Abstract
Background
Recent findings around long noncoding RNAs (lncRNAs) have opened novel areas of research around their prospective use in overcoming chemoresistance. Herein, we aimed to investigate the role of lncRNA H19 in temozolomide (TMZ) resistance of human glioma cells and the possible mechanisms.
Methods
Short-/long-term oxidative stress was induced, and TMZ-resistant glioma cells (U251TMZ and LN229TMZ) were established. Small interfering RNA (siRNA) and overexpression plasmids were used to modulate the expression of H19 and/or luciferase the reporters. The MTT assay and immunoblotting of cleaved caspase-3, cyclin D1, XIAP and Bcl-2 were conducted to evaluate TMZ sensitivity. Luciferase reporter and quantitative real-time PCR (qRT-PCR) assays were used to verify the activation of NF-κB pathways by H19.
Results
Knockdown of H19 in U251TMZ and LN229TMZ cells decreased half maximal inhibitory concentration (IC50) values for TMZ and increased cell apoptosis, and H19 overexpression in U251 and LN229 cells led to the opposite effects, indicating that the H19 confers TMZ resistance to glioma cells. Furthermore, knockdown of H19 decreased the NF-κB signaling, which was revealed by repressed reporter activity and declined expression of its downstream targets in TMZ-resistant glioma cells. In contrast, H19 overexpression in U251 and LN229 cells resulted in an increase in NF-κB activation. Blockage of NF-κB activation by its inhibitor abolished TMZ resistance caused by H19 overexpression. Addition of H2O2 to induce oxidative stress largely reversed TMZ sensitivity caused by H19 knockdown.
Conclusion
H19 confers TMZ resistance through activating NF-κB signaling and may represent a novel therapeutic target for TMZ-resistant gliomas.
Keywords: H19, oxidative stress, TMZ resistance, NF-κB pathway, glioma
Introduction
Glioma is the most common and invasive type of primary brain tumor in the central nervous system, accounting for about 80% of primary malignant brain tumors.1,2 Among the gliomas, glioblastoma (GBM) is the most aggressive subtype with most poor survival rates.3,4 Constant treatment options include surgical resection, radiotherapy and chemotherapy. Despite much progress achieved in glioma therapy during the last few years, the overall survival of glioma patients remains merely 12–15 months.5–7 Temozolomide (TMZ) is a prevalent chemotherapeutic agent for glioma, which induces DNA strand breaks in growing tumor cells. The mechanism of action of TMZ is attributed to cell apoptosis due to induction of DNA O6-methylguanine. TMZ leads to repair failure due to the MGMT promoter methylation. Therefore, treatment with TMZ alone is insensitive to specific patients who highly express the MGMT.8,9 However, similar to any other cancer, chemoresistance contributes majorly to cancer recurrence; TMZ resistance makes the therapeutic actuality worse for glioma patients.10 Overcoming TMZ resistance becomes a challenge before treating this disease, and more intensive studies are required to investigate the internal molecular mechanism and finally find a cure.
Oxidative stress is strongly correlated with the survival of cancer cells and is also implicated in glioma development.11,12 Excessive ROS levels produced by oxidative stress can affect cellular functions and alter gene expression, which may promote glioma progression.13
Long noncoding RNA (lncRNA) is a class of RNA with the length of over 200 nucleotides without protein-coding capacity. Along with the growing importance of short non-coding RNAs, such as microRNAs, realized by researchers, recently, lncRNAs are becoming hot reported. They are now identified to take part in various biological processes, for instance, cell proliferation, apoptosis, differentiation and caicinogenesis.14–16 They regulate gene expression via transcriptional, posttranscriptional and epigenetic pathways.17 Significantly, studies of lncRNAs in human cancers attract the most widely attention: 1) abnormal expression of lncRNA is frequently observed in cancers and might be used as a diagnostic or prognostic biomarker and 2) some lncRNAs play critical roles in cancer development and progression.18,19 The involvement of lncRNAs in human cancers provides another possibility to consider lncRNAs as therapeutic targets in cancer drug development.
LncRNA H19 encodes a 2.3 kb long transcript, and it has been characterized as an oncogenic lncRNA in a few cancers, including glioma.20–22 Specifically, several independent groups have verified that H19 is overexpressed in gliomas.23–25 Functionally, H19 could drive malignant transformation and promote proliferation, invasion, stemness and chemoresistance.23–26 Mechanistically, most studies attribute its oncogenic functions to its interaction with c-Myc transcription factor or microRNAs.24–26 Although these findings give possible explanations of H19’s roles in glioma, the mechanisms of H19 in governing TMZ resistance remain unclear. Meanwhile, the linkage between oxidative stresses and H19 expression in glioma stays a mystery.
In the present study, we analyzed the expression of H19 in oxidative stressed and TMZ-resistant U251 and LN229 glioma cells. Then, functional mechanism and mechanism exploring work were done to determine the role and mechanism of H19 in TMZ resistance, respectively. Our findings add more knowledge on the role of H19 in TMZ resistance and possible modulations regulating H19 levels in glioma.
Materials and methods
Cell culture
Human glioma U251 and LN229 cell lines were obtained from the cell bank of the Chinese Academy of Sciences (Shanghai, People’s Republic of China). Cells were cultured using DMEM containing 10% FBS (Thermo Fisher Scientific, Waltham, MA, USA) and maintained in a humidified atmosphere of 5% CO2 in air at 37°C.
Cell transfections and reagents
An H19 overexpression plasmid was synthesized and cloned into plasmid pcDNA3.1, and the empty plasmid pcDNA3.1 served as a control. Small interfering RNA (siRNA) targeting H19 was purchased from GenePharma (Suzhou, People’s Republic of China), with a scrambled siRNA used as the negative control. Transfection was performed using Lipofectamine®3000 or Lipofectamine®RNAiMAX reagent (Thermo Fisher Scientific) as per the manufacturer’s protocol.
Oxidative stress treatment in glioma cells
Glioma cells were treated with 1 µM H2O2 for 1 hour for short-term incubation in vitro. Glioma cells were treated with 1 µM glucose oxidase for 24 hours for long-term oxidation in vitro.
Establishment of TMZ-resistant glioma cell lines
1×105 U251 or LN229 cells were exposed to an initial TMZ concentration of 1 µM for 15 days. The surviving population of cells was grown to 80% confluence and passaged twice over 3 weeks. The concentration of TMZ was then sequentially increased in the same manner to 5 µM for 15 days, 25 µM for 15 days, 50 µM for 15 days, 100 µM for 20 days, 200 µM for 20 days, 500 µM for 25 days, 1,000 µM for 25 days and finally to the concentration of 2,000 µM for the last 30 days. The half maximal inhibitory concentration (IC50) of U251 cell was 1,093 µM, while the IC50 of TMZ-resistant U251 cell was 1,722 µM. The IC50 of LN229 cell was 1,018 µM, while the IC50 of TMZ-resistant LN229 cell was 1,571 µM. The established TMZ-resistant U251 or LN229 cells were designated as U251TMZ or LN229TMZ, respectively.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from cells using TRIzol reagent (Thermo Fisher Scientific). LncRNA and gene expression in glioma cells were detected using a SYBR Green qRT-PCR Master Mix kit (Toyobo, Osaka, Japan) as per the manufacturer’s instructions. Expression of α-Tubulin was used as an endogenous control. qRT-PCR was performed using the method.
Immunoblotting
Proteins were extracted from cells using RIPA lysis buffer (Beyotime, Guangzhou, People’s Republic of China), which was supplemented with a phenylmethanesulfonyl fluoride (Beyotime). A total of 20 µg of proteins were loaded on SDS-PAGE, followed by transferring to an nitrocellulose filter membrane (EMD Millipore, Billerica, MA, USA). The membrane was blocked with 5% skim milk at room temperature (RT) for 1 hour and was continuously probed with indicated primary antibodies at 4°C overnight. Then, the membranes were washed with PBS and incubated with specific HRP-conjugated secondary antibodies at RT for 1 hour. α-Tubulin was used as an internal control. α-Tubulin (1:4,000), cyclin D1 (1:1,000), XIAP (1:1,000), Bcl-2 (1:1,000) and cleaved caspase 3 (1:1,000) antibodies were purchased from Cell Signaling Technology (Trask Lane Danvers, MA, USA).
MTT assay
Cell viability was evaluated by using MTT assay. Glioma cells were seeded into 96-well plates at the concentration of 2.5×103 cells/well. Cells were treated with different concentrations of TMZ (0, 100, 250, 500, 1,000, 1,500 and 2,000 µM) for 24 hours. Then, 10 µL MTT (10 mg/mL) was added to each well and incubated in the dark at 37°C for another 2 hours. Absorbance was determined at a wavelength of 570 nm.
Luciferase reporter assay
For luciferase assay, U251 or LN229 cells were seeded into 24-well plates. After culturing for 24 hours, pNF-κB-luc vector (Beyotime) and Renilla vector (pRL-TK; Promega Corporation, Fitchburg, WI, USA) with or without H19 knockdown or overexpression were co-transfected into glioma cells using Lipofectamine®2000. Luciferase activity levels were measured 48 hours after transfection using the Dual-Luciferase Reporter Assay System (Promega Corporation). Firefly luciferase activity was normalized using the corresponding Renilla luciferase activity. Data are representative of three independent experiments.
Statistical analyses
Data are expressed as mean ± SD values. Differences between two groups were performed using two-tailed Student’s t-test. Differences were considered to be statistically significant when P-value was <0.05.
Results
Induced H19 expression by oxidative stress and TMZ resistance
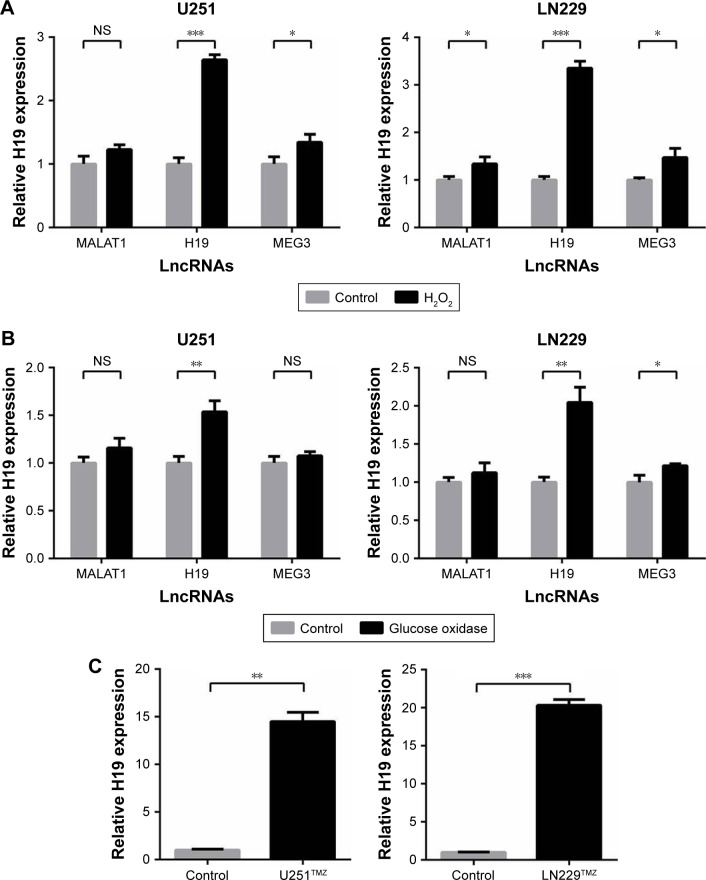
In glioma, two main events are involved with the progression of gliomas: epigenetics and oxidative stress elicited by generation of ROS.27 We started this study from the speculation that lncRNAs induced by oxidative stress may stimulate TMZ resistance in glioma cells. Two human glioma cell lines, U251 and LN229, were used and treated with short-term (H2O2) or long-term (glucose) oxidative stress, and expression of selected lncRNAs (MALAT1, H19, MEG3) was determined by qRT-PCR. The results showed that H19 was the most significantly increased lncRNA induced by oxida-tive stress (Figure 1A and B). Additionally, we established TMZ-resistant U251 and LN229 (U251TMZ and LN229TMZ) cells by constant exposure to a high concentration of TMZ as described previously.28 We also analyzed the levels of H19 in the two TMZ-resistant cells. The results showed that, in comparison with parental non-treated cells, H19 was increased in U251TMZ and LN229TMZ cells (Figure 1C), which is in accordance with the previous reports.28,29
Figure 1.
H19 expression is induced by oxidative stress and TMZ resistance.
Notes: (A) LncRNAs (MALAT1, H19 and MEG3) stimulated by short-term oxidative stress using 1 µM H2O2 in U251 and LN229 cells. (B) LncRNAs (MALAT1, H19 and MEG3) stimulated by long-term oxidative stress using 1 µM glucose oxidase in U251 and LN229 cells. (C) The mRNA expression of H19 was assessed by qRT-PCR analysis, and α-Tubulin was used as the internal control. *P<0.05, **P<0.01 and ***P<0.001 vs the control group.
Abbreviations: TMZ, temozolomide; lncRNA, long noncoding RNA; qRT-PCR, quantitative real-time PCR; NS, not significant.
H19 confers TMZ resistance to glioma cells
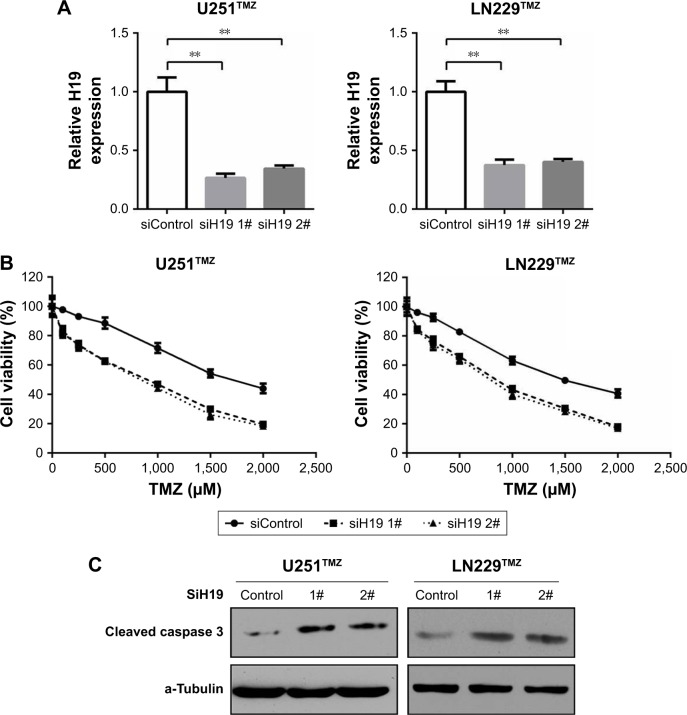
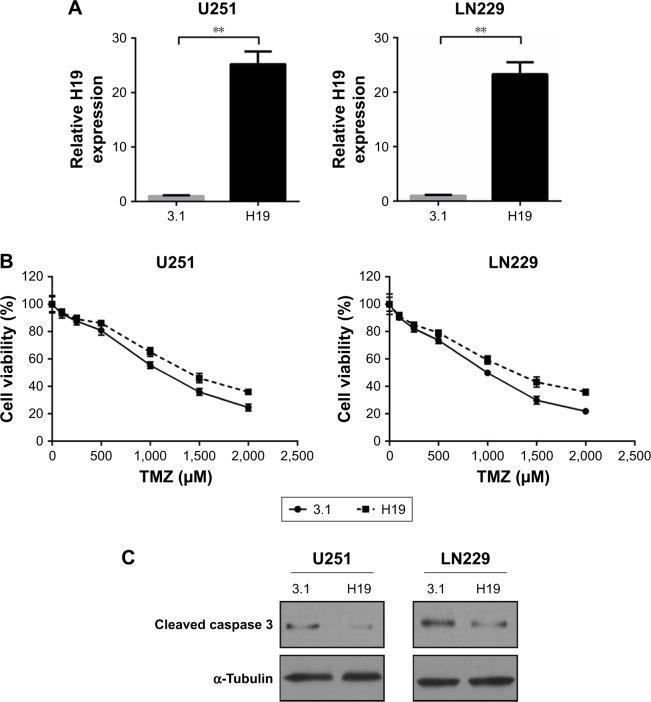
To validate the role of H19 in TMZ resistance of glioma cells, we first used two independent siRNAs targeting against H19 in U251TMZ and LN229TMZ cells. qRT-PCR confirmed the knockdown efficiency (Figure 2A). In all, 2 days after siRNA transfection, U251TMZ and LN229TMZ cells were treated by increasing doses of TMZ and cell viabilities were detected by MTT assay 24 hours post TMZ exposure. As shown in Figure 2B, H19 inhibition decreased IC50 values for TMZ in both U251TMZ and LN229TMZ cells. Immunoblotting analyses of cleavaged caspase 3, cyclin D1, XIAP and Bcl-2 expression indicated enhanced cell apoptosis by TMZ in H19-inhibited cells (Figure 2C). To further strengthen our results, we constructed H19-overexpressing cells by introducing H19-expressing plasmid (Figure 3A). In line with the abovementioned findings, H19 overexpression in wild-type U251 and LN229 cells increased TMZ resistance as accessed by elevated IC50 values (Figure 3B) and reduced cell apoptosis as revealed by less cleavage of caspase 3 and more cyclin D1, XIAP and Bcl-2 expression values (Figure 3C). In a sentence, these findings suggest that H19 confers TMZ resistance to glioma cells.
Figure 2.
Knockdown of H19 decreases chemoresistance of glioma cells to TMZ.
Notes: (A) Relative expression of H19 was detected by qRT-PCR in U251TMZ and LN229TMZ cells after transfected with control siRNAs or two individual H19 siRNAs. (B) MTT assay was performed to determine the cell viabilities of transfected U251TMZ and LN229TMZ cells with TMZ (0, 100, 250, 500, 1,000, 1,500 or 2,000 µM) treatments. (C) Transfected U251TMZ and LN229TMZ cells were treated with 1,750 µM TMZ for 24 hours. The protein level of cleaved caspase 3, cyclin D1, XIAP and Bcl-2 was assessed by immunoblotting. α-Tubulin was used as the internal control. **P<0.01 vs the control group.
Abbreviations: TMZ, temozolomide; qRT-PCR, quantitative real-time PCR; siRNA, small interfering RNA.
Figure 3.
Overexpression of H19 increases chemoresistance of glioma cells to TMZ.
Notes: (A) Relative expression of H19 was detected by qRT-PCR in U251 and LN229 cells after transfected with control plasmid or H19 overexpression plasmid. (B) MTT assay was performed to determine the cell viabilities of transfected U251 and LN229 cells with TMZ (0, 100, 250, 500, 1,000, 1,500 or 2,000 µM) treatments. (C) Transfected U251 and LN229 cells were treated with 1,000 µM TMZ for 24 hours. The protein level of cleaved caspase 3, cyclin D1, XIAP and Bcl-2 was assessed by immunoblotting. α-Tubulin was used as the internal control. **P<0.01 vs the control group.
Abbreviations: TMZ, temozolomide; qRT-PCR, quantitative real-time PCR.
H19 activates NF-κB signaling and therefore TMZ resistance
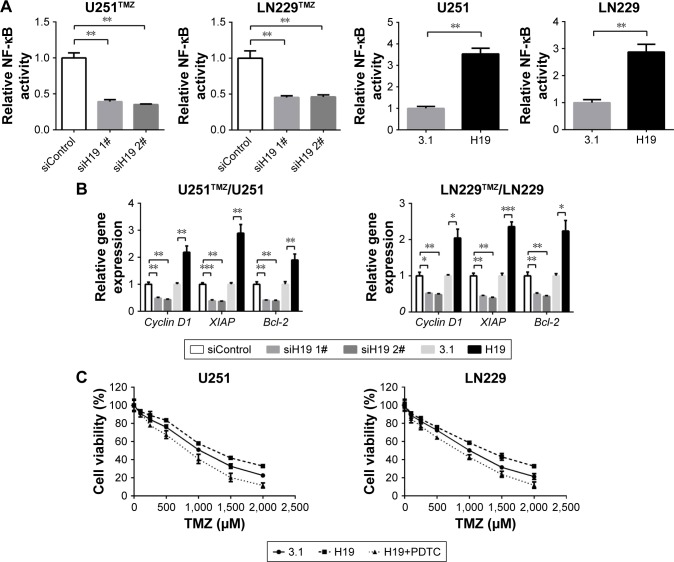
As is known, activation of NF-κB signaling pathway is accompanied by the acquisition of TMZ resistance in glioma.30 To test the involvement of NF-κB signaling in the H19-induced TMZ resistance in our study, we analyzed the activity of NF-κB pathway by measuring its reporter and its downstream target expression. pNF-κB-luc vector was purchased from Beyotime. The vector contains three copies of NF-κB response element GGGAATTTCC, which can be used to indicate the level of transcription activity of NF-κB. As shown in Figure 4A, co-transfection of H19 siRNA and NF-κB reporter decreased the luciferase activity in TMZ-resistant glioma cells (U251TMZ and LN229TMZ), whereas co-transfection of H19 plasmid and NF-κB reporter increased the luciferase activity in wild-type glioma cells (U251 and LN229). We next examined the mRNA levels of targets driven by NF-κB, including cyclin D1, XIAP and Bcl-2. qRT-PCR results showed that H19 knockdown led to reduced expression of these targets in U251TMZ and LN229TMZ cells, whereas H19 overexpression made the opposite effects in U251 and LN229 cells (Figure 4B). To build relationship between NF-κB activation and H19-induced TMZ resistance, we used a specific NF-κB inhibitor, PDTC, to inhibit its intracellular activation. As shown in Figure 4C, pretreatment of PDTC significantly impairs TMZ resistance caused by H19 overexpression, as accessed by MTT assay. These results indicate that H19 could activate NF-κB signaling, which contributes to TMZ resistance in glioma cells.
Figure 4.
H19 confers TMZ resistance through NF-κB signaling pathway.
Notes: (A) The NF-κB luciferase activity was inhibited or enhanced in H19-silenced U251TMZ and LN229TMZ cells or H19-overexpressed U251 and LN229 cells. (B) The downstream targets of NF-κB pathway such as cyclin D1, XIAP and Bcl-2 were downregulated in U251TMZ and LN229TMZ cells after H19 knockdown or upregulated in U251 and LN229 cells after H19 overexpression. (C) MTT assay was performed to determine the cell viabilities of transfected U251 and LN229 cells with TMZ (0, 100, 250, 500, 1,000, 1,500 or 2,000 µM) treatments combined with or without 30 µM PDTC. *P<0.05, **P<0.01 and ***P<0.001 vs the control group.
Abbreviation: TMZ, temozolomide.
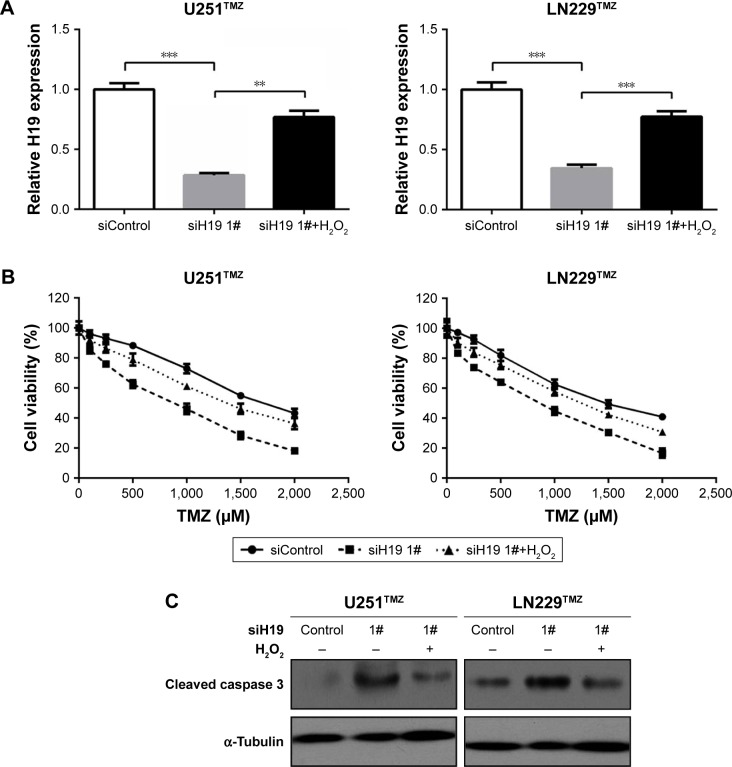
Oxidative stress reversed TMZ sensitivity caused by H19 knockdown
As described earlier, H19 knockdown sensitized established TMZ-resistant glioma cells toward TMZ exposure (Figure 2B and C). We also showed that oxidative stress was an important inducer of H19 expression in glioma cells (Figure 1A and B). Then, we speculated that short-term oxidative stress triggered by H2O2 might reverse TMZ sensitivity caused by H19 knockdown, since it recovered H19 expression. To test this hypothesis, we first detected H19 expression by qRT-PCR and validated that H19 siRNAs could inhibit its endogenous expression, which could be recovered by H2O2 treatment for 1 hour (Figure 5A). Moreover, pretreatment of 1 µM H2O2 for 1 hour elevated IC50 and decreased cell apoptosis compared with that in the H19 knockdown group, nearly to the control siRNA-treated cells (Figure 5B and C). These results elucidated that H19 induced by oxidative stress contributes to TMZ resistance in glioma cells.
Figure 5.
H2O2-induced oxidative stress reversed TMZ sensitivity caused by H19 knockdown.
Notes: (A) qRT-PCR was performed to determine the H19 expression of transfected U251TMZ and LN229TMZ cells with H19 knockdown combined with or without 1 µM H2O2. (B) MTT assay was performed to determine the cell viabilities of transfected U251TMZ and LN229TMZ cells with TMZ (0, 100, 250, 500, 1,000, 1,500 or 2,000 µM) treatments combined with or without 1 µM H2O2. (C) The protein level of cleaved caspase 3 was assessed by immunoblotting as described in (A). α-Tubulin was used as the internal control. **P<0.01 and ***P<0.001 vs the control group.
Abbreviations: TMZ, temozolomide; qRT-PCR, quantitative real-time PCR.
Discussion
Glioma is the most common type of primary brain tumor in the central nervous system, in which GBM is the most aggressive subtype.1–4 The current therapy efficacy remains poor mostly because of chemoresistance, which contributes majorly in cancer recurrence.10 Anti-chemoresistance, especially anti-TMZ resistance, has become a promising treatment strategy for glioma patients. Recently, one of the most hot targets in glioma TMZ resistance is the discovery of lncRNAs and their key roles.31,32
Using representative cell lines (U251 and LN229), we herein investigated the role of lncRNA H19 in glioma TMZ resistance. Our results revealed that the H19 level was induced by oxidative stress and was increased in U251TMZ and LN229TMZ cells. Cellular assays confirmed that knockdown of H19 in TMZ resistance cells sensitized them to TMZ exposure. Meanwhile, H19 overexpression in U251 and LN229 cells caused TMZ resistance, strengthening that H19 confers TMZ resistance to glioma cells. Furthermore, we found that H19 knockdown inhibited NF-κB signaling, whereas H19 overexpression resulted in activated NF-κB signaling. Blockage of NF-κB activation by its inhibitor obviously abolished TMZ resistance caused by H19 over-expression. Finally, we verified that oxidative stress largely reversed TMZ sensitivity caused by H19 knockdown by inducing more H19 expression.
In fact, numerous studies have shown that H19 is upregulated in gliomas and could drive malignant transformation and promote chemoresistance.23–26 Most recently, Jia et al29 pointed out that H19 was upregulated in TMZ-resistant glioma cells and silencing of H19 decreased TMZ chemoresistance of glioma cells by suppressing epithelial–mesenchymal transition (EMT) via the Wnt/β-catenin pathway. However, whether there are other mechanisms of H19 in governing TMZ resistance remains unclear. In the present study, we provided another pathway that might be linked with H19-induced TMZ resistance, that is, the NF-κB signaling pathway. Constitutively activate NF-κB is a major transcription factor linked with glioma, and it may be responsible for multiple cellular functions including apoptosis inhibition and promotion of survival, invasion and immune response.32 Activation of NF-κB signaling is also accompanied by the acquisition of TMZ resistance in glioma.30 Our results showed that H19 knockdown inhibited NF-κB signaling as revealed by decreased NF-κB reporter activity and target expression, whereas H19 overexpression resulted in the activated NF-κB signaling. Importantly, blockage of NF-κB activation by its inhibitor obviously abolished TMZ resistance caused by H19 overexpression.
As is known, epigenetics and oxidative stress are associated with the progression of gliomas.27 In the present study, we found that H19 could be stimulated by both short-term and long-term oxidative stress. Notably, applying H2O2 to induce oxidative stress majorly reversed TMZ sensitivity caused by H19 knockdown, suggesting that there is a penetrating pathway: oxidative stress produced more H19, which led to TMZ resistance via activating NF-κB signaling.
Our study provides more insights into the potential role and mechanism of H19 in glioma TMZ resistance. Nevertheless, there are still some limitations in our study. For instance, our results are based on cellular experiments. More in vitro primary cultured cell work or in vivo data are needed to strengthen our conclusion.
Conclusion
Our study revealed that H19 could be stimulated by oxidative stress, and H19 confers TMZ resistance through activating NF-κB signaling. LncRNA H19 may represent a novel therapeutic target for TMZ-resistant gliomas. Our results provide new insights into the mechanism linking H19 with glioma chemoresistance.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cuddapah VA, Robel S, Watkins S, Sontheimer H. A neurocentric perspective on glioma invasion. Nat Rev Neurosci. 2014;15(7):455–465. doi: 10.1038/nrn3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 3.Omuro A, Deangelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 4.Weller M. Novel diagnostic and therapeutic approaches to malignant glioma. Swiss Med Wkly. 2011;141:w13210. doi: 10.4414/smw.2011.13210. [DOI] [PubMed] [Google Scholar]
- 5.Tabatabai G, Stupp R, van den Bent MJ, et al. Molecular diagnostics of gliomas: the clinical perspective. Acta Neuropathol. 2010;120(5):585–592. doi: 10.1007/s00401-010-0750-6. [DOI] [PubMed] [Google Scholar]
- 6.Lara-Velazquez M, Al-Kharboosh R, Jeanneret S, et al. Advances in Brain Tumor Surgery for Glioblastoma in Adults. Brain Sci. 2017;7(12):166. doi: 10.3390/brainsci7120166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamandis P, Aldape KD. Insights From Molecular Profiling of Adult Glioma. J Clin Oncol. 2017;35(21):2386–2393. doi: 10.1200/JCO.2017.73.9516. [DOI] [PubMed] [Google Scholar]
- 8.Thomas A, Tanaka M, Trepel J, Reinhold WC, Rajapakse VN, Pommier Y. Temozolomide in the Era of Precision Medicine. Cancer Res. 2017;77(4):823–826. doi: 10.1158/0008-5472.CAN-16-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dullea A, Marignol L. MGMT testing allows for personalised therapy in the temozolomide era. Tumour Biol. 2016;37(1):87–96. doi: 10.1007/s13277-015-4240-2. [DOI] [PubMed] [Google Scholar]
- 10.Nanegrungsunk D, Onchan W, Chattipakorn N, Chattipakorn SC. Current evidence of temozolomide and bevacizumab in treatment of gliomas. Neurol Res. 2015;37(2):167–183. doi: 10.1179/1743132814Y.0000000423. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert MR, Liu Y, Neltner J, et al. Autophagy and oxidative stress in gliomas with IDH1 mutations. Acta Neuropathol. 2014;127(2):221–233. doi: 10.1007/s00401-013-1194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta RK, Patel AK, Shah N, et al. Oxidative stress and antioxidants in disease and cancer: a review. Asian Pac J Cancer Prev. 2014;15(11):4405–4409. doi: 10.7314/apjcp.2014.15.11.4405. [DOI] [PubMed] [Google Scholar]
- 13.Kim SH, Kwon CH, Nakano I. Detoxification of oxidative stress in glioma stem cells: mechanism, clinical relevance, and therapeutic development. J Neurosci Res. 2014;92(11):1419–1424. doi: 10.1002/jnr.23431. [DOI] [PubMed] [Google Scholar]
- 14.Lu C, Huang YH. Progress in long non-coding RNAs in animals. Yi Chuan. 2017;39(11):1054–1065. doi: 10.16288/j.yczz.17-120. [DOI] [PubMed] [Google Scholar]
- 15.Marchese FP, Raimondi I, Huarte M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017;18(1):206. doi: 10.1186/s13059-017-1348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ard R, Allshire RC, Marquardt S. Emerging Properties and Functional Consequences of Noncoding Transcription. Genetics. 2017;207(2):357–367. doi: 10.1534/genetics.117.300095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun W, Yang Y, Xu C, Guo J. Regulatory mechanisms of long non-coding RNAs on gene expression in cancers. Cancer Genet. 2017;216–217:105–110. doi: 10.1016/j.cancergen.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Lin C, Yang L. Long noncoding RNA in cancer: wiring signaling circuitry. Trends Cell Biol. 2018;28(4):287–301. doi: 10.1016/j.tcb.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao AKDM, Rajkumar T, Mani S. Perspectives of long non-coding RNAs in cancer. Mol Biol Rep. 2017;44(2):203–218. doi: 10.1007/s11033-017-4103-6. [DOI] [PubMed] [Google Scholar]
- 20.Matouk IJ, Halle D, Raveh E, Gilon M, Sorin V, Hochberg A. The role of the oncofetal H19 lncRNA in tumor metastasis: orchestrating the EMT-MET decision. Oncotarget. 2016;7(4):3748–3765. doi: 10.18632/oncotarget.6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collette J, Le Bourhis X, Adriaenssens E. Regulation of Human Breast Cancer by the Long Non-Coding RNA H19. Int J Mol Sci. 2017;18(11):2319. doi: 10.3390/ijms18112319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang X, Yan Y, Hu M, et al. Increased level of H19 long noncoding RNA promotes invasion, angiogenesis, and stemness of glioblastoma cells. J Neurosurg. 2016;124(1):129–136. doi: 10.3171/2014.12.JNS1426. [DOI] [PubMed] [Google Scholar]
- 23.Shi Y, Wang Y, Luan W, et al. Long Non-Coding RNA H19 Promotes Glioma Cell Invasion by Deriving miR-675. PLoS One. 2014;9(1):e86295. doi: 10.1371/journal.pone.0086295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barsyte-Lovejoy D, Lau SK, Boutros PC, et al. The c-Myc Oncogene Directly Induces the H19 Noncoding RNA by Allele-Specific Binding to Potentiate Tumorigenesis. Cancer Res. 2006;66(10):5330–5337. doi: 10.1158/0008-5472.CAN-06-0037. [DOI] [PubMed] [Google Scholar]
- 25.Zhang T, Wang YR, Zeng F, Cao HY, Zhou HD, Wang YJ. LncRNA H19 is overexpressed in glioma tissue, is negatively associated with patient survival, and promotes tumor growth through its derivative miR-675. Eur Rev Med Pharmacol Sci. 2016;20(23):4891–4897. [PubMed] [Google Scholar]
- 26.Jia P, Cai H, Liu X, et al. Long non-coding RNA H19 regulates glioma angiogenesis and the biological behavior of glioma-associated endothelial cells by inhibiting microRNA-29a. Cancer Lett. 2016;381(2):359–369. doi: 10.1016/j.canlet.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Rangel-Lopez E, Sanchez-Perez Y, Soto-Reyes E, Garcia-Cuellar CM, Cacho-Diaz B, Santamaria A. Role of epigenetics and oxidative stress in gliomagenesis. CNS Neurol Disord Drug Targets. 2018 doi: 10.2174/1871527317666180110124645. [DOI] [PubMed] [Google Scholar]
- 28.Jiang P, Wang P, Sun X, et al. Knockdown of long noncoding RNA H19 sensitizes human glioma cells to temozolomide therapy. Onco Targets Ther. 2016;9:3501–3509. doi: 10.2147/OTT.S96278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia L, Tian Y, Chen Y, Zhang G. The silencing of LncRNA-H19 decreases chemoresistance of human glioma cells to temozolomide by suppressing epithelial-mesenchymal transition via the Wnt/β-Catenin pathway. Onco Targets Ther. 2018;11:313–321. doi: 10.2147/OTT.S154339. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Li ZY, Li QZ, Chen L, Chen BD, Wang B, Zhang XJ, Li WP. Histone Deacetylase Inhibitor RGFP109 Overcomes Temozolomide Resistance by Blocking NF-κB-Dependent Transcription in Glioblastoma Cell Lines. Onco Targets Ther Neurochem Res. 2016;11(12):313–321. doi: 10.1007/s11064-016-2043-5. [DOI] [PubMed] [Google Scholar]
- 31.du P, Zhao H, Peng R, et al. LncRNA-XIST interacts with miR-29c to modulate the chemoresistance of glioma cell to TMZ through DNA mismatch repair pathway. Biosci Rep. 2017;37(5):BSR20170696. doi: 10.1042/BSR20170696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng H, Xu N, Liu Y, et al. Genomic profiling of long non-coding RNA and mRNA expression associated with acquired temozolomide resistance in glioblastoma cells. Int J Oncol. 2017;51(2):445–455. doi: 10.3892/ijo.2017.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]