricaulis on aluminium induced neurotoxicity in rat brain. J Ethnopharmacol. 2009;124:409–415. doi: 10.1016/j.jep.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 22.Bihaqi S.W., Singh A.P., Tiwari M. In vivo investigation of the neuroprotective property of Convolvulus pluricaulis in scopolamine-induced cognitive impairments in Wistar rats. Indian J Pharmacol. 2011;43:520. doi: 10.4103/0253-7613.84958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhuna K., Dhuna V., Bhatia G., Singh J., Kamboj S.S. Neuroprotective Effect of Convolvulus pluricaulis methanol extract on Hydrogen Peroxide induced oxidative stress in human IMR32 neuroblastoma cell line. Br Biotechnol J. 2012;1(2):192. [Google Scholar]
- 24.Kujala T.S., Loponen J.M., Klika K.D., Pihlaja K. Phenolics and betacyanins in red beetroot (Beta vulgaris) root: distribution and effect of cold storage on the content of total phenolics and three individual compounds. J Agric Food Chem. 2000;48:5338–5342. doi: 10.1021/jf000523q. [DOI] [PubMed] [Google Scholar]
- 25.Sakanaka S., Tachibana Y., Okada Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha) Food Chem. 2005;89:569–575. [Google Scholar]
- 26.Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;26:1199–1200. [Google Scholar]
- 27.Dinis T.C., Madeira V.M., Almeida L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 28.Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 29.Prieto P., Pineda M., Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 30.Kalita S., Kumar G., Karthik L., Rao K.V. In vitro antioxidant and DNA damage inhibition activity of aqueous extract of Lantana camara L. (Verbenaceae) leaves. Asian Pac J Trop Biomed. 2012;2:S1675–S1679. [Google Scholar]
- 31.Mayo J.C., Tan D.X., Sainz R.M., Natarajan M., Lopez-Burillo S., Reiter R.J. Protection against oxidative protein damage induced by metal-catalyzed reaction or alkylperoxyl radicals: comparative effects of melatonin and other antioxidants. Biochim Biophys Acta Gen Subj. 2003;1620:139–150. doi: 10.1016/s0304-4165(02)00527-5. [DOI] [PubMed] [Google Scholar]
- 32.Wright J.R., Colby H.D., Miles P.R. Cytosolic factors which affect microsomal lipid peroxidation in lung and liver. Arch Biochem Biophys. 1981;206:296–304. doi: 10.1016/0003-9861(81)90095-3. [DOI] [PubMed] [Google Scholar]
- 33.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 34.Cohen G., Dembiec D., Marcus J. Measurement of catalase activity in tissue extracts. Anal Biochem. 1970;34:30–38. doi: 10.1016/0003-2697(70)90083-7. [DOI] [PubMed] [Google Scholar]
- 35.Wang H., Joseph J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999;27:612–616. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 36.Singh N.P., McCoy M.T., Tice R.R., Schneider E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 37.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 38.Ferreira A., Proença C., Serralheiro M.L., Araujo M.E. The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J Ethnopharmacol. 2006;108:31–37. doi: 10.1016/j.jep.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Sadeghnia H.R., Kamkar M., Assadpour E., Boroushaki M.T., Ghorbani A. Protective effect of safranal, a constituent of Crocus sativus, on quinolinic acid-induced oxidative damage in rat hippocampus. Iran j basic med sci. 2013;16:73. [PMC free article] [PubMed] [Google Scholar]
- 40.Kumaran A., Karunakaran R.J. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT-Food Sci TechnoL. 2007;40:344–352. [Google Scholar]
- 41.Juraschek S.P., Guallar E., Appel L.J., Miller E.R. Effects of vitamin C supplementation on blood pressure: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;95:1079–1088. doi: 10.3945/ajcn.111.027995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dysken M.W., Sano M., Asthana S. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. Jama. 2014;311:33–44. doi: 10.1001/jama.2013.282834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mnafgui K., Derbali A., Sayadi S., Gharsallah N., Elfeki A., Allouche N. Anti-obesity and cardioprotective effects of cinnamic acid in high fat diet-induced obese rats. J Food Sci Technol. 2015;52:4369–4377. doi: 10.1007/s13197-014-1488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Banu L.A., Kudrat-E-Zahan M., Bashar M.A., Haque M.M., Quamruzzaman M., Islam M.S. Studies on synthesis and characterization with antimicrobial activity of mixed ligand coordinating co (ii) Complexes with phthalic acid and heterocyclic amines. Int J Commun Syst. 2015;2:38–41. [Google Scholar]
- 45.Liu X., Zhang Y., Li J. Cognitive deficits and decreased locomotor activity induced by single-walled carbon nanotubes and neuroprotective effects of ascorbic acid. Int J Nanomed. 2014;9:823. doi: 10.2147/IJN.S56339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alzoubi K.H., Khabour O.F., Rashid B.A., Damaj I.M., Salah H.A. The neuroprotective effect of vitamin E on chronic sleep deprivation-induced memory impairment: the role of oxidative stress. Behav Brain Res. 2012;226:205–210. doi: 10.1016/j.bbr.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 47.Ibrahim A.T., Magdy M.A., Ahmed E.A., Omar H.M. The protective effects of vitamin E and zinc supplementation against lithium-induced brain toxicity of male albino rats. Environ Pollut. 2015;4:9. [Google Scholar]
- 48.Etminan M., Gill S.S., Samii A. Intake of vitamin E, vitamin C, and carotenoids and the risk of Parkinson's disease: a meta-analysis. Lancet Neurol. 2005;4:362–365. doi: 10.1016/S1474-4422(05)70097-1. [DOI] [PubMed] [Google Scholar]
- 49.Saxena M., Saxena J. Evaluation of phytoconstituents of Acorus calamus by FTIR and UV-VIS spectroscopic analysis. IJPBR. 2012;3:498–501. [Google Scholar]
- 50.Maobe M.A., Nyarango R.M. Fourier transformer infra-red spectrophotometer analysis of Warburgia ugandensis medicinal herb used for the treatment of diabetes, malaria and pneumonia in Kisii region, Southwest Kenya. Global J Pharmacol. 2013;7:61–68. [Google Scholar]
- 51.Ashokkumar R., Ramaswamy M. Phytochemical screening by FTIR spectroscopic analysis of leaf extracts of selected Indian medicinal plants. Int J Curr Microbiol Appl Sci. 2014;3:395–396. [Google Scholar]
- 52.Kumar P.V., Pammi S.V., Kollu P., Satyanarayana K.V., Shameem U. Green synthesis and characterization of silver nanoparticles using Boerhaavia diffusa plant extract and their anti-bacterial activity. Ind Crop Prod. 2014;52:562–566. [Google Scholar]
- 53.Adisakwattana S., Sompong W., Meeprom A., Ngamukote S., Yibchok-Anun S. Cinnamic acid and its derivatives inhibit fructose-mediated protein glycation. Int J Mol Sci. 2012;13(2):1778–1789. doi: 10.3390/ijms13021778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taner G., Özkan Vardar D., Aydin S., Aytaç Z., Başaran A., Başaran N. Use of in vitro assays to assess the potential cytotoxic, genotoxic and antigenotoxic effects of vanillic and cinnamic acid. Drug Chem Toxicol. 2017;40(2):183–190. doi: 10.1080/01480545.2016.1190740. [DOI] [PubMed] [Google Scholar]
- 55.Conforti F., Statti G., Loizzo M.R., Sacchetti G., Poli F., Menichini F. In vitro antioxidant effectand inhibition of a-amylase of two varieties of Amaranthus caudatus seeds. Biol Pharmaceut Bull. 2005;28:1098–1102. doi: 10.1248/bpb.28.1098. [DOI] [PubMed] [Google Scholar]
- 56.Behl C., Davis J., Cole G.M., Schubert D. Vitamin E protects nerve cells from amyloid β protein toxicity. Biochem Biophys Res Commun. 1992;186(2):944–950. doi: 10.1016/0006-291x(92)90837-b. [DOI] [PubMed] [Google Scholar]
- 57.Kara Y., Doguc D.K., Kulac E., Gultekin F. Acetylsalicylic acid and ascorbic acid combination improves cognition; via antioxidant effect or increased expression of NMDARs and nAChRs? Environ Toxicol Pharmacol. 2014;37(3):916–927. doi: 10.1016/j.etap.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 58.Sawicka-Glazer E., Czuczwar S.J. Vitamin C: a new auxiliary treatment of epilepsy? Pharmacol Rep. 2014;66(4):529–533. doi: 10.1016/j.pharep.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 59.Dong Y., Wang S., Zhang T. Ascorbic acid ameliorates seizures and brain damage in rats through inhibiting autophagy. Brain Res. 2013;1535:115–123. doi: 10.1016/j.brainres.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 60.Jayashree G.V., Kumar K.H., Krupashree K., Rachitha P., Khanum F. LC–ESI–MS/MS analysis of Asparagus racemosus Willd. roots and its protective effects against t-BHP induced oxidative stress in rats. Ind Crop Prod. 2015;78:102–109. [Google Scholar]
- 61.Razack S., Kumar K.H., Nallamuthu I., Naika M., Khanum F. Antioxidant, biomolecule oxidation protective activities of Nardostachys jatamansi DC and its phytochemical analysis by RP-HPLC and GC-MS. Antioxidants. 2015;4(1):185–203. doi: 10.3390/antiox4010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kandikattu H.K., Venuprasad M.P., Pal A., Khanum F. Phytochemical analysis and exercise enhancing effects of hydroalcoholic extract of Celastrus paniculatus Willd. Ind Crop Prod. 2014;55:217–224. [Google Scholar]
- 63.Soobrattee M.A., Neergheen V.S., Luximon-Ramma A., Aruoma O.I., Bahorun T. Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutat Res Fund Mol Mech Mutagen. 2005;579:200–213. doi: 10.1016/j.mrfmmm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 64.Golla U., Bhimathati S.S. Evaluation of antioxidant and DNA damage protection activity of the hydroalcoholic extract of Desmostachya bipinnata L. Stapf. Sci World J. 2014;2014:1–8. doi: 10.1155/2014/215084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krupashree K., Kumar K.H., Rachitha P., Jayashree G.V., Khanum F. Chemical composition, antioxidant and macromolecule damage protective effects of Picrorhiza kurroa Royle ex Benth. South Afr J Bot. 2014;94:249–254. [Google Scholar]
- 66.Jayashree G.V., Rachitha P., Krupashree K., Kumar K.H., Khanum F. Antioxidant and DNA damage protective effects of Asparagus racemosus in human colon and mice muscle cells. Phcog J. 2015;(3):7. [Google Scholar]
- 67.Suboh S.M., Bilto Y.Y., Aburjai T.A. Protective effects of selected medicinal plants against protein degradation, lipid peroxidation and deformability loss of oxidatively stressed human erythrocytes. Phytother Res. 2004;18:280–284. doi: 10.1002/ptr.1380. [DOI] [PubMed] [Google Scholar]
- 68.Venuprasad M.P., Kumar K.H., Khanum F. Neuroprotective effects of hydroalcoholic extract of Ocimum sanctum against H2O2 induced neuronal cell damage in SH-SY5Y cells via its antioxidative defence mechanism. Neurochem Res. 2013;38:2190–2200. doi: 10.1007/s11064-013-1128-7. [DOI] [PubMed] [Google Scholar]
- 69.Kumar K.H., Tamatam A., Pal A., Khanum F. Neuroprotective effects of Cyperus rotundus on SIN-1 induced nitric oxide generation and protein nitration: ameliorative effect against apoptosis mediated neuronal cell damage. Neurotoxicology. 2013;34:150–159. doi: 10.1016/j.neuro.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 70.Tenkerian C., El-Sibai M., Daher C.F., Mroueh M. Hepatoprotective, antioxidant, and anticancer effects of the tragopogon porrifolius methanolic extract. Evid base Compl Alternative Med. 2015;2015:1–10. doi: 10.1155/2015/161720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cho N., Lee K.Y., Huh J. Cognitive-enhancing effects of Rhus verniciflua bark extract and its active flavonoids with neuroprotective and anti-inflammatory activities. Food Chem Toxicol. 2013;58:355–361. doi: 10.1016/j.fct.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 72.Seoposengwe K., Van Tonder J.J., Steenkamp V. In vitro neuroprotective potential of four medicinal plants against rotenone-induced toxicity in SH-SY5Y neuroblastoma cells. BMC Compl Alternative Med. 2013;13(1):1. doi: 10.1186/1472-6882-13-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ghaffari H., Venkataramana M., Ghassam B.J. Rosmarinic acid mediated neuroprotective effects against H2O2-induced neuronal cell damage in N2A cells. Life Sci. 2014;113:7–13. doi: 10.1016/j.lfs.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 74.Tamilselvam K., Braidy N., Manivasagam T. Neuroprotective effects of hesperidin, a plant flavanone, on rotenone-induced oxidative stress and apoptosis in a cellular model for Parkinson's disease. Oxidative med cell longev. 2013;2013:1–11. doi: 10.1155/2013/102741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sree N.V., Sri P.U., Ramarao N. Neuro-protective properties of orthosiphon staminus (benth) leaf methanolic fraction through antioxidant mechanisms on sh-sy5y cells: an in-vitro evaluation. Int J Pharm Sci Res. 2015;6:1115. [Google Scholar]
- 76.Kalantari Heibatullah. Determination of the mutagenicity potential of supermint herbal medicine by single cell gel electrophoresis in rat hepatocytes. Adv Pharmaceut Bull. 2012;2(2):245. doi: 10.5681/apb.2012.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paul S., Chakraborty S., Mukherjee A., Kundu R. Evaluation of cytotoxicity and DNA damaging activity of three plant extracts on cervical cancer cell lines. Int J Pharmaceut Sci Rev Res. 2015;31:183–189. [Google Scholar]
- 78.Prehn J.H., Bindokas V.P., Jordán J. Protective effect of transforming growth factor-beta 1 on beta-amyloid neurotoxicity in rat hippocampal neurons. Mol Pharmacol. 1996;49:319–328. [PubMed] [Google Scholar]
- 79.Lee C.H., Hwang D.S., Kim H.G. Protective effect of Cyperi rhizoma against 6-hydroxydopamine-induced neuronal damage. J Med Food. 2010;13:564–571. doi: 10.1089/jmf.2009.1252. [DOI] [PubMed] [Google Scholar]
- 80.Cregan S.P., MacLaurin J.G., Craig C.G. Bax-dependent caspase-3 activation is a key determinant in p53-induced apoptosis in neurons. J Neurosci. 1999;19(18):7860–7869. doi: 10.1523/JNEUROSCI.19-18-07860.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ye J., Liu Z., Wei J. Protective effect of SIRT1 on toxicity of microglial-derived factors induced by LPS to PC12 cells via the p53-caspase-3-dependent apoptotic pathway. Neurosci Lett. 2013;553:72–77. doi: 10.1016/j.neulet.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 82.Radi E., Formichi P., Battisti C., Federico A. Apoptosis and oxidative stress in neurodegenerative diseases. J Alzheim Dis. 2014;42:S125–S152. doi: 10.3233/JAD-132738. [DOI] [PubMed] [Google Scholar]
- 83.Migliore L., Coppedè F. Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutat Res Genet Toxicol Environ Mutagen. 2009;674:73–84. doi: 10.1016/j.mrgentox.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 84.Kwon S.H., Kim M.J., Ma S.X. Eucommia ulmoides Oliv. Bark. protects against hydrogen peroxide-induced neuronal cell death in SH-SY5Y cells. J Ethnopharmacol. 2012;142:337–345. doi: 10.1016/j.jep.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 85.Azmi N.H., Ismail N., Imam M.U., Ismail M. Ethyl acetate extract of germinated brown rice attenuates hydrogen peroxide-induced oxidative stress in human SH-SY5Y neuroblastoma cells: role of anti-apoptotic, pro-survival and antioxidant genes. BMC Compl Alternative Med. 2013;13:1. doi: 10.1186/1472-6882-13-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Law B.N., Ling A.P., Koh R.Y., Chye S.M., Wong Y.P. Neuroprotective effects of orientin on hydrogen peroxide-induced apoptosis in SH-SY5Y cells. Mol Med Rep. 2014;9:947–954. doi: 10.3892/mmr.2013.1878. [DOI] [PubMed] [Google Scholar]
- 87.Jiang X.W., Bai J.P., Zhang Q. Caffeoylquinic acid derivatives protect SH-SY5Y neuroblastoma cells from hydrogen peroxide-induced injury through modulating oxidative status. Cell Mol Neurobiol. 2016 doi: 10.1007/s10571-016-0387-7. 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]