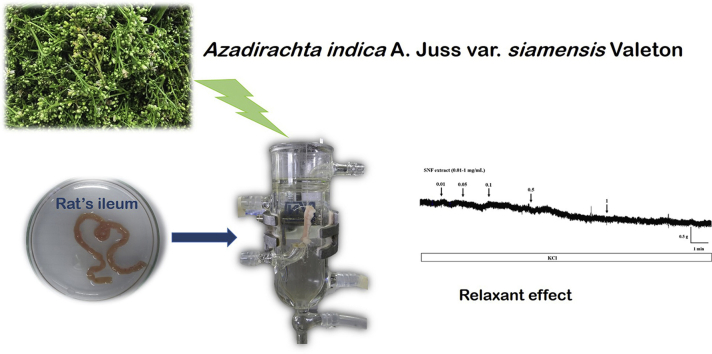
Abstract
Azadirachta indica A. Juss var. siamensis Valeton or commonly known as Siamese neem is one of the most well-known plant in traditional Ayurvedic medicine. The aim of the present study was to investigate the relaxant effects of A. indica on isolated rat ileum contractions and its potential underlying mechanisms involved. The isometric contractions of ileum segments were investigated in organ baths for spontaneous activity and response to aqueous extract of Siamese neem flower (SNF). The spasmolytic action of the extract was also assessed on contraction induced by acetylcholine and high potassium. Our findings indicate that cumulative concentrations of SNF aqueous extract induced relaxant effect on spontaneous rat ileum contractions. The extract has also suppressed the cumulative concentration response curve for acetylcholine and pottasium ions-induced contraction. The presence and absence of propranol (antagonist of β-adrenergic receptor) and l-Name (antagonist of nitric oxide synthase) in SNF aqeous extract co-treatment demonstrated no significant different in term of contraction activity when compared to SNF extract treatment alone. The treatment of SNF extract caused a significant inhibition in tissue contraction stimulated by accumulation of calcium ions. Our results showed the relaxant effect of SNF aqueous extract on the isolated rat ileum. In short, the SNF aqueous extract exhibited an inhibitory effect on the spontaneous ileum contactions particularly on the contraction stimulated by acetylcholine and high potassium. The observed effect might acted through the modulation of calcium channels. This findings provide a pharmacological basis for the traditional use of SNF for the treatment of gastrointestinal spasms.
Keywords: Siamese neem, Ileum, l-name, Propanolol, CaCl2
Graphical abstract
1. Introduction
Gastrointestinal (GI) diseases and symptoms are known to cause an effect on quality of life, work and activity impairments, and health care costs.1 A disturbance in coordination between the motor, sensory or secretory function of GI tract are known to cause GI functional and motility disorders.2 Although the etiology of some cases of GI functional and motility disorders are still remain unclear, the common observed symptoms are including nausea, vomiting, bloating, abdominal discomfort or pain, constipation or diarrhea.2 The onset of GI motility disorders has been attributed to several causes, which including the consequence complications of other systemic illnesses, such as diabetes.2,3
Generally, the pharmacological interventions for GI motility disorders are involved in altering the regulation of GI smooth muscle, enteric nervous system (ENS), autonomic ganglia, and central nervous system. Radulovic M et al. have suggested that release of neurotransmitters and several ion channels in GI tissues including ATP-sensitive potassium (KATP) channel, calcium ions (Ca2+)-activated potassium ions (K+) channels, voltage-sensitive Ca2+ channels and chloride ion channels play an important role in regulating GI motility.4 In fact, those mechanisms have served as the fundamental knowledge in new discoveries and development of new therapeutic agents. Cholinergic agonists or laxative agents are widely used for gastrointestinal motility disorders management.4 However, these drugs have an undesirable side effect. Complementary and alternative medicines (CAM) have been associated with symptom management and quality of life in a common gastrointestinal disorder.5 Therefore, the attention has been shifted to bio-prospecting the natural products to overcome or control GI motility disorders.
For centuries, herbs have been used in traditional medicine to treat GI disorders. With the recent advancement of technologies or scientific techniques, it enables the essences of this traditional knowledge to be explored further. These investigations have opened up more avenues which either allows a more systematic usage of traditional medicine or alternatively to be developed to become a standard drug. For instance, Siamese neem tree (Azadirachta indica A. Juss. var. siamensis Valeton) which is belong to Meliaceae family has been used in Ayurvedic medicine for more than four thousand years.6 All parts of the neem tree (leaves, flowers, seeds, fruits, roots and bark) have been used for the treatment of inflammation, infections, fever, skin diseases and dental disorders.7 The robustness and well-studied biological activities has thus contributed to its commercial exploitation for treatment of various diseases. In particular, it is traditionally suggested and used to treat diarrhea and peptic ulcer.8, 9, 10, 11 However, to the best of our knowledge the role of Siamese neem flower (SNF) on intestinal smooth muscle motility is remaining unclear. Therefore, the current study aims to study the effect of SNF extract on isolated rat ileum contractions induced by high potassium and acetylcholine, and investigated the possible mechanisms involved. The findings might help to explain the extensively usage of SNF for the treatment of gastrointestinal diseases.
2. Materials and methods
2.1. Chemicals
Atropine sulphate, propranolol, acetylcholine chloride and l-NAME were purchased from Sigma (USA), KCl, CaCl2 and other reagents were obtained from Merck Company (Germany). The extract and chemicals were dissolved in distilled water for experimentation. The Krebs' solution used in these experiments had the following composition: 122 mM NaCl, 5 mM KCl, 10 mM HEPES, 0.5 mM KH2PO4, 0.5 mM NaH2PO4, 1 mM MgCl2, 1.8 mM CaCl,2 and 11 mM glucose; pH 7.3.
2.2. Plant materials
Siamese neem flowers (SNF) were collected from Tumbon BanMaetumboonyong Mueang Phayao, Thailand. The plant was identified by a botanist and a voucher specimen (No. 003805) was deposited at the herbarium of the Faculty of Biology, Naresuan University, Phitsanulok, Thailand.
2.3. Preparation of SNF aqueous extract
Siamese neem flowers were washed, cut in smaller pieces, and blended in an electric blender in distilled water (plants 100 g: water 300 mL). They were filtered to obtain a crude solution and subsequently freeze-dried by freeze dryer (Scam VacCoolSafe 110–4 Pro) until powder was obtained. The lyophilized sample powder or extract was then stored at −20 °C. Prior to experimentation, the powder extract was prepared by dissolving it in appropriate amount of distilled water.
2.4. Animals
Male Wistar rats (200–250 g) were obtained from the National Laboratory Animal Centre, Mahidol University, Salaya, Nakhornpathom, Thailand. Experiments were approved by the Animal Ethics Committee of University of Phayao, Phayao, Thailand. Animals were maintained at Laboratory Animal Research Center, University of Phayao under 12-h dark/12-h light condition and free access to water and standard rodent diet.
2.5. Tissue preparation and experimental procedure
After animals were fasted overnight, they were deeply anesthetized by 50 mg/kg BW zolazepam/tiletamine (zoletil) and 3 mg/kg BW xylazine. Two pieces of ileum were isolated from 2 cm above the ileocaecal junction. The intraluminal content was flushed out with cooled Krebs' solution and cleaned off connective tissue surrounding. The tissue was mounted in an organ bath (30 mL) (the lower region was tightly tied to the bottom inside the bath while the upper region was connected to an isotonic force transducer) containing Krebs'solution and maintained at 37 °C, pH7.4, and a continuous supply of oxygen from air bubbles. The ileum was equilibrated for 1 h under 1 g resting and the solution was replaced every 15 min. Ileum contraction were measured using force transducer connected to an iWorx214 A/D converter (LabScribe2; Instruments, Thailand) and the data was recorded.
2.6. Relaxation effects of siamese neem flower
To examine the relaxation effect of SNF on ileum contraction, SNF extract (0.01–10 mg/mL) was cumulatively added into the organ bath once the contraction plateau was reached by the KCl (80 mM) induction. The contractions induced by the extract was recorded and normalized to KCl induced activity which was considered as the maximum contraction (100% contraction). In order to investigated the mechanism of action involved in relaxant effect demonstrated by SNF, experimentations with ileum preincubation in 100 μM l-Name (antagonist of nitric oxide synthase) for 20 min or 1 μM propanolol (antagonist of β-adrenergic receptor) for 30 min prior to KCl 80 mM exposure were then conducted. Following the preincubation period as stated above, the extract (5 mg/mL) was then added into the organ bath when KCl induced contraction reached a constant activity. On the other hand, the addition experiments were also conducted in order to examine the acetylcholine chloride (10−5 M, agonist of acetylcholine receptor) induced ileum contraction activity in either the presence or absence of extract (5 mg/mL).
2.7. Effect of siamese neem flower on extracellular Ca2+ influx
In order, to determine the role of SNF on extracellular calcium, Ileum was initially equlibrated in Ca2+ free Krebs' solution (0.01 mM EGTA, 122 mM NaCl, 5 mM KCl, 10 mM HEPES, 0.5 mM KH2PO4, 0.5 mM NaH2PO4, 1 mM MgCl2, and 11 mM glucose; pH 7.3) for 30 min. Following the incubation, SNF extract was added into the solution. Subsequently, a cumulatively concentration of CaCl2 (1–40 mM) was then added into the organ bath to stimulate the contraction. The contraction activity was observed and recorded for 10 min following the addition. The activity was determined by comparing the extract presence group to the control group or known as extract absence group.
2.8. Statistical analysis
Data was expressed as Mean ± SEM (n = 6–8 for each set of experiments). For data with normal distribution, the Analysis of variance (ANOVA) was used to test of significant difference between the groups if there were more than 2 groups. Bonferroni multiple-comparison test was also used for post-hoc analysis. If data were skewness, Kruskal-Wallis test were used to test the difference and multiple-comparison among 3 groups. . P-values less than 0.05 were considered statistically significant.
3. Results
3.1. Effects of siamese neem flower extracts on ileum relaxations
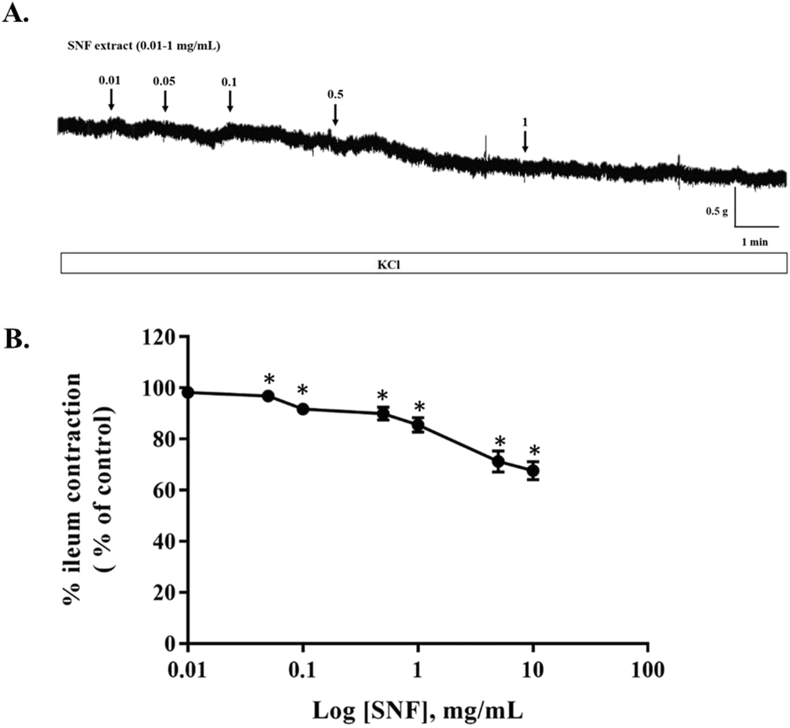
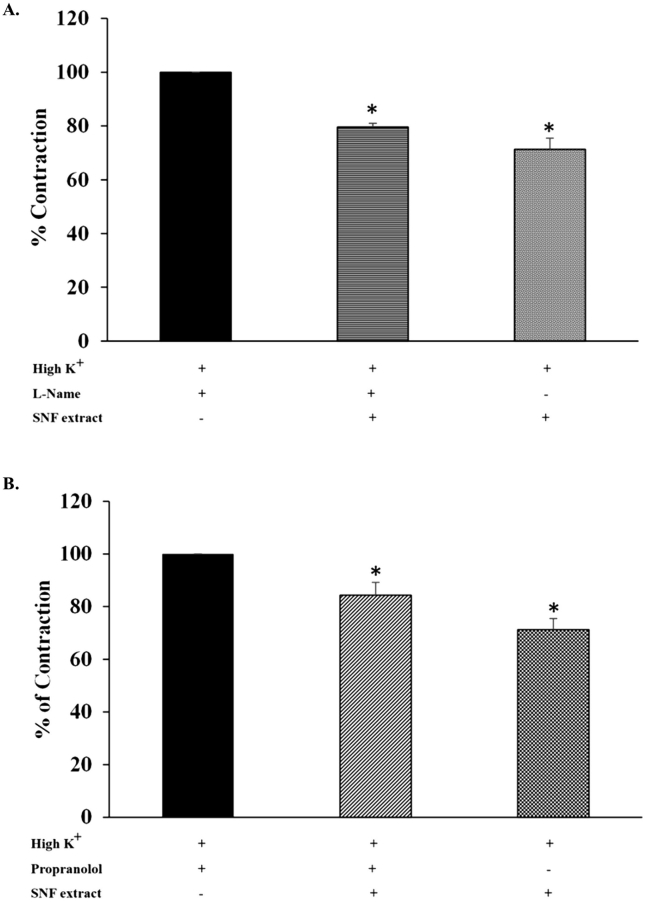
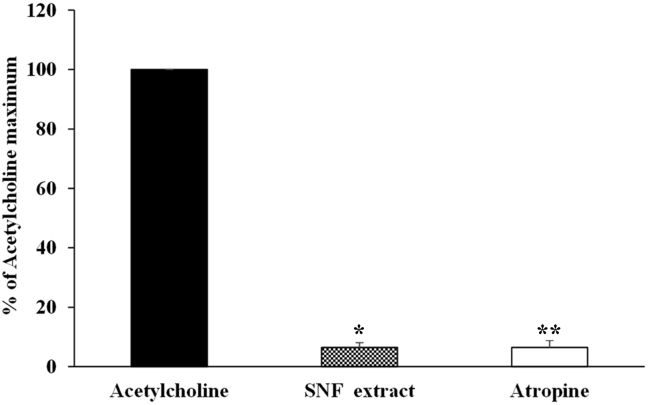
In order to study whether SNF extract reduced ileum contractions, 80 mM KCl was used for induction of the contraction. The cumulative concentrations of SNF extract (0.01–10 mg/mL) was added after KCl induced contraction reached to the plateau states. The extracts produced ileum relaxations in a dose dependent manner induced by KCl (Fig. 1). The extract at 5 and 10 mg/mL responsed to 71% and 68% of the contraction, respectively. However, there were no statistically significant difference of ileum contractions between 5 and 10 mg/mL of the extract. Therefore, 5 mg/mL of the extract was used in the subsequent study to define its potential mechanisms. To investigated the mechanism involved of the SNF extract on ileum relaxation, ileum was pretreated with 100 μM l-Name (antagonist of nitric oxide synthase) or 1 μM propanolol (antagonist of β-adrenergic receptor). The extract (5 mg/mL) induced relaxation in the presence and absence of l-Name (Fig. 2A). There were no statistically significant differences of ileum relaxtion between the presence and absence of l-Name. The relaxation effects of the extract responsed to 80% and 71%of ileum contraction induced by the presence and absence of l-Name, respectively. Similarly, preincubating of propranolol, the contraction was attenuated significantly in the presence of the extract (Fig. 2B). Moreover, pretreatment of the SNF extract (5 mg/mL) and atropine (100 nM) showed a significantly abolished the acetylcholine-effect (1 × 10−5 M) as shown in Fig. 3.
Fig. 1.
Effects of cumulative concentrations of SNF extract on rat's ileum contractions induced by KCl. 80 mM KCl were added to an organ bath to induce constant contractions. The extract (0.01–10 mg/mL) was then added cumulatively. (A) Representative trace of SNF extract and (B) summary of inhibitory effect of SNF extract on ileum contractions induced by KCl. Values are means ± SEM for 8 experiments. *Significantly different from values for control (P < .001).
Fig. 2.
The contractile effects of KCl on ileum and the inhibitory effect of SNF extract and (A) l-Name or (B) propanolol on KCl-induced contraction. Ileum was preincubated with 100 μM l-Name (antagonist of nitric oxide synthase) for 20 min or 1 μM propanolol (antagonist of β-adrenergic receptor) for 30 min prior to KCl 80 mM. Data are expressed as means ± SEM for 8 experiments. Panel (A), * significantly different among 3 groups compared with High K+ (P < .001). Panel (B), significantly different between propanolol and/or SNF extract compared with High K+ (#P < .001 and @P = .021, respectively).
Fig. 3.
Effect of SNF extract and atropine on acetylcholine-induced contraction. Ileum was preincubated with the extract (5 mg/mL) or atropine (100 nM) for 20 min prior to acetylcholine chloride (10−5 M). Values are means ± SEM for 6 experiments. SNF extract or atropine significantly different compared with acetylcholine (*P = .002 and **P = .001, respectively).
3.2. Effect of siamese neem flower on extracellular Ca2+ influx
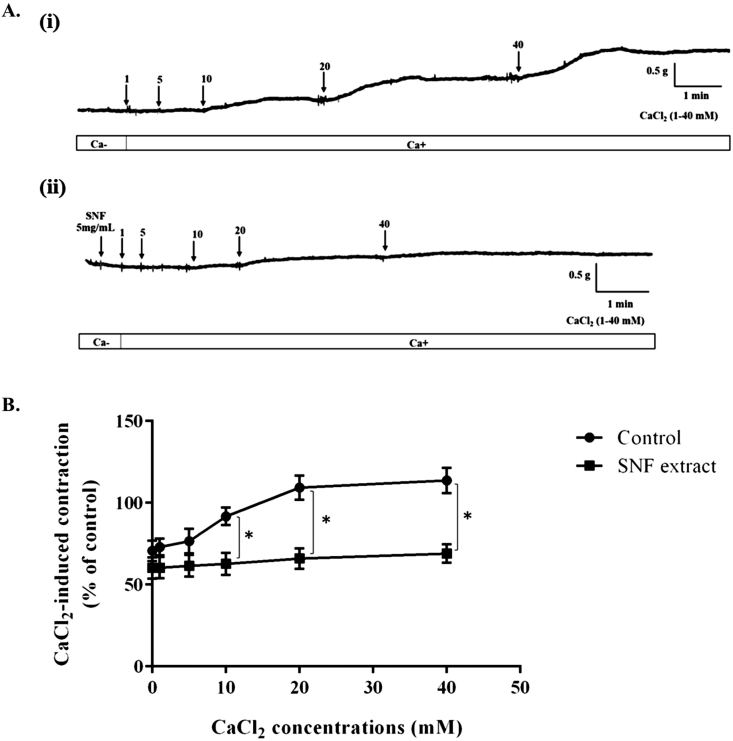
To investigated whether SNF extract produced ileum relaxation by interfering Ca2+ influx, the cumulative concentrations of CaCl2 (1–40 mM) were added in the bath containing Ca2+ free solution containing high K+ in the presence or absence SNF extract (5 mg/mL). The effect of Ca2+ induced ileum contraction in the absence and presence SNF extract on ileum contraction are shown in Fig. 4. Ca2+ induced a progressively increased the contraction in the depolarized tissue through KCL (80 mM). The contraction induced by CaCl2 was significantly decreased in the presence of SNF extract approximately 29%, 43%, and 45% at CaCl2 concentrations 10, 20, and 40 mM, respectively (Fig. 4B).
Fig. 4.
Effects on ileum contraction induced by CaCl2 in the absence (Control) and presence of SNF extract. (A) Representative traces of effects of absence (i) and presence of SNF extract (ii) and (B) summary of inhibitory effect of the extract on iluem contraction induced by cumulative Ca2+ in Ca2+ free solution containing 80 mM K+. Ileum was equlibrated in Ca2+ free Krebs' solution prior to incubate with the SNF extract (5 mg/mL), subsequently treated with cumulative doses of CaCl2 (1–40 mM) into the organ bath to stimulate the contraction. Values are means ± SEM for 8 experiments. *Significantly different from values for control (P < .001).
4. Discussion
The regulation of gastrointestinal motility and smooth muscle activity depends on hormones neurotransmitters, and numerous mediators including acetylcholine, noradrenaline and non-adrenergic non-cholinergic (NANC), which can be excitatory or inhibitory to gastrointestinal smooth muscle.12,13 The purpose of this study was to investigate the relaxant effects of siamese neem flower (A indica A. Juss var. siamensis Valeton) on rat ileal smooth muscle contractions.
KCl and acetylcholine (ACh) are known to induce smooth muscle contraction. The high level of K+ induces cell depolarization and consequently activates of L-type voltage-dependent calcium channels. The activation of calcium channels will cause the increase of Ca2+ influx into smooth muscle cell which eventually induced contraction.14 Meanwhile, Ach has known to play roles in the excitatory effect of parasympathetic nerves on intestinal smooth muscle. It binds to muscarinic receptors on the smooth muscle cell membrane15 and thereby increases the activity of phospholipase C which generates inositol triphosphate (IP3). The release of IP3 from the plasma membrane allowed it to stimulate the release of intracellular Ca2+ stores and subsequently induce contraction of smooth muscle cells.
However, under the influence of the competitive antagonist of muscarinic receptors such as atropine will lead to the response in inhibition of contraction. Therefore, substances that inhibit the K+ induced contraction may exert its effect through the blockage of the Ca2+ channel or preventing the entry of Ca2+ into smooth muscle cell. In our experiments, the presence of SNF extract significantly reduced the rat ileum contractions induced by KCl and ACh. Furthermore, SNF extract has also showed the atropine-like action by abolishing the stimulatory effect of acetylcholine. It could be possibly due to the fact that, SNF extract may possess an action as Ca2+ channel blocker or antagonist agent to ACh.
In order to further examine the relaxant effect of SNF extract, CaCl2 were then utilized in following study. Interestingly, the presence of SNF extract was observed to cause a significant decrease in ileum smooth muscle contraction induced by CaCl2. The result of the study implies the relaxant effect possess by SNF extract might acted through the blockage of calcium channel. The findings are found in agreement with many other studies, as plants such as Celastrus paniculatus16, Curcuma aeruginosa Roxb.17, and Rosmarinus officinalis L18 are known to exhibit calcium channel blocker-like activity.
Since nitric oxide (NO) and β-adrenoceptors are also known to affect gastrointestinal motility, the possible involvement of those receptors in observed SNF relaxant effect was then examined further as well. NO is synthesized from the l-arginine by nitric oxide synthase and it plays a major roles as an inhibitory NANC mediator in the gastrointestinal tract.12 It induces smooth muscle relaxation and hyperpolarization by activating the soluble guanylate cyclase and leading to the formation of cyclic guanosine monophosphate (cGMP). The increase in cGMP was then leads to the activation of cGMP dependent protein kinase G, which not only dephosphorylated the myosin light-chain but also activates potassium channels, and on the same time reducing the intracellular calcium concentrations.12,19 Therefore, the further study was conducted in order to understand whether the relaxant effect demonstrated by SNF extract might have acted through the modulation of nitric oxide production. However, the results showed that ileum relaxation induced by SNF extract was insensitive towards the stimulation of l-Name, a nitric oxide synthesis inhibitor. In addition, the simultaneous addition of l-Name and SNF extract resulted in a similar trend of response which was observed in SNF extract treatment group. It can be suggested that the relaxation response is not mediated through NO pathway. Meanwhile, another aspect of the study was focusing on the investigation on the possible mediation of β-adrenergic receptor in SNF extract stimulated relaxant effect. However, the experiment found there is no real correlation between the β-adrenergic receptor and relaxant effect showed by SNF extract. It was proven true as the results have shown no statically difference between the reduction of ileum contraction induced by SNF extract either in the presence or absence of propranolol, a β-adrenergic receptor antagonist. The results rule out the roles of β-adrenergic pathway in the relaxant effect of SNF extract.
Studies have showed the phytochemical constituents of many plants are able to attenuate the smooth muscle motility.20, 21, 22 Neem tree (Azadirachta indica A. Juss. var. siamensis Valeton) contains diterpenoids, triterpenoids, limonoids, proteins, polysaccharides, sulphurous compounds, polyphenolics, dihydrochalone, coumarin and tannins and aliphatic compounds7 alkaloids, flavonoids, steroids, gum, carbohydrates, saponins, and tannin.23 β-sitosterol, lupeol, rutin, ellagic acid, ferulic acid and quercetin,6 azadirachtin, nimbin, gallic acid, salanin, nimbolin, azadiradione, azadirone, mimbinin.7 It have been reported that phenolic compounds such as diterpene polyester and quercetin were involved in inducing ileal smooth muscle relaxation.20, 21, 22 On the other hand, flavonoids such as apigenin, genistein, quercetin, rutin, catechin, naringenin have known to exhibit relaxant effect on isolated gastric stomach.24 Furthermore, the flavonoid known as galetin 3,6-dimethyl ether has shown to decrease guinea pig ileum contraction through K+ channel activation and decrease in cytosolic calcium concentration.25 Therefore, the relaxant effect demonstrated by SNF might possibly due to the presence of these bioactive compounds in the extract.
However, further studies are needed to confirm bioavailability of its active compounds and in vivo studies will also be required to figure out the optimal dose for effective therapy.
5. Conclusions
The present study provides evidence that an aqueous extract of siamese neem flower possesses a relaxant effect in isolated rat ileum. The observed effect might have mediated through calcium channels. The findings of current study provide a pharmacological basis for the traditional use of SNF for the treatment of gastrointestinal spasms.
Conflicts of interest
The authors declare that they have no conflicts of interests.
Acknowledgements
The authors are very grateful Natakorn Kamkaew for support the instrument and wish to acknowledge School of Medical Science, University of Phayao for available to do research.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
Acharaporn Duangjai, Email: achara.phso@gmail.com.
Bey-Hing Goh, Email: goh.bey.hing@monash.edu.
Learn-Han Lee, Email: lee.learn.han@monash.edu.
Surasak Saokaew, Email: saokaew@gmail.com, surasak.sa@up.ac.th.
References
- 1.Peery A.F., Dellon E.S., Lund J. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179–1187. doi: 10.1053/j.gastro.2012.08.002. e1171–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beyder A., Farrugia G. Targeting ion channels for the treatment of gastrointestinal motility disorders. Therap Adv Gastroenterol. 2012;5(1):5–21. doi: 10.1177/1756283X11415892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kashyap P., Farrugia G. Diabetic gastroparesis: what we have learned and had to unlearn in the past 5 years. Gut. 2010;59(12):1716–1726. doi: 10.1136/gut.2009.199703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radulovic M., Anand P., Korsten M.A., Gong B. Targeting ion channels: an important therapeutic implication in gastrointestinal dysmotility in patients with spinal cord injury. J Neurogastroenterol Motil. 2015;21(4):494–502. doi: 10.5056/jnm15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grundmann O., Yoon S.L. Complementary and alternative medicines in irritable bowel syndrome: an integrative view. World J Gastroenterol. 2014;20(2):346–362. doi: 10.3748/wjg.v20.i2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pandey G., Verma K., Singh M. Evaluation of phytochemical, antibacterial and free radical scavenging properties of Azadirachta Indica (Neem) leaves. Int J Pharm Pharmaceut Sci. 2014;6(2):444–447. [Google Scholar]
- 7.Subapriya R., Nagini S. Medicinal properties of neem leaves: a review. Curr Med Chem Anticancer Agents. 2005;5(2) doi: 10.2174/1568011053174828. 149–146. [DOI] [PubMed] [Google Scholar]
- 8.Kumar V.S., Navaratnam V. Neem (Azadirachta indica): prehistory to contemporary medicinal uses to humankind. Asian Pac J Trop Biomed. 2013;3(7):505–514. doi: 10.1016/S2221-1691(13)60105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bandyopadhyay U., Biswas K., Sengupta A. Clinical studies on the effect of Neem (Azadirachta indica) bark extract on gastric secretion and gastroduodenal ulcer. Life Sci. 2004;75(24):2867–2878. doi: 10.1016/j.lfs.2004.04.050. [DOI] [PubMed] [Google Scholar]
- 10.Maity P., Biswas K., Chattopadhyay I., Banerjee R.K., Bandyopadhyay U. The use of neem for controlling gastric hyperacidity and ulcer. Phytother Res. 2009;23(6):747–755. doi: 10.1002/ptr.2721. [DOI] [PubMed] [Google Scholar]
- 11.Thakurta P., Bhowmik P., Mukherjee S., Hajra T.K., Patra A., Bag P.K. Antibacterial, antisecretory and antihemorrhagic activity of Azadirachta indica used to treat cholera and diarrhea in India. J Ethnopharmacol. 2007;111(3):607–612. doi: 10.1016/j.jep.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda N.M., Miller S.M. Non-adrenergic non-cholinergic inhibition of gastrointestinal smooth muscle and its intracellular mechanism(s) Fundam Clin Pharmacol. 2010;24(3):261–268. doi: 10.1111/j.1472-8206.2009.00761.x. [DOI] [PubMed] [Google Scholar]
- 13.Hansen M.B. Neurohumoral control of gastrointestinal motility. Physiol Res. 2003;52(1):1–30. [PubMed] [Google Scholar]
- 14.Karaki H., Ozaki H., Hori M. Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev. 1997;49(2):157–230. [PubMed] [Google Scholar]
- 15.Montgomery L.E., Tansey E.A., Johnson C.D., Roe S.M., Quinn J.G. Autonomic modification of intestinal smooth muscle contractility. Adv Physiol Educ. 2016;40(1):104–109. doi: 10.1152/advan.00038.2015. [DOI] [PubMed] [Google Scholar]
- 16.Borrelli F., Borbone N., Capasso R. Potent relaxant effect of a Celastrus paniculatus extract in the rat and human ileum. J Ethnopharmacol. 2009;122(3):434–438. doi: 10.1016/j.jep.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Thaina P., Tungcharoen P., Wongnawa M., Reanmongkol W., Subhadhirasakul S. Uterine relaxant effects of Curcuma aeruginosa Roxb. rhizome extracts. J Ethnopharmacol. 2009;121(3):433–443. doi: 10.1016/j.jep.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Ventura-Martínez R., Rivero-Osorno O., Gómez C., González-Trujano M.E. Spasmolytic activity of Rosmarinus officinalis L. involves calcium channels in the Guinea pig ileum. J Ethnopharmacol. 2011;137(3):1528–1532. doi: 10.1016/j.jep.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 19.Ragy M., Elbassuoni E. The role of nitric oxide and L-type calcium channel blocker in the contractility of rabbit ileum in vitro. J Physiol Biochem. 2012;68(4):521–528. doi: 10.1007/s13105-012-0167-x. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi J., Sekine T., Deguchi S. Phenolic compounds from Gastrodia rhizome and relaxant effects of related compounds on isolated smooth muscle preparation. Phytochemistry. 2002;59(5):513–519. doi: 10.1016/s0031-9422(02)00008-0. [DOI] [PubMed] [Google Scholar]
- 21.Ghanadian M., Sadraei H., Yousuf S., Asghari G., Choudhary M.I., Jahed M. New diterpene polyester and phenolic compounds from Pycnocycla spinosa Decne. Ex Boiss with relaxant effects on KCl-induced contraction in rat ileum. Phytochemistry Letters. 2014;7:57–61. [Google Scholar]
- 22.Hammad H.M., Abdalla S.S. Pharmacological effects of selected flavonoids on rat isolated ileum: structure-activity relationship. Gen Pharmacol Vasc Syst. 1997;28(5):767–771. doi: 10.1016/s0306-3623(96)00299-6. [DOI] [PubMed] [Google Scholar]
- 23.Emran T.B., Uddin M.M.N., Rahman A., Uddin Z., Islam M. Phytochemical, antimicrobial, cytotoxic, analgesic and anti-inflammatory properties of Azadirachta Indica: a therapeutic study. J Bioanal Biomed. 2015;S12:1–7. [Google Scholar]
- 24.Amira S., Rotondo A., Mulè F. Relaxant effects of flavonoids on the mouse isolated stomach: structure-activity relationships. Eur J Pharmacol. 2008;599(1–3):126–130. doi: 10.1016/j.ejphar.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 25.Vasconcelos L.H.C., Correia ACdC., Souza ILLd. Flavonoid galetin 3,6-dimethyl ether attenuates Guinea pig ileum contraction through K+ channel activation and decrease in cytosolic calcium concentration. Eur J Pharmacol. 2015;767:52–60. doi: 10.1016/j.ejphar.2015.10.007. [DOI] [PubMed] [Google Scholar]