Abstract
This study aimed to describe real-life microbiological testing of adults hospitalised with community-acquired pneumonia (CAP) and to assess concordance with the 2007 Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS) and 2011 European Respiratory Society (ERS) CAP guidelines.
This was a cohort study based on the Global Initiative for Methicillin-resistant Staphylococcus aureus Pneumonia (GLIMP) database, which contains point-prevalence data on adults hospitalised with CAP across 54 countries during 2015.
In total, 3702 patients were included. Testing was performed in 3217 patients, and included blood culture (71.1%), sputum culture (61.8%), Legionella urinary antigen test (30.1%), pneumococcal urinary antigen test (30.0%), viral testing (14.9%), acute-phase serology (8.8%), bronchoalveolar lavage culture (8.4%) and pleural fluid culture (3.2%). A pathogen was detected in 1173 (36.5%) patients. Testing attitudes varied significantly according to geography and disease severity. Testing was concordant with IDSA/ATS and ERS guidelines in 16.7% and 23.9% of patients, respectively. IDSA/ATS concordance was higher in Europe than in North America (21.5% versus 9.8%; p<0.01), while ERS concordance was higher in North America than in Europe (33.5% versus 19.5%; p<0.01).
Testing practices of adults hospitalised with CAP varied significantly by geography and disease severity. There was a wide discordance between real-life testing practices and IDSA/ATS/ERS guideline recommendations.
Short abstract
Testing practices vary based on geography and disease severity, and IDSA/ATS/ERS testing recommendations are rarely followed http://ow.ly/80Iy30lxo1c
Introduction
Community-acquired pneumonia (CAP) is a leading cause of hospitalisation worldwide. Mortality rates for patients hospitalised with CAP approach 30%, especially in those admitted to an intensive care unit (ICU) [1–4]. Diagnostic testing in CAP has the potential to improve individual patient management, reducing the risk of clinical failure and death, and to generate epidemiological data, informing the selection of appropriate empirical antibiotic therapy. Unfortunately, these advantages are counterbalanced by high healthcare costs associated with diagnostic testing and low sensitivity of these tests to identify pathogens causing CAP [5, 6].
Considering both the benefits and limitations of diagnostic testing, several international scientific societies have published guidelines on effective diagnostic testing strategies for hospitalised patients with CAP. However, important differences exist between recommendations of different societies [7]. These recommendations are mainly based on expert opinion given the scarcity of published evidence. In particular, substantial differences can be found between the two most cited international guidelines on CAP: the 2007 Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS) and the 2011 European Respiratory Society (ERS) guidelines [8, 9]. Limited data are available of real-life diagnostic testing practices for hospitalised patients with CAP and whether real-life diagnostic testing practices are concordant with the 2007 IDSA/ATS and 2011 ERS guideline recommendations [10–12].
This study aimed to describe real-life microbiological testing of adults hospitalised with CAP, evaluate the influence of geography and disease severity on microbiological testing practices, and assess the concordance of real-life microbiological testing with the two most cited international guidelines, specifically the 2007 IDSA/ATS and the 2011 ERS guidelines.
Methods
Study design, setting and participants
We performed a secondary analysis of an international, multicentre, observational, prospective cohort study using the Global Initiative for Methicillin-resistant Staphylococcus aureus Pneumonia (GLIMP) database [13]. GLIMP was conducted among 222 hospitals in 54 countries over 4 days, with 1 day per month randomly selected during March, April, May and June 2015. All adult patients (aged >18 years) hospitalised for CAP at the participating centres during the four study days were screened by GLIMP investigators and included in this secondary analysis. Patients hospitalised with a diagnosis of hospital-acquired or ventilator-associated pneumonia were excluded. The GLIMP coordinating centre was located at the University of Texas Health San Antonio (San Antonio, TX, USA). The coordinating centre received expedited project approval by the institutional review board (number HSC20150184E). The review board waived the need for receipt of informed consent due to the nature of the study. Institutional review board approval was obtained by the site investigators at each individual centre. A detailed description of the GLIMP organisation and methodologies has been previously published [13].
Study outcomes
The primary outcome of this study was describing real-life microbiological testing among patients hospitalised with CAP, including the frequency of testing, laboratory technique used and patients’ characteristics by testing status (tested patients versus not tested patients). This study had also two secondary outcomes. The first was to evaluate the influence of geography and disease severity on testing practices. ICU admission, invasive mechanical ventilation, vasopressors, and combined administration of vasopressors and invasive mechanical ventilation were used as measures of disease severity. The second was to evaluate the concordance of real-life microbiological testing with the 2007 IDSA/ATS and the 2011 ERS guidelines for CAP.
Study definitions
CAP was defined by evidence of new pulmonary infiltrates on thoracic imaging (chest radiograph, computed tomography or ultrasound) during the first 48 h of hospitalisation and at least one of the following criteria: new or increased cough with or without sputum production or with purulent respiratory secretions; fever or hypothermia (documented rectal or oral temperature ≥37.8°C or <36°C, respectively); and evidence of systemic inflammation, such as abnormal white blood cell count (leukocytosis (>10 000 cells·mL−1), leukopenia (<4000 cells·mL−1) or bandaemia (>10%)) and increased C-reactive protein or procalcitonin concentrations above the local upper limit of normal. Hospitalisation was defined as admission at an inpatient service and subsequent stay for ≥24 h. Methicillin-resistant Staphylococcus aureus was defined according to the Clinical and Laboratory Standards Institute (CLSI) guidelines, in which the minimum inhibitory concentration was ≥4 µg·mL−1 to oxacillin. Production of extended-spectrum β-lactamase was defined according to the CLSI guidelines via broth microdilution or disk diffusion clavulanate inhibition test.
Diagnostic testing was defined as concordant with the 2007 IDSA/ATS guidelines if recommended tests were performed and non-recommended tests were not performed. Similarly, diagnostic testing was defined as concordant with the 2011 ERS guidelines if recommended tests were performed and non-recommended tests were not performed. The tests considered in this study as recommended or non-recommended by the IDSA/ATS and ERS guidelines are presented in table 1. Over-testing was defined as a condition where tests not required were performed. Under-testing was defined as a condition where required tests were not performed.
TABLE 1.
Tests considered as recommended or not recommended by the Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS) and European Respiratory Society (ERS) guidelines
| IDSA/ATS recommendations | ERS recommendations | |
| Blood culture | Recommended in case of ICU admission, leukopenia, alcohol abuse, chronic severe liver disease, asplenia, positive pneumococcal urinary antigen test and presence of pleural effusion | Recommended in all patients hospitalised with CAP |
| Sputum culture | Recommended in case of ICU admission, alcohol abuse, severe obstructive or structural lung disease, positive Legionella urinary antigen test, positive pneumococcal urinary antigen test and presence of pleural effusion | Recommended in case of purulent sputum sample |
| Bronchoalveolar lavage culture | Recommended in case of intubation | Recommended in case of intubation |
| Legionella urinary antigen test | Recommended in case of ICU admission, alcohol abuse and presence of pleural effusion | Recommended in all patients hospitalised with severe CAP |
| Pneumococcal urinary antigen test | Recommended in case of ICU admission, leukopenia, alcohol abuse, chronic severe liver disease, asplenia and pleural effusion | Recommended in all patients hospitalised with severe CAP |
| Acute-phase serology for Chlamydophila pneumoniae, Mycoplasma pneumoniae, and Legionella species | Not recommended | Not recommended |
ICU: intensive care unit; CAP: community-acquired pneumonia.
Statistical analysis
Continuous variables were presented as medians with interquartile range. Categorical variables were presented as frequencies and percentages of the specified group. Comparisons between groups were made with the Fisher exact test or the Kruskal–Wallis test, as appropriate. A two-sided p-value of <0.05 was considered statistically significant. Statistical analyses were performed using SPSS Statistics, version 24, software (IBM, Armonk, NY, USA).
Results
Among 3702 patients, 3217 (86.9%) had at least one diagnostic test performed, with 1173 (36.5%) patients with at least one pathogen detected by diagnostic testing. Variables significantly associated with the performance of diagnostic testing are presented in table 2. When patients from whom at least one pathogen was detected were compared with patients from whom no pathogens were detected, we found the former group more commonly presented the following conditions: bronchiectasis, tracheostomy, at least one respiratory comorbidity, hypertension, HIV infection, pervious infections, previous healthcare exposure, severe CAP, ICU admission, mechanical ventilation and use of vasopressors (table 2).
TABLE 2.
Baseline characteristics of adult inpatients with community-acquired pneumonia by testing status and by pathogen detection
| Testing status | Pathogen detection | |||||
| Not tested | Tested | p-value | Not detected | Detected | p-value | |
| Patients | 485 | 3217 | 2044 | 1173 | ||
| Demographic characteristics | ||||||
| Age | 71.0 (54.0–81.0) | 68.0 (54.0–80.0) | 0.03 | 70.0 (55.0–81.0) | 65.0 (50.0–77.0) | <0.01 |
| Male sex | 255 (52.6) | 1888 (58.7) | 0.01 | 1173 (57.4) | 715 (61.0) | 0.04 |
| Underweight | 14 (5.2) | 152 (7.4) | 0.19 | 83 (6.5) | 69 (8.8) | 0.06 |
| Obesity | 64 (13.2) | 513 (15.9) | 0.12 | 314 (15.4) | 199 (17.0) | 0.23 |
| Respiratory past medical history | ||||||
| Active lung cancer | 17 (3.5) | 92 (2.9) | 0.43 | 62 (3.0) | 30 (2.6) | 0.44 |
| Asthma | 26 (5.4) | 235 (7.3) | 0.12 | 149 (7.3) | 86 (7.3) | 0.97 |
| Bronchiectasis | 9 (1.9) | 169 (5.3) | <0.01 | 76 (3.7) | 93 (7.9) | <0.01 |
| Chronic aspiration | 39 (8.0) | 218 (6.8) | 0.31 | 137 (6.7) | 81 (6.9) | 0.83 |
| COPD | 96 (19.8) | 840 (26.1) | 0.01 | 517 (25.3) | 323 (27.5) | 0.16 |
| Current or former smoker | 121 (24.9) | 1124 (34.9) | <0.01 | 709 (34.7) | 415 (35.4) | 0.69 |
| Interstitial lung disease | 4 (0.8) | 91 (2.8) | 0.01 | 65 (3.2) | 26 (2.2) | 0.11 |
| Obstructive sleep apnoea | 7 (1.4) | 123 (3.8) | 0.01 | 78 (3.8) | 45 (3.8) | 0.98 |
| Home oxygen therapy | 14 (2.9) | 210 (6.5) | <0.01 | 123 (6.0) | 87 (7.4) | 0.12 |
| Lung transplant | 0 (0.0) | 7 (0.2) | 0.30 | 2 (0.1) | 5 (0.4) | 0.06 |
| Tracheostomy | 3 (0.6) | 50 (1.6) | 0.11 | 14 (0.7) | 36 (3.1) | <0.01 |
| At least one respiratory comorbidity | 235 (48.5) | 1917 (59.6) | <0.01 | 1434 (56.7) | 718 (61.2) | 0.01 |
| Cardiovascular past medical history | ||||||
| Arrhythmia | 69 (14.2) | 458 (14.2) | 1.00 | 307 (15.0) | 151 (12.9) | 0.09 |
| Coronary artery disease | 69 (14.2) | 528 (16.4) | 0.22 | 325 (15.9) | 203 (17.3) | 0.30 |
| Heart failure | 64 (13.2) | 421 (13.1) | 0.95 | 270 (13.2) | 151 (12.9) | 0.79 |
| Hypertension | 201 (41.4) | 1454 (45.2) | 0.12 | 973 (47.6) | 481 (41.0) | <0.01 |
| Stroke | 55 (11.3) | 251 (7.8) | 0.01 | 168 (8.2) | 83 (7.1) | 0.25 |
| Immunosuppressive conditions | ||||||
| Active solid tumour | 40 (8.2) | 247 (7.7) | 0.66 | 164 (8.0) | 83 (7.1) | 0.33 |
| AIDS | 8 (1.6) | 57 (1.8) | 0.85 | 24 (1.2) | 33 (2.8) | <0.01 |
| Aplastic anaemia | 1 (0.2) | 13 (0.4) | 0.51 | 8 (0.4) | 5 (0.4) | 0.88 |
| Asplenia | 0 (0.0) | 12 (0.4) | 0.18 | 5 (0.2) | 7 (0.6) | 0.12 |
| Chemotherapy in the last 3 months | 9 (1.9) | 136 (4.2) | 0.01 | 83 (4.1) | 53 (4.5) | 0.54 |
| Haematological malignancy | 11 (2.3) | 151 (4.7) | 0.02 | 93 (4.5) | 58 (4.9) | 0.61 |
| HIV infection | 16 (3.3) | 107 (3.3) | 0.98 | 56 (2.7) | 51 (4.3) | 0.01 |
| Neutropenia | 4 (0.8) | 44 (1.4) | 0.32 | 29 (1.4) | 15 (1.3) | 0.74 |
| Steroids use | 24 (4.9) | 270 (8.4) | 0.01 | 174 (8.5) | 96 (8.2) | 0.75 |
| At least one immunosuppressive condition | 74 (15.3) | 591 (18.4) | 0.09 | 356 (17.4) | 235 (20.0) | 0.07 |
| Other chronic medical conditions | ||||||
| Chronic renal failure | 50 (10.3) | 350 (10.9) | 0.71 | 241 (11.8) | 109 (9.3) | 0.03 |
| Cirrhosis | 6 (1.2) | 64 (2.0) | 0.26 | 36 (1.8) | 28 (2.4) | 0.22 |
| Diabetes mellitus | 92 (19.0) | 690 (21.4) | 0.21 | 448 (21.9) | 242 (20.6) | 0.39 |
| Haemodialysis | 1 (0.2) | 51 (1.6) | 0.02 | 32 (1.6) | 19 (1.6) | 0.91 |
| Liver disease | 11 (2.3) | 129 (4.0) | 0.06 | 75 (3.7) | 54 (4.6) | 0.19 |
| Mental illness | 33 (6.8) | 221 (6.9) | 0.96 | 146 (7.1) | 75 (6.4) | 0.42 |
| Dementia | 74 (15.3) | 334 (10.4) | <0.01 | 228 (11.2) | 106 (9.0) | 0.06 |
| Previous infections | ||||||
| Prior ESBL | 0 (0.0) | 55 (1.7) | <0.01 | 27 (1.3) | 28 (2.4) | 0.03 |
| Prior MRSA | 5 (1.0) | 81 (2.5) | 0.04 | 34 (1.7) | 47 (4.0) | <0.01 |
| Prior mycobacterial disease | 7 (1.4) | 89 (2.8) | 0.09 | 42 (2.1) | 47 (4.0) | <0.01 |
| Prior Pseudomonas | 4 (0.8) | 97 (3.0) | <0.01 | 32 (1.6) | 65 (5.5) | <0.01 |
| Previous healthcare exposure# | ||||||
| Lower respiratory tract infection | 106 (21.9) | 935 (29.1) | <0.01 | 667 (26.4) | 374 (31.9) | <0.01 |
| Emergency room admission | 120 (24.7) | 981 (30.5) | 0.01 | 708 (28.0) | 393 (33.5) | <0.01 |
| Hospitalisation | 128 (26.4) | 1035 (32.2) | 0.01 | 771 (30.5) | 392 (33.4) | 0.07 |
| Home antibiotic infusion therapy | 21 (4.3) | 141 (4.4) | 0.96 | 94 (3.7) | 68 (5.8) | <0.01 |
| Intravenous antibiotics | 89 (18.4) | 816 (25.4) | <0.01 | 569 (22.5) | 336 (28.6) | <0.01 |
| Oral antibiotics | 166 (34.2) | 1226 (38.1) | 0.10 | 917 (36.3) | 475 (40.5) | 0.01 |
| Current pneumonia episode | ||||||
| ICU admission | 33 (6.8) | 601 (18.7) | <0.01 | 275 (13.5) | 326 (27.8) | <0.01 |
| Mechanical ventilation | 28 (5.8) | 634 (19.7) | <0.01 | 312 (15.3) | 322 (27.5) | <0.01 |
| Vasopressors | 9 (1.9) | 233 (7.2) | <0.01 | 91 (4.5) | 142 (12.1) | <0.01 |
Data are presented as n, median (interquartile range) or n (%), unless otherwise stated. COPD: chronic obstructive pulmonary disease; ESBL: extended-spectrum β-lactamase; MRSA: methicillin-resistant Staphylococcus aureus; ICU: intensive care unit. #: in the previous 12 months. Bold indicates statistical significance at p<0.05.
Of the 3702 patients hospitalised with CAP and included in this study, diagnostic testing was as follows: 2633 (71.1%) had blood cultures, 2287 (61.8%) had sputum cultures, 1113 (30.1%) had Legionella urinary antigen testing, 1110 (30.0%) had pneumococcal urinary antigen testing, 553 (14.9%) had viral testing, 325 (8.8%) had acute-phase serology, 312 (8.4%) had bronchoalveolar lavage (BAL) cultures and 117 (3.2%) had pleural fluid cultures. Blood, sputum, BAL cultures, pleural fluid cultures and viral testing were more frequently obtained among patients undergoing invasive mechanical ventilation compared to patients not receiving invasive mechanical ventilation. In contrast, when ICU admission, vasopressor administration, or combined vasopressor and invasive mechanical ventilation administration were used as measures of disease severity, only blood cultures, BAL cultures and viral testing were significantly more common among patients with a severe disease (table 3). Of the 8450 diagnostic tests performed, 12.9% yielded a positive result. Specifically, 38.8% of the BAL cultures, 28.2% of the viral testing, 19.4% of acute-phase serology testing, 17.7% of sputum cultures, 11.7% of pneumococcal urinary antigen testing, 11.1% of pleural fluid cultures, 6.7% of blood cultures and 2.2% of Legionella urinary antigen testing led to the detection of at least one pathogen, for a total of 1362 pathogens detected. Bacteria, viruses and fungi accounted for 83.0%, 14.1% and 2.9% of the pathogens detected, respectively. Streptococcus pneumoniae was the most frequently encountered pathogen (n=268), followed by Staphylococcus aureus (n=188), influenza viruses (n=154) and Pseudomonas aeruginosa (n=133).
TABLE 3.
Microbiological tests performed among adult inpatients with community-acquired pneumonia by disease severity
| Total | ICU | Invasive mechanical ventilation and vasopressors | |||||
| No | Yes | p-value | No | Yes | p-value | ||
| Patients | 3702 | 3068 | 634 | 3529 | 173 | ||
| Blood culture | 2633 (71.1) | 2110 (68.8) | 523 (82.5) | <0.01 | 2477 (70.2) | 156 (90.2) | <0.01 |
| Sputum culture | 2287 (61.8) | 1895 (61.8) | 392 (61.8) | 0.98 | 2182 (61.8) | 105 (60.7) | 0.76 |
| Bronchoalveolar lavage culture | 312 (8.4) | 179 (5.8) | 133 (21.0) | <0.01 | 268 (7.6) | 44 (25.4) | <0.01 |
| Pleural fluid culture | 117 (3.2) | 90 (2.9) | 27 (4.3) | 0.08 | 108 (3.1) | 9 (5.2) | 0.12 |
| Pneumococcal urinary antigen | 1110 (30.0) | 939 (30.6) | 171 (27.0) | 0.07 | 1054 (29.9) | 56 (32.4) | 0.48 |
| Legionella urinary antigen | 1113 (30.1) | 939 (30.6) | 174 (27.4) | 0.11 | 1051 (29.8) | 62 (35.8) | 0.09 |
| Acute-phase serology | 325 (8.8) | 258 (8.4) | 67 (10.6) | 0.08 | 307 (8.7) | 18 (10.4) | 0.44 |
| Viral testing | 553 (14.9) | 390 (12.7) | 163 (25.7) | <0.01 | 482 (13.7) | 71 (41.0) | <0.01 |
Data are presented as n or n (%), unless otherwise stated. ICU: intensive care unit. Bold indicates statistical significance at p<0.05.
When the performance of diagnostic testing was compared among patients admitted at participating hospitals in North America, South America, Africa, Asia, Europe and Oceania, significant differences were identified (table 4). Performance of at least one test ranged from 82.1% among patients hospitalised in Africa to 97.8% among patients hospitalised in Asia. While blood cultures were obtained in approximately 70–90% of patients hospitalised in North America, South America, Europe, Asia and Oceania, only 41.7% of patients in participating African hospitals had blood cultures (p<0.01). Similarly, viral testing was performed in 10–20% of patients hospitalised in North America, South America, Europe, Asia and Oceania, and in 1.3% of patients admitted to African hospitals (p<0.01). Pneumococcal and Legionella urinary antigen tests were performed in >40% of the patients admitted in European hospitals and in <20% of the patients hospitalised in the remaining continents (p<0.01). Acute-phase serology for Chlamydophila pneumoniae, Mycoplasma pneumoniae and Legionella species was more common in Europe (11.2%) than elsewhere (4.6%) (p<0.01).
TABLE 4.
Microbiological tests performed among adult inpatients with community-acquired pneumonia by geographic area
| Continent | Rest of the world | p-value | |
| Blood culture | |||
| North America | 434 (82.0) | 2199 (69.3) | <0.01 |
| South America | 202 (92.7) | 2431 (69.8) | <0.01 |
| Africa | 65 (41.7) | 2568 (72.4) | <0.01 |
| Asia | 294 (70.8) | 2339 (71.2) | 0.89 |
| Europe | 1609 (68.6) | 1024 (75.4) | <0.01 |
| Oceania | 29 (72.5) | 2604 (71.1) | 0.85 |
| Sputum culture | |||
| North America | 286 (54.1) | 2001 (63.1) | <0.01 |
| South America | 95 (43.6) | 2192 (62.9) | <0.01 |
| Africa | 92 (59.0) | 2195 (61.9) | 0.46 |
| Asia | 305 (73.5) | 1982 (60.3) | <0.01 |
| Europe | 1496 (63.8) | 791 (58.2) | <0.01 |
| Oceania | 13 (32.5) | 2274 (62.1) | <0.01 |
| Bronchoalveolar lavage culture | |||
| North America | 68 (12.9) | 244 (7.7) | <0.01 |
| South America | 15 (6.9) | 297 (8.5) | 0.40 |
| Africa | 10 (6.4) | 302 (8.5) | 0.35 |
| Asia | 48 (11.6) | 264 (8.0) | 0.02 |
| Europe | 171 (7.3) | 141 (10.4) | <0.01 |
| Oceania | 0 (0.0) | 312 (8.5) | 0.05 |
| Pleural fluid culture | |||
| North America | 13 (2.5) | 104 (3.3) | 0.32 |
| South America | 13 (6.0) | 104 (3.3) | 0.02 |
| Africa | 12 (7.7) | 105 (3.0) | <0.01 |
| Asia | 12 (2.9) | 105 (3.2) | 0.74 |
| Europe | 67 (2.9) | 50 (3.7) | 0.17 |
| Oceania | 0 (0.0) | 117 (3.2) | 0.25 |
| Pneumococcal urinary antigen | |||
| North America | 55 (10.4) | 1055 (33.2) | <0.01 |
| South America | 17 (7.8) | 1093 (31.4) | <0.01 |
| Africa | 2 (1.3) | 1108 (31.2) | <0.01 |
| Asia | 22 (5.3) | 1088 (33.1) | <0.01 |
| Europe | 1014 (43.3) | 96 (7.1) | <0.01 |
| Oceania | 0 (0.0) | 1110 (30.3) | <0.01 |
| Legionella urinary antigen | |||
| North America | 93 (17.6) | 1020 (32.1) | <0.01 |
| South America | 1 (0.5) | 1112 (31.9) | <0.01 |
| Africa | 1 (0.6) | 1112 (31.4) | <0.01 |
| Asia | 30 (7.2) | 1083 (32.9) | <0.01 |
| Europe | 988 (42.2) | 125 (9.2) | <0.01 |
| Oceania | 0 (0.0) | 1113 (30.4) | <0.01 |
| Acute-phase serology | |||
| North America | 22 (4.2) | 303 (9.5) | <0.01 |
| South America | 11 (5.0) | 314 (9.0) | 0.04 |
| Africa | 9 (5.8) | 316 (8.9) | 0.18 |
| Asia | 17 (4.1) | 308 (9.4) | <0.01 |
| Europe | 263 (11.2) | 62 (4.6) | <0.01 |
| Oceania | 3 (7.5) | 322 (8.8) | 0.77 |
| Viral testing | |||
| North America | 83 (15.7) | 470 (14.8) | 0.60 |
| South America | 25 (11.5) | 528 (15.2) | 0.14 |
| Africa | 2 (1.3) | 551 (15.5) | <0.01 |
| Asia | 78 (18.8) | 475 (14.5) | 0.02 |
| Europe | 361 (15.4) | 192 (14.1) | 0.30 |
| Oceania | 4 (10.0) | 549 (15.0) | 0.38 |
| At least one test done | |||
| North America | 489 (92.4) | 2728 (86.0) | <0.01 |
| South America | 204 (93.6) | 3013 (86.5) | <0.01 |
| Africa | 128 (82.1) | 3089 (87.1) | 0.07 |
| Asia | 406 (97.8) | 2811 (85.5) | <0.01 |
| Europe | 1955 (83.4) | 1262 (92.9) | <0.01 |
| Oceania | 35 (87.5) | 3182 (86.9) | 0.91 |
Data are presented as n (%), unless otherwise stated. Bold indicates statistical significance at p<0.05.
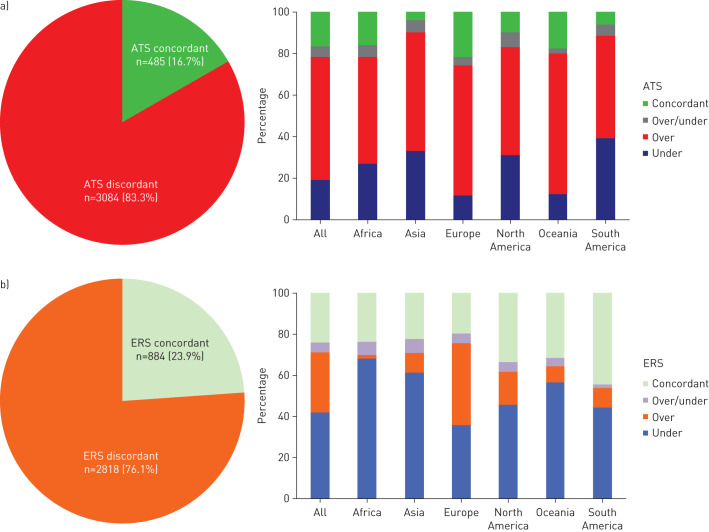
Diagnostic testing was concordant with the IDSA/ATS and ERS recommendations in 16.7% and 23.9% of the patients, respectively. When the overall study population was analysed, over-testing and under-testing were reported in 59.3% and 19.1% of the IDSA/ATS-discordant patients, respectively (figure 1). Among IDSA/ATS-discordant patients, blood cultures performed without indications accounted for the majority of over-testing, while the lack of pneumococcal urinary antigen test was responsible for a significant percentage of under-testing. Over-testing and under-testing were documented in 29.3% and in 42.0% of the ERS-discordant patients, respectively (figure 1). Among ERS-discordant patients, pneumococcal and Legionella urinary antigen tests performed without an indication accounted for the majority of over-testing, while under-testing was mainly due to the lack of obtaining blood cultures when indicated. IDSA/ATS concordance was more common in Europe than in North America (21.5% versus 9.8%; p<0.01), while ERS concordance was more common in North America than in Europe (33.5% versus 19.5%; p<0.01).
FIGURE 1.
Discordance of diagnostic testing with the a) 2007 Infectious Diseases Society of America/American Thoracic Society (ATS) guidelines and b) 2011 European Respiratory Society (ERS) guidelines for community-acquired pneumonia by geographic area. Over: over-testing; under: under-testing; over/under: over-testing and under-testing.
Discussion
This international, multicentre, point-prevalence study provides a high-quality, real-life picture of diagnostic testing in patients hospitalised with CAP. At least one microbiological test was performed in the vast majority of patients hospitalised with CAP and led to an aetiological diagnosis in one-third of patients tested. Geographic area and disease severity influenced testing frequency. Our study also highlights a significant discordance of real-life diagnostic testing compared to recommended testing in the 2007 IDSA/ATS and 2011 ERS guidelines for CAP management in adults. Several crucial points could be raised by our findings.
First, a pathogen was identified in one-third of adult inpatients with CAP. This pathogen-detection yield is similar to previously reported investigations [14–21] and consistent with the EPIC (Etiology of Pneumonia in the Community) study [1]. Despite extensive laboratory testing, the EPIC study identified a pathogen in 38% of 2259 patients hospitalised with CAP in the USA. The low pathogen-detection yield reported in this and other CAP studies highlights how limited our understanding is of CAP aetiologies among adult inpatients and how current empirical antimicrobial recommendations are based on weak evidence. Studies aimed at collecting as much data as possible to identify the aetiology using state-of-the-art diagnostic techniques and innovative pathogen-discovery approaches are urgently needed. Furthermore, aetiological studies should use novel analytical techniques in order to incorporate evidence from multiple specimens to account for the imperfect sensitivity and specificity of the diagnostic tests used [22]. Once a more accurate estimate of the aetiological distribution of CAP among adult inpatients is available, empirical antimicrobial recommendations should be updated.
Secondly, our study showed the existence of a strong association between pneumonia severity and performance of diagnostic testing, in accordance with IDSA/ATS and ERS recommendations. As a consequence, pneumonia severity was also associated with an increased probability of pathogen detection. Exploring the true determinants of pathogen detection would have required a systematic and universal testing strategy and, for this reason, it was out of the scope of this study.
Thirdly, our study confirmed the differing diagnostic yield of various diagnostic tests. Specifically, only 6.7% of blood cultures yielded a positive result, confirming the low sensitivity of blood cultures for revealing the aetiology of CAP, similar to the findings of other studies [16–23]. In contrast, BAL cultures were characterised by a high diagnostic yield (38.8%). While impractical and potentially associated with complications, BAL cultures represent an effective diagnostic tool for patients with severe infections, who may benefit the most from a targeted antimicrobial regimen. Indeed, a randomised trial by van der Eerden et al. [24] showed a statistically significant difference in mortality among ICU patients receiving empirical broad-spectrum antimicrobials (91%) versus patients receiving pathogen-directed antimicrobials (45%).
Fourthly, our analysis described a significant geographic variation in diagnostic testing strategies. We could speculate that the economical restraints of African health systems accounted for the reduced number of blood cultures and viral tests performed in this setting. Laboratory infrastructure to support diagnostic microbiological testing is limited in most African countries: bacteriological culture or molecular techniques that form the mainstay of CAP diagnostics in well-resourced settings are often lacking in Africa [25, 26]. In contrast, the seasonality and the epidemiological relevance of respiratory viruses, such as avian-origin influenza A and Middle East respiratory syndrome coronavirus, may have favoured the performance of viral testing in Asia [27, 28].
Fifthly, our study is among the first to evaluate the concordance of real-life diagnostic testing with international guidelines. To the best of our knowledge, only Jenkins et al. [12] made a similar attempt. They analysed the concordance of diagnostic testing practices retrospectively in a cohort of adult inpatients with CAP with the 2007 IDSA/ATS guidelines and reported over-testing with blood cultures. Our study revealed that real-life diagnostic testing was not concordant with IDSA/ATS or ERS guidelines in the vast majority of the patients. Specifically, the discordance with the IDSA/ATS guidelines was mainly due to over-testing. This situation may be explained by the restrictive testing recommended by the IDSA/ATS guidelines. Of note, under-testing was also a cause of discordance with the IDSA/ATS guidelines and was more frequently encountered in North America than in Europe. Discordance with ERS guidelines was mainly due to under-testing, as a result of the extensive testing approach recommended by these guidelines. Over-testing was also identified as a cause of discordance with the ERS guidelines. This event was more frequently reported in Europe than in North America. We were intrigued by European clinicians’ extensive ordering of diagnostic tests, even beyond what is recommended by the ERS guidelines. Insurance and healthcare system-related factors may have shaped the diagnostic approach both of European and North American clinicians. Nonetheless, the significant discrepancies between real-life diagnostic testing and IDSA/ATS/ERS recommended testing is worrisome and further studies aimed at assessing the clinical and economic implications of the testing approach proposed by the IDSA/ATS guidelines, the testing approach proposed by the ERS guidelines, and real-life diagnostic testing are needed.
Finally, this study has important strengths and limitations. To our knowledge, GLIMP is the first study to enrol a large and diverse group of adult patients hospitalised with CAP across six continents, providing a detailed, real-world picture of CAP diagnostic testing around the world. Our ability to assess the concordance of real-life diagnostic testing with IDSA/ATS and ERS guideline recommendations was affected by the lack of information about recent travel, failure of outpatient antibiotic therapy, presence of cavitary infiltrates, lymphopenia, and feasibility of sputum collection in the GLIMP database. Similarly, incomplete data regarding presence of pleural effusions and clinical and epidemiological determinants of Legionella infection limited the accuracy our findings, probably leading to an inflation of our over-testing estimates. Finally, due to its cross-sectional design, our study did not provide CAP outcome data.
In conclusion, our understanding of the aetiologies of CAP among hospitalised adults is scarce, limiting the accuracy of empirical antimicrobial regimens. Disease severity and geography are associated with differences in testing approaches. The wide discordance between IDSA/ATS/ERS recommendations and real-life testing strategies should prompt future studies to evaluate the clinical and economic implications of different testing approaches and investigate the reasons for these differences.
Footnotes
GLIMP investigators: We would like to thank the following study contributors for their valuable collaboration. Argentina: Patricia Karina Aruj, Dept of Internal Medicine, University Hospital Alfredo Lanari, Buenos Aires, Argentina; Silvia Attorri, Hospital Luis Lago Maggiore, Mendoza, Argentina; Enrique Barimboim, Hospital Central de Mendoza, Argentina; Juan Pablo Caeiro and María I. Garzón, Hospital Privado Universitario, Córdoba, Argentina; Victor Hugo Cambursano, V.H. Dr Cazaux A. Servicio de Neumologia, Hospital Rawson, Córdoba, Argentina; Adrian Ceccato, Hospital Nacional Prof Alejandro Posadas, Argentina; Julio Chertcoff, Florencia Lascar and Fernando Di Tulio, Critical Care Unit and Respiratory Medicine, Buenos Aires British Hospital, Buenos Aires, Argentina; Ariel Cordon Díaz, Hospital General Alvear, Ciudad, Mendoza, Argentina; Lautaro de Vedia, Respiratory Intensive Care Unit, Hospital Muñiz, Buenos Aires, Argentina; Maria Cristina Ganaha, Infectious Diseases Ward, Hospital Interzonal General de Agudos “Vicente Lopez y Planes” from General Rodriguez, Buenos Aires, Argentina; Sandra Lambert, Hospital El Cruce – Alta Complejidad en Red, Argentina; Gustavo Lopardo, Hospital Bernardo Houssay, Vicente López, Argentina; Carlos M. Luna, Pulmonary Medicine Division, Dept of Medicine, Hospital de Clínicas, Universidad de Buenos Aires, Argentina; Alessio Gerardo Malberti, Hospital Nuestra Señora del Carmen, Argentina; Nora Morcillo and Silvina Tartara, Hospital Zonal Especializado de Agudos y Crónicos Dr Antonio A. Cetrangolo, Argentina; Claudia Pensotti, Infectious Diseases and Infection Control Dept, Buenos Aires, Clinica Privada Monte Grande, Argentina; Betiana Pereyra, Hospital San Roque, Córdoba, Argentina; Pablo Gustavo Scapellato, Infectious Diseases Dept, Hospital D.F. Santojanni, Argentina; Juan Pablo Stagnaro, HZGA Mi Pueblo, Florencio Varela, Argentina. Australia: Sonali Shah, Dept of General Medicine, Austin hospital, Heidelberg, Australia. Austria: Felix Lötsch and Florian Thalhammer, Division of Infectious Diseases and Tropical Medicine, Dept of Medicine I, Medical University of Vienna, Austria. Belgium: Kurt Anseeuw, ZNA Campus Stuivenberg, Antwerp, Belgium; Camille A. Francois, Anesthesia and Critical Care Dept, Erasme University Hospital, Brussels, Belgium; Eva Van Braeckel, Dept of Respiratory Medicine, Ghent University Hospital, Belgium; Jean Louis Vincent, Dept of Intensive Care, Erasme University Hospital, Université Libre de Bruxelles, Brussels, Belgium. Benin: Marcel Zannou Djimon, Jules Bashi and Roger Dodo, Centre Hospitalier Universitaire HKM of Cotonou, Benin. Brazil: Simone Aranha Nouér, Federal University of Rio de Janeiro, Brazil. Bulgaria: Peter Chipev and Milena Encheva, Clinic of Pulmonary Diseases, Military Medical Academy, Sofia, Bulgaria; Darina Miteva, UMHAT “St Marina”, Varna, Bulgaria; Diana Petkova, University Hospital Varna, Bulgaria. Cameroon: Adamou Dodo Balkissou, Yaounde Jamot Hospital, Yaounde, Cameroon; Eric Walter Pefura Yone, Département de Médecine Interne, University of Yaounde, Yaoundé, Cameroon; Bertrand Hugo Mbatchou Ngahane, Douala General Hospital, Douala, Cameroon. China: Ning Shen, Respiratory Medicine, Peking University Third Hospital, Beijing, China; Jin-fu Xu, Dept of Respiratory Medicine, Shanghai Pulmonary Hospital, Tongji University, China. Colombia: Carlos Andres Bustamante Rico and Ricardo Buitrago, Clinica Shaio, Bogota, Colombia; Fernando Jose Pereira Paternina, Las Americas Clinic, Medellin, Colombia. Congo: Jean-Marie Kayembe Ntumba, Cliniques Universitaires de Kinshasa, DR Congo. Croatia: Vesna Vladic Carevic, Interne Medicine, Dubrovnik, Croatia; Marko Jakopovic, Medical School, University of Zagreb, Dept for Respiratory Diseases Jordanovac, University Hospital Centre Zagreb, Zagreb, Croatia; Mateja Jankovic, University Hospital Center Zagreb, Dept for Respiratory Diseases, Zagreb, Croatia; Zinka Matkovic, University Hospital Dubrava, Zagreb, Croatia; Ivan Mitrecic, Karlovac general hospital, Karlovac, Croatia. Denmark: Marie-Laure Bouchy Jacobsson, Emergency Dept, Nordsjællands Hospital, Hillerød, Denmark; Anette Bro Christensen, Dept of Anaethesiology, Viborg Region Hospital, Denmark; Uffe Christian Heitmann Bødtger, Dept of Pulmonology, Naestved Hospital, Denmark; Christian Niels Meyer, Dept of Internal Medicine, Roskilde Hospital, Copenhagen University Hospital, Roskilde, Denmark; Andreas Vestergaard Jensen, Gertrud Baunbæk-Knudsen, Pelle Trier Petersen and Stine Andersen, Dept of Lung and Infectious Diseases, Nordsjællands Hospital, Hillerød, Denmark. Egypt: Ibrahim El-Said Abd El-Wahhab, Thoracic Medicine, Faculty of Medicine, Mansoura University, Egypt; Nesreen Elsayed Morsy, Pulmonary, Critical Care and Sleep Medicine, Faculty of Medicine, Mansoura University, Mansoura, Egypt; Hanaa Shafiek, Chest Diseases Dept, Faculty of Medicine, Alexandria University, Egypt; Eman Sobh, Chest Diseases Dept, Al-Azhar University, Cairo, Egypt. Ethiopia: Kedir Abdella Abdulsemed, Dept of Medical Laboratory Science and Pathology, College of Health Sciences, Mycobacteriology Research Centre, Institute of Biotechnology Research, Jimma University, Jimma, Ethiopia. France: Fabrice Bertrand, Critical Care Unit, Robert Ballanger Hospital, Aulnay sous Bois, France; Christian Brun-Buisson, Univ Hospital Henri Mondor, 94000 Créteil, France; Etienne de Montmollin, Intensive Care Unit, Hôpital Delafontaine, Centre hospitalier de Saint-Denis, Saint-Denis, France; Muriel Fartoukh, Unité de réanimation médico-chirurgicale, Pôle Thorax Voies aériennes, Hôpital Tenon, Groupe Hospitalier Est Parisien, France; Jonathan Messika, Publique-Hôpital de Paris, Service de Réanimation Médico-chirurgicale, Hôpital Louis Mourier, Colombes, France, and Université Paris Diderot, IAME, UMR 1137, Sorbonne Paris Cité, Paris, France; Pierre Tattevin, Infectious Diseases and ICU, Pontchaillou University Hospital, Rennes, France; Abdo Khoury, Dept of Emergency Medicine and Critical Care, University of Franche – Comté, Medical Center, France. Gambia: Bernard Ebruke, Medical Research Council Unit, Gambia. Germany: Michael Dreher, Dept of Cardiology, Pneumology, Vascular Medicine and Intensive Care Medicine, University Hospital Aachen, Aachen, Germany; Martin Kolditz, Division of Pulmonology, Medical Dept I, University Hospital Carl Gustav Carus, Technische Universität Dresden, Germany; Matthias Meisinger, Klinikum Niederlausitz GmbH, Klinik für Innere Medizin und Intensivmedizin, Senftenberg, Germany; Mathias W. Pletz and Stefan Hagel, Center for Infectious Diseases and Infection Control, Jena University Hospital, Germany; Jan Rupp, Dept of Molecular and Infectious Diseases, University of Lübeck, Lübeck, Germany; Tom Schaberg, Zentrum für Pneumologie, Agaplesion Diakonieklinikum Rotenburg, Germany; Marc Spielmanns, Internal Medicine Dept, Pulmonary Rehabilitation and Dept of Health, School of Medicine, University Witten-Herdecke, St Remigius-Hospital, Leverkusen, Germany; Petra Creutz and Norton Suttorp, Dept of Infectious Disease and Respiratory Medicine, Charité – University Medicine, Berlin, Germany. Ghana: Beatrice Siaw-Lartey, Komfo-Anokye Teaching Hospital, Kumasi, Ghana. Greece: Katerina Dimakou, 5th Respiratory Medicine Dept, “SOTIRIA” Chest Hospital, Athens, Greece; Dimosthenis Papapetrou, Medical Group of Athens (Paleo Faliro Clinic), Athens, Greece; Evdoxia Tsigou and Dimitrios Ampazis, Agioi Anargiroi Hospital, Kifissia, Athens, Greece; Evangelos Kaimakamis, Intensive Care Unit, “G. Papanikolaou” General Hospital of Thessaloniki, Greece; Mina Gaga, 7th Respiratory Medicine Dept and Asthma Center, Athens Chest Hospital, Greece. India: Mohit Bhatia, S.S. Hospital IMS BHU Varanasi, India; Raja Dhar, Fortis Hospitals, Kolkata, India; George D'Souza, Dept of Pulmonary Medicine, St John's Medical College Hospital, Bangalore, India; Rajiv Garg, Dept of Respiratory Medicine, King George's Medical University UP, Lucknow, India; Parvaiz A. Koul, Dept of Internal and Pulmonary Medicine, SheriKashmir Institute of Medical Sciences, Srinagar, India; P.A. Mahesh and B.S. Jayaraj, Dept of Pulmonary Medicine, JSS Medical College, JSS University, Mysore, India; Kiran Vishnu Narayan, Pulmonary Medicine, Government Medical College Kozhikode, Kerala, India; Hirennappa B. Udnur and Shashi Bhaskara Krishnamurthy, Columbia Asia Hospital, Hebbal, Bengaluru, Karnataka, India; Surya Kant, Dept of Respiratory Medicine, King George's Medical University, Chowk, Lucknow, Uttar Pradesh, India; Rajesh Swarnakar, Getwell Hospital and Research Institute, Dhantoli, Nagpur, India; Sneha Limaye and Sundeep Salvi, on behalf of the Respiratory Research Network of India (RRNI) from the Chest Research Foundation in Pune, India. Iran: Keihan Golshani, Isfahan University of Medical Sciences; Iran. Ireland: Vera M. Keatings, Letterkenny General Hospital, Co Donegal, Ireland; Ignacio Martin-Loeches, Multidisciplinary Intensive Care Research Organization (MICRO), St James's University Hospital, Trinity Centre for Health Sciences Dublin, Ireland. Israel: Yasmin Maor, Infectious Disease Unit, Affiliated to Tel Aviv University, Wolfson Medical Center, Holon, Israel; Jacob Strahilevitz, Dept of Clinical Microbiology and Infectious Diseases, Hadassah-Hebrew University, Jerusalem, Israel. Italy: Salvatore Battaglia, University of Palermo, Pneumologia DiBiMIS, Palermo, Italy; Maria Carrabba, Internal Medicine Dept, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy; Piero Ceriana, Pulmonary Rehabilitation, IRCCS Fondazione Maugeri, Pavia, Italy; Marco Confalonieri, Dept of Pulmunology, University Hospital, Trieste, Italy; Antonella d'Arminio Monforte, Dept of Health Sciences, Clinic of Infectious Disease, San Paolo Hospital, University of Milan, Italy; Bruno Del Prato, Interventional Pneumology, Hospital Antonio Cardarelli, Naples, Italy; Marino De Rosa, UOC Pneumologia P.O. San Filippo Neri ASL RM E Roma, Italy; Riccardo Fantini, Respiratory Diseases clinic, Policlinico di Modena, Modena, Italy; Giuseppe Fiorentino, UOC Fisiopatologia e Riabilitazione Respiratoria AO Ospedali dei Colli PO Monaldi, Italy; Maria Antonia Gammino, Pulmonary Medicine Unit, San Martino Hospital, ASL 5 Oristano, Sardegna, Italy; Francesco Menzella, Dept of Cardiac-Thoracic-Vascular and Intensive Care Medicine, Pneumology Unit, IRCCS- Arcispedale Santa Maria Nuova, Reggio Emilia, Italy; Giuseppe Milani, Azienda Ospedaliera Sant Anna di Como, Presidio Ospedale S. Anna Nuovo, Unità Operativa di Pneumologia, Como, Italy; Stefano Nava, Alma Mater University of Bologna, DIMES, Respiratory and Critical Care Unit Sant'Orsola Malpighi Hospital, Italy; Gerardo Palmiero, Respiratory Unit, Versilia Hospital, Azienda USL 12 Viareggio, Lido di Camaiore, Lucca, Italy; Roberta Petrino and Barbra Gabrielli, Emergency Medicine Unit, S. Andrea Hospital, Vercelli, Italy; Paolo Rossi, Internal Medicine Dept, Azienda Ospedaliero-Universitaria S. Maria della Misericordia, Udine, Italy; Claudio Sorino, Pulmonology Unit, A.O. Sant Anna di Como, Italy; Gundi Steinhilber, Spedali Civili Brescia, U.O. Pneumologia e Fisiopatologia Respiratoria, Brescia, Italy; Alessandro Zanforlin, ULSS 18 Rovigo, Ospedale San Luca, Trecenta, Italy; Fabio Franzetti, Manuela Carugati, Manuela Morosi and Elisa Monge, Dept of Biomedical and Clinical Sciences, Division of Infectious Diseases, Luigi Sacco Hospital, Università degli Studi di Milano, Milan, Italy; Mauro Carone, Fondazione Salvatore Maugeri, IRCCS, Cassano Murge, Italy; Vincenzo Patella, Allergology and Clinical Immunology Unit, Dept of Medical Sciences, Battipaglia Hospital, Battipaglia, Salerno, Italy; Simone Scarlata, Geriatrics, Unit of Respiratory Pathophysiology and Thoracic Endoscopy, Campus Bio Medico University and Teaching Hospital, Rome, Italy; Andrea Comel, UO Pneumologia, Ospedale Pederzoli, Peschiera del Garda, Italy. Japan: Kiyoyasu Kurahashi, Yokohama City University Medical Center, Japan. Lebanon: Zeina Aoun Bacha, Medicine School, St Joseph University, Beyrouth, Lebanon. Mexico: Daniel Barajas Ugalde, National Institute of Respiratory Diseases, Mexico; Omar Ceballos Zuñiga, Hospital General de Mexicali, Mexicali, Baja California, Mexico; José F. Villegas, Hospital Universitario, Monterrey, Mexico. Montenegro: Milic Medenica, Hospital for Lung Diseases – Brezovik, Niksic, Montenegro. The Netherlands: E.M.W. van de Garde, Dept Clinical Pharmacy, St Antonius Hospital, Utrecht/Nieuwegein, The Netherlands. Nepal: Deebya Raj Mihsra, Internal Medicine, BP Koirala Institute of Health Sciences, Nepal; Poojan Shrestha, Oxford University Clinical Research Unit, Patan Hospital, Nepal. New Zealand: Elliott Ridgeon, Medical Research Institute of New Zealand. Nigeria: Babatunde Ishola Awokola, Dept of Family Medicine and Primary Care, Lily Hospitals Limited, Warri, Nigeria; Ogonna N.O. Nwankwo, University of Calabar Teaching Hospital, Calabar, Nigeria; Adefuye Bolanle Olufunlola, Olabisi Onabanjo University teaching hospital, Sagamu, Ogun State, Nigeria; Segaolu Olumide, Dept of Medicine (Pulmonary Unit), University College Hospital, Ibadan, Nigeria; Kingsley N. Ukwaja, Dept of Medicine, Federal Teaching Hospital Abakaliki, Ebonyi State, Nigeria. Pakistan: Muhammad Irfan, Section of Pulmonary and Critical Care Medicine, Dept of Medicine, Aga Khan University, Karachi, Pakistan. Poland: Lukasz Minarowski, Dept of Lung Diseases and Tuberculosis, Medical University of Bialystok, Poland; Skoczyński Szymon, Dept of Pneumology, School of Medicine in Katowice, Medical University of Silesia, Katowice, Institute of Occupational Medicine and Environmental Health, Sosnowiec, Poland. Portugal: Felipe Froes, Hospital Pulido Valente – CHLN, Lisbon, Portugal; Pedro Leuschner, Centro Hospitalar do Porto, Porto, Portugal; Mariana Meireles, Cláudia Ferrão, Pedro Leuschner and João Neves, Serviço de Medicina, Centro Hospitalar do Porto, Largo Prof Abel Salazar, Porto, Portugal; Sofia B Ravara, Faculty of Health Sciences, University of Beira Interior; Cova da Beira Hospital Center, Covilhã, Portugal. Republic of Moldova: Victoria Brocovschii, Dept of Pneumology and Allergology, State University of Medicine and Pharmacy “Nicolae Testemitanu” Republic of Moldova; Chesov Ion, Clinic of Anesthesia and Intensive Care “Valeriu Ghrerg”, Institute of Emergency Medicine, State University of Medicine and Pharmacy “Nicolae Testemitanu”, Chisinau, Republic of Moldova; Doina Rusu, SMFU "N. Testemitanu", Chisinau, Republic of Moldova; Cristina Toma, Dept of Pneumology and Allergology, State University of Medicine and Pharmacy "Nicolae Testemitanu”, Chisinau, Republic of Moldova. Romania: Daniela Chirita, Hospital Sfantul Stefan, Bucharest, Romania; Carmen Mihaela Dorobat, Universitatea de Medicină şi Farmacie “Gr. T. Popa” Iaşi Facultatea de Medicină Stomatologică, Spitalul Clinic de Boli Infecţioase “Sfânta Parascheva”, Iaşi, Romania. Russia: Alexei Birkun, Dept of Anesthesiology, Critical Care and Emergency Medicine, Medical Academy named after S.I. Georgievsky, Russian Federation; Anna Kaluzhenina, Volgograd State Medical University, Russia. Saudi Arabia: Abdullah Almotairi, King Fahad medical City (KFMC), Riyadh, KSA; Zakeya Abdulbaqi Ali Bukhary, College of Medicine, Taibah University, Medina, KSA; Jameela Edathodu, Al Faisal University, King Faisal Specialist Hospital, Riyadh, KSA; Amal Fathy, Pulmonary and respiratory critical care Medicine, Mansoura University Egypt, Affiliate at Taibah University, KSA; Abdullah Mushira Abdulaziz Enani and Nazik Eltayeb Mohamed, Infectious Diseases Section, Medical Specialties Dept, King Fahad Medical City, Riyadh, KSA; Jawed Ulhadi Memon, Pulmonology Division, Dept of Internal Medicine, King Fahad Hospital, Hofuf, Al Ahasa, KSA; Abdelhaleem Bella, Dammam University-Saudi Arabia and King Fahad Hospital, KSA. Serbia: Nada Bogdanović, Pulmonary Dept of KHC Dr Dragiša Mišović, Belgrade, Serbia; Branislava Milenkovic, Clinic for Pulmonary Diseases, Clinical Centre of Serbia, Faculty of Medicine, University of Belgrade, Belgrade, Serbia; Dragica Pesut, University of Belgrade School of Medicine, Teaching Hospital of Pulmonology, Clinical Centre of Serbia, Belgrade, Serbia. South Africa: Charles Feldman, Division of Pulmonology, Dept of Internal Medicine, Charlotte Maxeke Johannesburg Academic Hospital, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa. South Korea: Ho Kee Yum, Inje Univ Seoul Paik Hospital, South Korea. Spain: Luis Borderìas, Respiratory and Sleep Unit, Hospital San Jorge, Huesca, Spain; Noel Manuel Bordon Garcia, Barcelona Policlínic and Moises Broggi Hospital at Sant Joan Despí, Spain; Hugo Cabello Alarcón, Sant Hospital Seu de Urgell, Catalonia, Spain; Catia Cilloniz and Antoni Torres, Dept of Pneumology, Institut Clinic del Tórax, Hospital Clinic of Barcelona, Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), University of Barcelona, Spain; Vicens Diaz-Brito and Xavier Casas, Infectious Diseases Unit and Pneumology Service, Parc Sanitari Sant Joan de Deu, Sant Boi, Barcelona, Spain; Alicia Encabo González, Hospital Complex of Pontevedra, Spain; Maria Luisa Fernández-Almira, Medicina Interna, Hospital Universitario Central de Asturias, Spain; Miguel Gallego, Dept of Respiratory Medicine, Hospital de Sabadell, Institut Universitari Parc Taulí-UAB, Sabadell, CIBER de Enfermedades Respiratorias, CIBERES, Bunyola, Spain; Inmaculada Gaspar-GarcÍa, Dept of Respiratory Medicine, Hospital Costa del Sol, Marbella, Málaga, Spain; Juan González del Castillo, Emergency Dept, Hospital Universitario Clínico San Carlos, Madrid, Spain; Patricia Javaloyes Victoria, Hospital General Universitario de Alicante, Alicante, Spain; Elena Laserna Martínez, Hospital Mollet, Barcelona, Spain; Rosa Malo de Molina, University Hospital Puerta de Hierro Majadahonda, Madrid; Pedro J. Marcos, Servicio de Neumología, Complejo Hospitalario Universitario de A Coruña (CHUAC), INIBIC, Sergas, Universidade de A Coruña (UDC), Spain; Rosario Menéndez, Pneumology Service, University and Polytechnic Hospital La Fe, Valencia, Spain; Ana Pando-Sandoval, Hospital Universitario Central de Asturias, Area de Gestion Clinica de Pulmon, Servicio de Neumologia, Oviedo, Spain; Cristina Prat Aymerich, Alicia Lacoma de la Torre and Ignasi García-Olivé, Microbiology Dept and Pneumology Dept, Hospital Universitari Germans Trias i Pujol, Institut d'Investigació Germans Trias i Pujol, Badalona, Universitat Autònoma de Barcelona, CIBER Enfermedades Respiratorias (CIBERES), Instituto de Salud Carlos III, Spain; Jordi Rello and Silvia Moyano, Critical Care Dept, Hospital Vall d'Hebron, Barcelona, Spain; Francisco Sanz, Servicio de Neumología, Consorci Hospital General Universitari de Valencia, Valencia, Spain; Oriol Sibila and Ana Rodrigo-Troyano, Servei de Pneumologia, Hospital de la Santa Creu i Sant Pau, IIB-Sant Pau, Barcelona, Spain; Jordi Solé-Violán, Hospital Universitario de Gran Canaria Dr Negrín, Las Palmas de Gran Canaria, Spain; Ane Uranga, Pulmology Dept, Hospital of Galdakao-Usansolo, Spain; Job F.M. van Boven, Hospital Universitari Son Espases, Palma de Mallorca, Spain; Ester Vendrell Torra and Jordi Almirall Pujol, Intensive Care Medicine, Hospital de Mataró, Spain. Togo: Arnauld Attannon Fiogbe, Pulmonology and Infectious Diseases Service/University Hospital of Sylvanus Olympio, Lomé, Togo. Tunisia: Ferdaous Yangui, Dept of Pneumology, Hospital of Internal Forces Security (IFS), Marsa, Tunis, Tunisia. Turkey: Semra Bilaceroglu, Izmir Dr Suat Seren Training and Research Hospital for Thoracic Medicine and Surgery, Izmir, Turkey; Levent Dalar, Pulmonary Medicine, Istanbul Bilim University, Istanbul, Turkey; Ufuk Yilmaz, Suat Seren Chest Disease and Surgery Training and Research Hospital, İzmir, Turkey. Ukraine: Artemii Bogomolov, Vinnitsa National Pirogov Memorial Medical University, Vinnitsa Regional Antituberculosis Hospital, Vinnitsa, Ukraine. United Arab Emirates: Naheed Elahi, Dubai Hospital, UAE. UK: Devesh J. Dhasmana, Victoria Hospital, Kirkcaldy, NHS Fife, UK; Andrew Feneley, Rhiannon Ions, Julie Skeemer and Gerrit Woltmann, University Hospitals of Leicester NHS Trust and University of Leicester, Leicester, UK; Carole Hancock, Royal Respiratory Research Team, Royal Liverpool University Hospital, Liverpool, UK; Adam T. Hill, Royal Infirmary and University of Edinburgh, UK; Banu Rudran, The Royal London Hospital, Barts Health Trust, London, UK; Silvia Ruiz-Buitrago and Marion Campbell, Hairmyres Hospital, Eaglesham Road, East Kilbride, UK; Paul Whitaker, Dept of Respiratory Medicine, St James's Hospital, Leeds, UK; Alexander Youzguin, Southport and Ormskirk Hospitals NHS Trust, UK; Anika Singanayagam, Imperial College Healthcare NHS Trust, London, UK. USA: Karen S. Allen, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA; Veronica Brito, Texas A&M Health Science Center, Division of Pulmonary, Critical Care and Sleep Medicine, Baylor Scott & White Health, TX, USA; Jessica Dietz, Fargo VA Health Care System, Fargo, ND, USA; Claire E. Dysart and Susan M. Kellie, Clement J. Zablocki VA Medical Center, Milwaukee, WI, USA, Division of Infectious Diseases, University of New Mexico School of Medicine, Raymond G. Murphy VA Medical Center, Albuquerque, NM, USA; Ricardo A. Franco-Sadud and Garnet Meier, Division of Hospital Medicine, Cook County Hospital, Chicago, IL, USA; Thomas L. Holland and Stephen P. Bergin, Dept of Medicine, Duke University Medical Center and School of Medicine, Duke Clinical Research Institute, Durham, NC, USA; Fayez Kheir, Dept of Pulmonary Diseases, Critical Care and Environmental Medicine, Tulane University Health Sciences Center, New Orleans, LA, USA; Mark Landmeier, Division of Pulmonary and Critical Care Medicine, Northwestern Memorial Hospital, Chicago, IL, USA; Manuel Lois, John Peter Smith Hospital, Fort Worth, TX, USA; Girish B. Nair, Interstitial Lung Disease Program and Pulmonary Rehabilitation, SUNY Stony Brook Winthrop University Hospital, Mineola, NY, USA; Hemali Patel, Dept of Medicine, Division of General Internal Medicine, Hospital Medicine Group, University of Colorado, CO, USA; Katherine Reyes, Henry Ford Hospital, Detroit, IL, USA; William Rodriguez-Cintron, Pulmonary/Critical Care Medicine, VA Caribbean Healthcare System, USA; Shigeki Saito, Tulane University, New Orleans, LA, USA; Nilam J. Soni, Julio Noda, Cecilia I. Hinojosa, Stephanie M. Levine, Luis F. Angel and Antonio Anzueto, Divisions of Hospital Medicine and Pulmonary/Critical Care Medicine, South Texas Veterans Health Care System, University of Texas Health Science Center San Antonio, San Antonio, TX, USA; K. Scott Whitlow, John Hipskind, Kunal Sukhija and Vicken Totten, Kaweah Delta Health Care District, Dept of Emergency Medicine, Visalia, CA, USA; Richard G. Wunderink and Ray D. Shah, Northwestern University Feinberg School of Medicine, Chicago, IL, USA. Zambia: Kondwelani John Mateyo, Dept of Internal Medicine, University Teaching Hospital, Lusaka, Zambia. Other investigators: Lorena Noriega, Ezequiel Alvarado, Mohamed Aman and Lucía Labra.
Conflict of interest: M. Carugati has nothing to disclose.
Conflict of interest: S. Aliberti reports grants and personal fees from Bayer Healthcare, Aradigm Corporation, Grifols, Chiesi and Insmed, and personal fees from AstraZeneca, Basilea, Zambon, Novartis, Raptor, Actavis UK Ltd and Horizon, all outside the submitted work.
Conflict of interest: L.F. Reyes has nothing to disclose.
Conflict of interest: R. Franco Sadud has nothing to disclose.
Conflict of interest: M. Irfan has nothing to disclose.
Conflict of interest: C. Prat has nothing to disclose.
Conflict of interest: N.J. Soni has nothing to disclose.
Conflict of interest: P. Faverio has nothing to disclose.
Conflict of interest: A. Gori reports receipt of grants/research support from Abbvie, Astellas, BMS, Boehringer, Gilead, Janssen, MSD, Novartis, Pfizer, Roche, ViiV and ANLAIDS Sezione Lombarda, receipt of honoraria or consultation fees from BMS, Gilead, Janssen, MSD, Novartis and ViiV, participation in a company sponsored speakers’ bureau from Gilead, and travel grants/support from BMS, Gilead, Janssen and ViiV.
Conflict of interest: F. Blasi reports personal fees from AstraZeneca, Guidotti Malesci, GSK and Novartis, and grants and personal fees from Bayer and Chiesi, Grifols, Insmed, Menarini, Pfizer, Teva and Zambon, all outside the submitted work.
Conflict of interest: M.I. Restrepo has nothing to disclose.
Support statement: This project was not funded and relied solely on voluntary site and investigator participation.
Contributor Information
Collaborators: GLIMP investigators, Patricia Karina Aruj, Silvia Attorri, Enrique Barimboim, Juan Pablo Caeiro, María I. Garzón, Victor Hugo Cambursano, Adrian Ceccato, Julio Chertcoff, Florencia Lascar, Fernando Di Tulio, Ariel Cordon Díaz, Lautaro de Vedia, Maria Cristina Ganaha, Sandra Lambert, Gustavo Lopardo, Carlos M. Luna, Alessio Gerardo Malberti, Nora Morcillo, Silvina Tartara, Claudia Pensotti, Betiana Pereyra, Pablo Gustavo Scapellato, Juan Pablo Stagnaro, Florencio Varela, Sonali Shah, Felix Lötsch, Florian Thalhammer, Kurt Anseeuw, Camille A. Francois, Eva Van Braeckel, Jean Louis Vincent, Marcel Zannou Djimon, Jules Bashi, Roger Dodo, Simone Aranha Nouér, Peter Chipev, Milena Encheva, Darina Miteva, Diana Petkova, Adamou Dodo Balkissou, Eric Walter Pefura Yone, Bertrand Hugo Mbatchou Ngahane, Ning Shen, Jin-fu Xu, Carlos Andres Bustamante Rico, Ricardo Buitrago, Fernando Jose Pereira Paternina, Jean-Marie Kayembe Ntumba, Vesna Vladic Carevic, Marko Jakopovic, Mateja Jankovic, Zinka Matkovic, Ivan Mitrecic, Marie-Laure Bouchy Jacobsson, Anette Bro Christensen, Uffe Christian Heitmann Bødtger, Christian Niels Meyer, Andreas Vestergaard Jensen, Gertrud Baunbæk-Knudsen, Pelle Trier Petersen, Stine Andersen, Ibrahim El-Said Abd El-Wahhab, Nesreen Elsayed Morsy, Hanaa Shafiek, Eman Sobh, Kedir Abdella Abdulsemed, Fabrice Bertrand, Christian Brun-Buisson, Etienne de Montmollin, Muriel Fartoukh, Jonathan Messika, Pierre Tattevin, Abdo Khoury, Bernard Ebruke, Michael Dreher, Martin Kolditz, Matthias Meisinger, Mathias W. Pletz, Stefan Hagel, Jan Rupp, Tom Schaberg, Marc Spielmanns, Petra Creutz, Norton Suttorp, Beatrice Siaw-Lartey, Katerina Dimakou, Dimosthenis Papapetrou, Evdoxia Tsigou, Dimitrios Ampazis, Evangelos Kaimakamis, Mina Gaga, Mohit Bhatia, Raja Dhar, George D'Souza, Rajiv Garg, Parvaiz A. Koul, P.A. Mahesh, B.S. Jayaraj, Kiran Vishnu Narayan, Hirennappa B. Udnur, Shashi Bhaskara Krishnamurthy, Surya Kant, Rajesh Swarnakar, Sneha Limaye, Sundeep Salvi, Keihan Golshani, Vera M. Keatings, Ignacio Martin-Loeches, Yasmin Maor, Jacob Strahilevitz, Salvatore Battaglia, Maria Carrabba, Piero Ceriana, Marco Confalonieri, Antonella d'Arminio Monforte, Bruno Del Prato, Marino De Rosa, Riccardo Fantini, Giuseppe Fiorentino, Maria Antonia Gammino, Francesco Menzella, Giuseppe Milani, Stefano Nava, Gerardo Palmiero, Roberta Petrino, Barbra Gabrielli, Paolo Rossi, Claudio Sorino, Gundi Steinhilber, Alessandro Zanforlin, Fabio Franzetti, Manuela Carugati, Manuela Morosi, Elisa Monge, Mauro Carone, Vincenzo Patella, Simone Scarlata, Andrea Comel, Kiyoyasu Kurahashi, Zeina Aoun Bacha, Daniel Barajas Ugalde, Omar Ceballos Zuñiga, José F. Villegas, Milic Medenica, E.M.W. van de Garde, Deebya Raj Mihsra, Poojan Shrestha, Elliott Ridgeon, Babatunde Ishola Awokola, Ogonna N.O. Nwankwo, Adefuye Bolanle Olufunlola, Segaolu Olumide, Kingsley N. Ukwaja, Muhammad Irfan, Lukasz Minarowski, Skoczyński Szymon, Felipe Froes, Pedro Leuschner, Mariana Meireles, Sofia B Ravara, Victoria Brocovschii, Chesov Ion, Doina Rusu, Cristina Toma, Daniela Chirita, Carmen Mihaela Dorobat, Alexei Birkun, Anna Kaluzhenina, Abdullah Almotairi, Zakeya Abdulbaqi Ali Bukhary, Jameela Edathodu, Amal Fathy, Abdullah Mushira Abdulaziz Enani, Nazik Eltayeb Mohamed, Jawed Ulhadi Memon, Abdelhaleem Bella, Nada Bogdanović, Branislava Milenkovic, Dragica Pesut, Charles Feldman, Ho Kee Yum, Luis Borderìas, Noel Manuel Bordon Garcia, Hugo Cabello Alarcón, Catia Cilloniz, Antoni Torres, Vicens Diaz-Brito, Xavier Casas, Alicia Encabo González, Maria Luisa Fernández-Almira, Miguel Gallego, Inmaculada Gaspar-GarcÍa, Juan González del Castillo, Patricia Javaloyes Victoria, Elena Laserna Martínez, Rosa Malo de Molina, Pedro J. Marcos, Rosario Menéndez, Ana Pando-Sandoval, Cristina Prat Aymerich, Jordi Rello, Silvia Moyano, Francisco Sanz, Oriol Sibila, Ana Rodrigo-Troyano, Jordi Solé-Violán, Ane Uranga, Job F.M. van Boven, Ester Vendrell Torra, Jordi Almirall Pujol, Arnauld Attannon Fiogbe, Ferdaous Yangui, Semra Bilaceroglu, Levent Dalar, Ufuk Yilmaz, Artemii Bogomolov, Naheed Elahi, Devesh J. Dhasmana, Andrew Feneley, Rhiannon Ions, Julie Skeemer, Gerrit Woltmann, Carole Hancock, Adam T. Hill, Banu Rudran, Silvia Ruiz-Buitrago, Marion Campbell, Paul Whitaker, Alexander Youzguin, Anika Singanayagam, Karen S. Allen, Veronica Brito, Jessica Dietz, Claire E. Dysart, Susan M. Kellie, Ricardo A. Franco-Sadud, Garnet Meier, Thomas L. Holland, Stephen P. Bergin, Fayez Kheir, Mark Landmeier, Manuel Lois, Girish B. Nair, Hemali Patel, Katherine Reyes, William Rodriguez-Cintron, Shigeki Saito, Nilam J. Soni, Julio Noda, Cecilia I. Hinojosa, Stephanie M. Levine, Luis F. Angel, Antonio Anzueto, K. Scott Whitlow, John Hipskind, Kunal Sukhija, Vicken Totten, Richard G. Wunderink, Ray D. Shah, Kondwelani John Mateyo, Lorena Noriega, Ezequiel Alvarado, Mohamed Aman, and Lucía Labra
References
- 1.Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373: 415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997; 336: 243–250. [DOI] [PubMed] [Google Scholar]
- 3.Johnstone J, Eurich DT, Majumdar SR, et al. Long-term morbidity and mortality after hospitalization with community-acquired pneumonia: a population-based cohort study. Medicine 2008; 87: 329–334. [DOI] [PubMed] [Google Scholar]
- 4.Metersky ML, Waterer G, Nsa W, et al. Predictors of in-hospital vs postdischarge mortality in pneumonia. Chest 2012; 142: 476–481. [DOI] [PubMed] [Google Scholar]
- 5.Musher DM, Roig IL, Cazares G, et al. Can an etiologic agent be identified in adults who are hospitalized for community-acquired pneumonia: results of a one-year study. J Infect 2013; 67: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jokinen C, Heiskanen L, Juvonen H, et al. Microbial etiology of community-acquired pneumonia in the adult population of 4 municipalities in eastern Finland. Clin Infect Dis 2001; 32: 1141–1154. [DOI] [PubMed] [Google Scholar]
- 7.Niederman MS, Luna CM. Community-acquired pneumonia guidelines: a global perspective. Semin Respir Crit Care Med 2012; 33: 298–310. [DOI] [PubMed] [Google Scholar]
- 8.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44: Suppl. 2, S27–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections – summary. Clin Microbiol Infect 2011; 17: Suppl. 6, 1–24. [DOI] [PubMed] [Google Scholar]
- 10.Bartlett JG. Diagnostic tests for agents of community-acquired pneumonia. Clin Infect Dis 2011; 52: Suppl. 4, S296–S304. [DOI] [PubMed] [Google Scholar]
- 11.Hollenbeck B, Dupont I, Mermel LA. How often is a work-up for Legionella pursued in patients with pneumonia? A retrospective study. BMC Infect Dis 2011; 11: 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenkins TC, Stella SA, Cervantes L, et al. Targets for antibiotic and healthcare resource stewardship in inpatient community-acquired pneumonia: a comparison of management practices with National Guideline Recommendations. Infection 2013; 41: 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aliberti S, Reyes LF, Faverio P, et al. Global initiative for meticillin-resistant Staphylococcus aureus pneumonia (GLIMP): an international, observational cohort study. Lancet Infect Dis 2016; 16: 1364–1376. [DOI] [PubMed] [Google Scholar]
- 14.Marston BJ, Plouffe JF, File TM Jr, et al. Incidence of community-acquired pneumonia requiring hospitalization. Results of a population-based active surveillance study in Ohio. Arch Intern Med 1997; 157: 1709–1718. [PubMed] [Google Scholar]
- 15.Jennings LC, Anderson TP, Beynon KA, et al. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax 2008; 63: 42–48. [DOI] [PubMed] [Google Scholar]
- 16.Johansson N, Kalin M, Tiveljung-Lindell A, et al. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis 2010; 50: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnstone J, Majumdar SR, Fox JD, et al. Viral infection in adults hospitalized with community-acquired pneumonia: prevalence, pathogens, and presentation. Chest 2008; 134: 1141–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lieberman D, Shimoni A, Shemer-Avni Y, et al. Respiratory viruses in adults with community-acquired pneumonia. Chest 2010; 138: 811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Templeton KE, Scheltinga SA, van den Eeden WC, et al. Improved diagnosis of the etiology of community-acquired pneumonia with real-time polymerase chain reaction. Clin Infect Dis 2005; 41: 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charles PG, Whitby M, Fuller AJ, et al. The etiology of community-acquired pneumonia in Australia: why penicillin plus doxycycline or a macrolide is the most appropriate therapy. Clin Infect Dis 2008; 46: 1513–1521. [DOI] [PubMed] [Google Scholar]
- 21.Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med 2014; 371: 1619–1628. [DOI] [PubMed] [Google Scholar]
- 22.Deloria Knoll M, Fu W, Shi Q, et al. Bayesian estimation of pneumonia etiology: epidemiologic considerations and applications to the Pneumonia Etiology Research for Child Health study. Clin Infect Dis 2017; 64: Suppl. 3, S213–S227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodhead MA, Macfarlane JT, McCracken JS, et al. Prospective study of the aetiology and outcome of pneumonia in the community. Lancet 1987; 1: 671–674. [DOI] [PubMed] [Google Scholar]
- 24.van der Eerden MM, Vlaspolder F, de Graaff CS, et al. Comparison between pathogen directed antibiotic treatment and empirical broad spectrum antibiotic treatment in patients with community acquired pneumonia: a prospective randomised study. Thorax 2005; 60: 672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Integrated Management of Adolescent and Adult Illness (IMAI) District Clinician Manual: Hospital Care for Adolescents and Adults. Guidelines for the Management of Common Illnesses with Limited Resources. Volume 1. Geneva, World Health Organization, 2011. Available from: www.who.int/hiv/pub/imai/imai2011/en/ [Google Scholar]
- 26.Aston SJ, Rylance J. Community-acquired pneumonia in sub-Saharan Africa. Semin Respir Crit Care Med 2016; 37: 855–867. [DOI] [PubMed] [Google Scholar]
- 27.Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis 2013; 13: 752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao HN, Lu HZ, Cao B, et al. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med 2013; 368: 2277–2285. [DOI] [PubMed] [Google Scholar]