Abstract
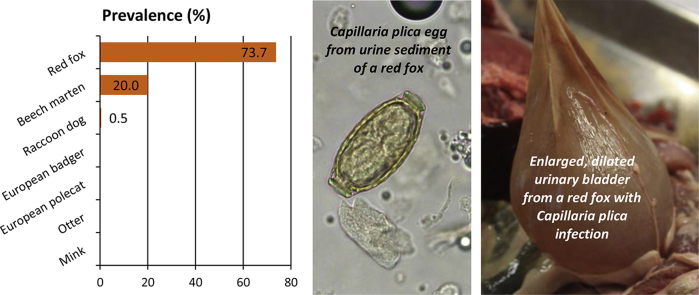
Capillaria plica is a parasitic nematode belonging to the family Capillariidae. The adult parasites reside in the urinary tract of wild and domestic canines. The infection is most often asymptomatic, but can cause a wide range of symptoms including urinary bladder inflammation, pollacisuria, dysuria and hematuria. Canines acquire the infection by ingesting the intermediate host, the earthworm (Lumbricidae). Epidemiological studies on C. plica infection in wildlife are few and only one previous Danish study examined the prevalence in red foxes, while studies on prevalence in other animals are limited. We examined the urine sediment or urinary bladder from 375 Raccoon dogs (Nyctereutes procyonoides), 247 red foxes (Vulpes vulpes), 20 beech martens (Martes foina), 16 wild mink (Neovison vison), 14 otters (Lutra lutra), nine European polecats (Mustela putorius), three European badgers (Meles meles) and one golden jackal (Canis aureus) received as a part of Danish wildlife surveillance. Capillaria plica was detected in 73.7% of red foxes, 20.0% of beech martens, 0.5% of raccoon dogs, and in the Golden jackal. Red foxes originating from all 5 regions of Denmark were infected, although with a significantly higher prevalence in the three regions in Jutland compared to Region Zealand.
Keywords: Capillaria plica, Bladderworm, Wild carnivores, Reservoir hosts
Graphical abstract
Highlights
-
•
Capillaria plica prevalence was high in Danish red foxes.
-
•
Capillaria plica prevalence was low or non-existent in other wild carnivores.
-
•
This study documents C. plica infection in beech martens for the first time.
1. Introduction
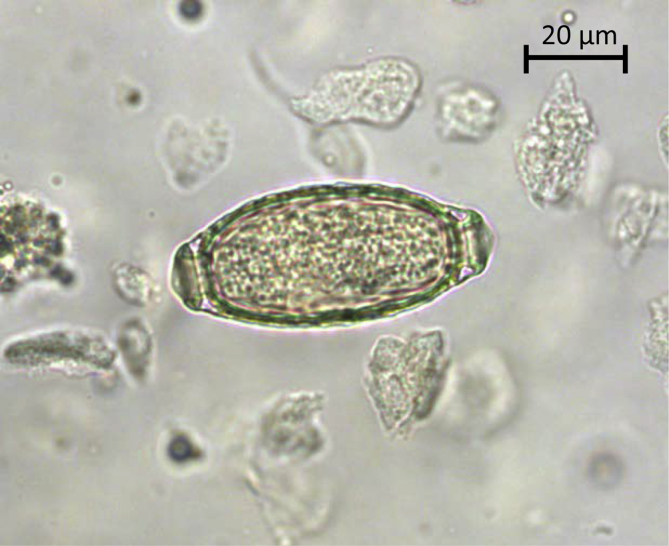
The bladderworm Capillaria plica (syn. Pearsonoma plica) is a threadlike nematode belonging to Capillariidae family (Butterworth and Beverley-Burton, 1980). The life-cycle is indirect, involving wild and domestic canines as final host, and earthworms (Lumbricidae) as intermediate host. The final host become infected when ingesting earthworms containing first-stage larvae (L1). The L1 develop to third-stage larvae (L3) in the small intestine, migrate to the urinary bladder and moult to adult worms within two months. Adults are 13–60 mm long and embedded in the bladder mucosa. Occasionally, adults reside in urethra and renal pelvis. The female worm lays 55–67 × 26–29 μm barrel-shaped eggs with buttons on both poles (Fig. 1). The eggs are spread to the environment with urine.
Fig. 1.
A typical barrel-shaped Capillaria plica egg in urine sediment from a red fox. The egg show a slightly pitted shell and two opercules with polar plugs. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Capillaria plica infection is generally asymptomatic, but severe urinary bladder inflammation, glomerular amyloidosis, oedema, hyperplasia of the mucosal membranes leading to pollacisuria, dysuria and hematuria are documented in dogs (Callegari et al., 2010; Lamrna and Main, 1964; Senior et al., 1980).
In Europe, C. plica infection is common in red foxes with reported prevalences of 78% in Germany (Bork-Mimm and Rinder, 2011), 53% in Norway (Davidson et al., 2006) and 52% in Hungary (Sréter et al., 2003). In Denmark, the prevalence has previously been documented to 80.5% in 748 red foxes collected over a five-year period in 1997–2002 (Saeed et al., 2006). Studies on C. plica infections in other carnivores are scarce, and no other Danish wild carnivores have previously been examined for C. plica.
The purpose of this study was to carry out a nation-wide cross-sectional study of C. plica prevalence in Danish wild carnivores, to determine their role as reservoir host for infection in Danish dogs.
2. Material and methods
The urinary bladder was obtained from 375 raccoon dogs (Nyctereutes procyonoides), 247 red foxes (Vulpes vulpes), 20 beech martens (Martes foina), 16 mink (Neovison vison), 14 otters (Lutra lutra), nine European polecats (Mustela putorius), three European badgers (Meles meles) and one golden jackal (Canis aureus).
The carcasses of the animals were submitted to the National Veterinary Institute, Technical University of Denmark (DTU-VET) as a part of general wildlife surveillance in 2017. The animals were either found dead, euthanized for animal welfare reasons, or hunted. The dead carnivores were transported in sealed plastic bags at −20 °C to DTU-VET and left at −80 °C for ≥2 days to inactivate potential infective parasites before necropsy. The region was listed along with information on date of death if recorded. Urinary bladders were recovered and placed in liquid-tight plastic boxes. The bladders were carefully opened and 10 ml of urine collected. If urine was absent, the bladder was cut open (n = 586), washed with 10 ml milliQ water and the water collected in 15 ml plastic tubes. All samples were centrifuged (178 × g) for 10 min, the supernatant discarded to 1.5 ml, flotation fluid added to 3 ml, and samples screened for C. plica eggs (Fig. 1). The egg quantity per ml was recorded.
The prevalence of C. plica infection per animal species, and per region for red foxes was calculated together with the 95% confidence intervals. Difference in C. plica prevalence for red foxes between the five regions was determined by a binary logistic regression model with C. plica infection as the dependent variable and region of origin as the independent variable. A p-value of 0.05 was considered significant.
3. Results
Capillaria plica eggs were isolated from red foxes, raccoon dogs, beech martens and the golden jackal. Mink, otters, European polecats and European badgers were negative for C. plica. In total, 182 red foxes harboured C. plica, corresponding to a prevalence of 73.7%. Of the 375 examined raccoon dogs, only two were positive, corresponding to a prevalence of 0.5%. Of the 20 beech martens examined, four were positive (20%).
Table 1 shows the egg load (eggs per ml) in the urine or urinary bladder of positive animals (n = 189). More than half of the positive red foxes (n = 94, 52.8%) had an egg load above 30 eggs per ml.
Table 1.
Capillaria plica egg load in urine sediment or sediment from washing of the bladder.
| Species | No examined | No positive animals (%) | 95% confidence interval | 1-10 eggs per ml (%) | 11-20 eggs per ml (%) | 21-30 eggs per ml (%) | >30 eggs per ml (%) |
|---|---|---|---|---|---|---|---|
| Red foxes (Vulpes vulpes) | 247 | 182 (73.7) | 68.2–79.2 | 41 (23.0) | 31 (17.4) | 12 (6.7) | 94 (52.8) |
| Raccoon dogs (Nyctereutes procyonoides) | 375 | 2 (0.5) | 0.2–1.3 | 1 (50.0) | 1 (50.0) | – | – |
| Golden jackal (Canis aureus) | 1 | 1 (100.0) | – | – | – | – | 1 (100.0) |
| Beech martens (Martes foina) | 20 | 4 (20.0) | 0.8–39.2 | – | 4 (100.0) | – | – |
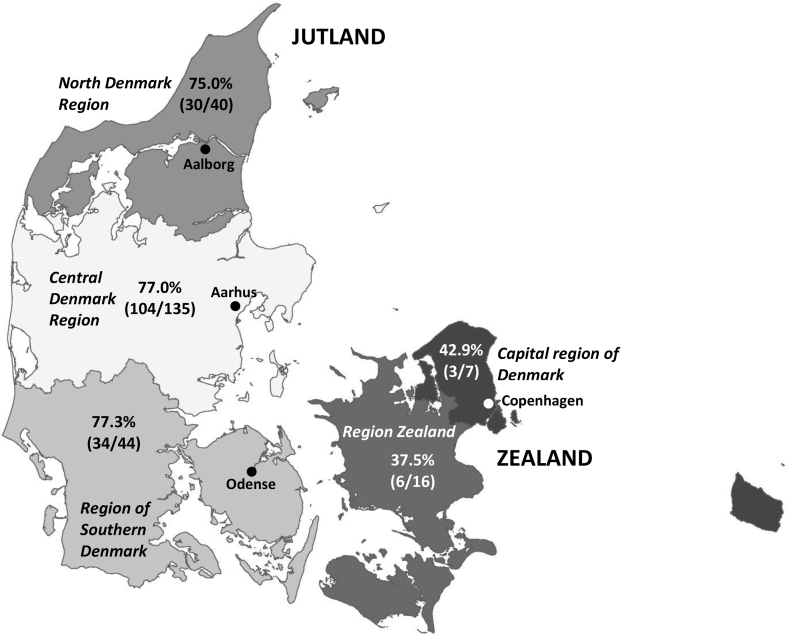
Fig. 2 show the prevalence of C. plica positive red foxes in the five Danish regions. In the three regions of Jutland (north Denmark region, central Denmark region and region of southern Denmark), C. plica prevalence was above 75%, while the prevalence in red foxes from the two regions on Zealand (region Zealand and capital region of Denmark) it was below 50% (Fig. 2). However, only in region Zealand, the C. plica prevalence was significant lower (p < 0.05) than the prevalence in the three regions in Jutland.
Fig. 2.
The prevalence of Capillaria plica infections in red foxes per region of Denmark (positive red foxes/total number of red foxes examined). The origin of five red foxes was unknown.
4. Discussion
In this cross-sectional study of C. plica infection in Danish wild carnivores, we identified red foxes as the most likely reservoir host for infection in dogs, as C. plica infection was common in red foxes (73.7%). In contrast, C. plica infection was rare in raccoon dogs, and absent in wild mink, otters, polecats and badgers. Lastly, we report C. plica infection in beech martens for the first time.
The high prevalence in red foxes in our study corresponds with the previous finding in Denmark (80.5%), although data were collected 16–21 years ago (Saeed et al., 2006). This demonstrates that C. plica continues to be highly prevalent in Danish red foxes and that red foxes most likely are the main definitive host of C. plica in Denmark. The C. plica prevalence of Danish red foxes correspond with a study from Germany with a prevalence of 78% in red foxes (Bork-Mimm and Rinder, 2011). However, the prevalence in our study was lower than in Lithuania (98,3%) and higher than in Norway (53.0%) and Hungary (52.0%) (Bork-Mimm and Rinder, 2011; Bružinskaitė-Schmidhalter et al., 2012; Davidson et al., 2006; Magi et al., 2015; Saeed et al., 2006). In addition, red foxes are becoming increasingly more “urbanized” wildlife (Deplazes et al., 2004; Gloor et al., 2002; Scott et al., 2014), carrying the parasite close to premises where pet animals are roaming. Thus, red foxes are seemingly the most important factor in the epidemiology and risk of spread of the parasite to pet dogs.
In our study, the C. plica prevalence in raccoon dogs (0.5%) is considerably lower than in red foxes (73.7%). This observation correspond to a Lithuanian study documenting a prevalence of 11.3% in raccoon dogs and 93.3% in red foxes (Bružinskaitė-Schmidhalter et al., 2012). To our knowledge, the Lithuanian study is the only other study where C. plica infection in raccoon dogs have been analysed.
Ingestion of infected earthworms is the sole documented infection method for C. plica in canines. Elmeros et al. (2018) and Mikkelsen et al. (2016) demonstrated that Danish raccoon dogs to a large extent eat earthworms (68.6% and 34.7% of animals). In contrast, red foxes rarely eat earthworms, but prefer microtine rodents (Kauhala et al., 1998; Pagh et al., 2015a, 2015b). Hence, the C. plica prevalence herein observed for both raccoon dogs and red foxes contradicts the feed preference. This could indicate that raccoon dogs are less suitable as final host for C. plica. This applies for badgers, too. A Spanish study documented a prevalence of 2.1% in Eurasian badgers (Torres et al., 2001), although badgers frequently consume earthworms (Kauhala et al., 1998; Madsen et al., 2002). In addition to acquire the infection through consumption of earthworms, it is likely that red foxes can acquire the infection through paratenic hosts like rodents and birds. When rodents and birds consume C. plica-infected earthworms, the parasite seemingly enters these animals without undergoing any further development, but might remain alive till it gains entry to the red fox through consumption of the paratenic host. However, further studies are needed to identify paratenic hosts. A second possibility is that another, hitherto undocumented intermediate host exist. Lastly, C. plica infection might accumulate in red fox, so once infected, the parasite reside within the bladder for an extended time. This could be the case if an age effect was noted. Unfortunately, the age was not recorded in this study. A last possibility is that Capillaria eggs documented from the various hosts are indeed different species. However, our study was limited by lack of molecular analysis of Capillaria eggs from the various species.
The red foxes from Jutland had significantly higher C. plica prevalence compared to red foxes form Zealand. Jutland is the peninsula that forms the continental portion of Denmark. The population density is 84 persons per km2. In comparison, the population density is 358 persons per km2 on Zealand. Since only few red foxes originated from Zealand, we refrain to conclude that the difference in prevalence between regions is affected by the availability of infected earthworms or the feeding habits of red foxes.
This is the first report of C. plica infection in beech martens (4 positive out of 20). Capillaria plica have been recorded in American martens with a prevalence of 6% (Seville and Addison, 1995), which determine that the genus martes are final hosts of C. plica.
In summary, our findings confirm that most Danish red foxes excrete C. plica eggs and that red foxes are the most likely reservoir host for infection in dogs. Moreover, further studies are needed to identify other possible intermediate hosts and infection through transport hosts.
Acknowledgements
This work was supported by the Ministry of Environment and Food of Denmark [grant numbers MST-854-00060]. A special thanks to laboratory technicians Boi Tien Thi Pharm and Aleksandra Tofteby. Without their hard work, this study was not a reality.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2018.09.006.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Bork-Mimm S., Rinder H. High prevalence of Capillaria plica infections in red foxes (Vulpes vulpes) in Southern Germany. Parasitol. Res. 2011;108:1063–1067. doi: 10.1007/s00436-010-2196-0. [DOI] [PubMed] [Google Scholar]
- Bružinskaitė-Schmidhalter R., Šarkūnas M., Malakauskas A., Mathis A., Torgerson P.R., Deplazes P. Helminths of red foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides) in Lithuania. Parasitology. 2012;139:120–127. doi: 10.1017/S0031182011001715. [DOI] [PubMed] [Google Scholar]
- Butterworth E.W., Beverley-Burton M. The taxonomy of Capillaria spp. (Nematoda: Trichuroidea) in carnivorous mammals from Ontario, Canada. Syst. Parasitol. 1980;1:211–236. [Google Scholar]
- Callegari D., Kramer L., Cantoni A.M., Di Lecce R., Dodi P.L., Grandi G. Canine bladderworm (Capillaria plica) infection associated with glomerular amyloidosis. Vet. Parasitol. 2010;168:338–341. doi: 10.1016/j.vetpar.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Davidson R.K., Gjerde B., Vikøren T., Lillehaug A., Handeland K. Prevalence of Trichinella larvae and extra-intestinal nematodes in Norwegian red foxes (Vulpes vulpes) Vet. Parasitol. 2006;136:307–316. doi: 10.1016/j.vetpar.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Deplazes P., Hegglin D., Gloor S., Romig T. Wilderness in the city: the urbanization of Echinococcus multilocularis. Trends Parasitol. 2004;20:77–84. doi: 10.1016/j.pt.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Elmeros M., Malene D., Mikkelsen G., Nørgaard L.S., Pertoldi C., Hammer Jensen T., Chriél M. The diet of feral raccoon dog (Nyctereutes procyonoides) and native badger (Meles meles) and red fox (Vulpes vulpes) in Denmark. Mamm. Res. 2018:1–9. [Google Scholar]
- Gloor S., Von B., Ziswiler V., Breitenmoser U., Funk S. The rise of urban foxes (Vulpes vulpes) in Switzerland and ecological and parasitological aspects of a fox population in the recently colonised city of Zurich. Zeitschrift für Säugetierkd. 2002;66:155–164. [Google Scholar]
- Kauhala K., Laukkanen P., von Rége I. Summer food composition and food niche overlap of the racoon dog, red fox and badger in Finland. Ecography. 1998;21:457–463. [Google Scholar]
- Lamrna J., Main F.A. Der Parasitenbefall bei Rotfiichsen in Siidhessen. Z. Jagdwiss. 1964;10:137–142. [Google Scholar]
- Magi M., Guardone L., Prati M.C., Mignone W., Macchioni F. Extraintestinal nematodes of the red fox Vulpes vulpes in north-west Italy. J. Helminthol. 2015;89:506–511. doi: 10.1017/S0022149X1400025X. [DOI] [PubMed] [Google Scholar]
- Madsen S.A., Madsen A.B., Elmeros M. Seasonal food of badgers (Meles meles) in Denmark. Mammalia. 2002;3:341–352. [Google Scholar]
- Mikkelsen D.M.G., Nørgaard L.S., Jensen T.H., Chriél M., Pertoldi C., Elmeros M. Mårhundens (Nyctereutes procyonoides) føde og fødeoverlap med hjemmehørende rovdyr i Danmark [In Danish] Flora og Fauna. 2016;122:101–114. [Google Scholar]
- Pagh S., Skjold Tjørnløv R., Illemann J.K., Tolsgaard S., Chriel M. Habitatrelateret føde hos ræv (Vulpes vulpes) i land-brugsområder. Flora Fauna. 2015;121:1–14. [Google Scholar]
- Pagh S., Skjold Tjørnløv R., Olesen C.R., Chriel M. The diet of Danish red foxes (Vulpes vulpes) in relation to a changing agricultural ecosystem. A historical perspective. Mammal Res. 2015;60:319–329. [Google Scholar]
- Saeed I., Maddox-Hyttel C., Monrad J., Kapel C.M.O. Helminths of red foxes (Vulpes vulpes) in Denmark. Vet. Parasitol. 2006;139:168–179. doi: 10.1016/j.vetpar.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Scott D.M., Berg M.J., Tolhurst B.A., Chauvenet A.L.M., Smith G.C., Neaves K., Lochhead J., Baker P.J. Changes in the distribution of red foxes (Vulpes vulpes) in urban areas in Great Britain: findings and limitations of a media-driven nationwide survey. PLoS One. 2014;9 doi: 10.1371/journal.pone.0099059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior D.F., Solomon G.B., Goldschmidt M.H., Joyce T., Bovee K.C. Capillaria plica infection in dogs. J. Am. Vet. Med. Assoc. 1980;176:901–905. [PubMed] [Google Scholar]
- Seville R.S., Addison E.M. Nongastrointestinal helminths in marten (Martes americana) from Ontario, Canada. J. Wildl. Dis. 1995;31:529–533. doi: 10.7589/0090-3558-31.4.529. [DOI] [PubMed] [Google Scholar]
- Sréter T., Széll Z., Marucci G., Pozio E., Varga I. Extraintestinal nematode infections of red foxes (Vulpes vulpes) in Hungary. Vet. Parasitol. 2003;115:329–334. doi: 10.1016/s0304-4017(03)00217-6. [DOI] [PubMed] [Google Scholar]
- Torres J., Miquel J., Motjeâ M. Helminth parasites of the eurasian badger (Meles meles L.) in Spain: a biogeographic approach. Parasitol. Res. 2001;87:259–263. doi: 10.1007/s004360000316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.