Abstract
Background
Regorafenib is a novel multikinase inhibitor (MKI) approved for use in the treatment of metastatic colorectal cancer (CRC), treatment-refractory gastrointestinal stromal tumors, and other solid tumor malignancies. However, the adverse events (AEs) associated with regorafenib have not been systematically investigated. Hence, we performed a meta-analysis to identify AEs associated with regorafenib in patients with advanced solid tumors.
Methods
The databases of PubMed, MEDLINE, and Embase and abstracts presented in American Society of Clinical Oncology annual meetings were searched for relevant publications from January 2004 to September 2017. Eligible studies were limited to prospective randomized controlled trials (RCTs) that evaluate the use of regorafenib in patients with advanced solid tumors. Incidence, relative risk (RR), and 95% CIs were calculated using a random or fixed effects model on the basis of the heterogeneity of the included studies.
Results
A total of 2,065 patients from six RCTs were included, and 1,340 of them received regorafenib and 725 received a placebo. Sixteen all-grade AEs and 15 high-grade AEs were investigated for their association with regorafenib. Results showed that hand–foot skin reaction (HFSR; 54%), diarrhea (33%), fatigue (32%), hypertension (31%), oral mucositis (28%), and anorexia (23%) were the most frequent clinical AEs. The most common high-grade (grade, ≥3) AEs were HFSR (16%), hypertension (13%), fatigue (6%), increased aspartate aminotransferase (AST; 6%), and hypophosphatemia (6%). Pooled RR showed that the use of regorafenib was associated with an increased risk of developing AEs. Subgroup analysis based on the prior MKI treatment showed that prior MKI treatment was associated with an increased incidence of all-grade anorexia (P=0.03) and a reduced incidence of high-grade increased AST (P=0.04). However, subgroup analysis based on the tumor type showed that no significant differences were found when comparing the RR of all-grade and high-grade AEs in patients with CRC or non-CRC.
Conclusion
The meta-analysis systematically investigated regorafenib-associated AEs. Knowledge of these AEs is essential for minimizing treatment-related toxicities and improving clinical outcomes.
Keywords: regorafenib, adverse event, AE, safety, multikinase inhibitor, meta-analysis
Introduction
Regorafenib (BAY 73-4506), a novel multikinase inhibitor (MKI) taken orally, blocks the activity of various protein kinases associated with tumor angiogenesis, oncogenesis, and tumor microenvironment.1 These protein kinases include vascular endothelial growth factor receptors 1–3 (VEGFR1–3), TIE2, KIT, RET, RAF-1, BRAF, FGF, and platelet-derived growth factor receptor.2 A great quantity of studies demonstrated that regorafenib has antitumor activity in patients with a range of advanced solid tumors. An international, randomized, Phase III trial of regorafenib, named CORRECT study, showed that regorafenib provided significant survival benefits in metastatic colorectal cancer (mCRC), which had failed all standard therapies.3 A subsequent Phase III trial (named GRID) indicated that regorafenib was effective in advanced gastrointestinal stromal tumors (GISTs) after the failure of imatinib and sunitinib.4 As of September 2017, regorafenib has been approved by the United States Food and Drug Administration (FDA) for the treatment of mCRC and locally advanced GISTs. Moreover, several latest randomized controlled trials (RCTs) suggest that regorafenib also demonstrates clinical benefits to patients with advanced hepatocellular cancer (HCC), advanced gastric cancer (GC), and soft tissue sarcoma.5–8 At present, the antitumor activity of regorafenib is being explored for other solid tumors in more than 30 clinical trials. Therefore, the use of regorafenib is expected to rise in the near future.9–12
However, as with other MKIs (sorafenib, sunitinib, cabozantinib, axitinib, and pazopanib), regorafenib is associated with a new set of adverse events (AEs) different from the toxicity profiles of traditional cytotoxic agents, including hand–foot skin reaction (HFSR), fatigue, diarrhea, stomatitis, hypertension, liver abnormalities, and rash or desquamation. Although these AEs are not life threatening, they may affect patients’ psychological well-being and physiological function, leading to the nonadherence or discontinuation of regorafenib treatment.13–15 Hence, it is important for clinicians to recognize the AEs associated with regorafenib in clinical practice. However, to the best of our knowledge, no comprehensive meta-analysis focusing on the clinically relevant toxicities associated with regorafenib has been performed. Therefore, we conducted this meta-analysis of all available RCTs to identify and analyze the AEs related to regorafenib in the treatment of advanced solid tumor.
Methods
Study selection
A comprehensive review of PubMed, MEDLINE, and Embase databases from January 2004 and September 2017 was performed using the following keywords: “regorafenib”, “BAY 73-4506”, “stivarga”, and “clinical trials”. The search was limited to articles published in English. Relevant abstracts from the American Society of Clinical Oncology published between January 2004 and September 2017 were also searched to identify relevant clinical trials through a similar strategy. Only clinical trials with full publication were included. The most recent manufacturer’s package insert was also scrutinized for related clinical information. Each publication was reviewed, and only the most recent, complete report of a trial was included when multiple publications of the same clinical trial were identified.
Clinical trials that met the following criteria were included in the meta-analysis: 1) prospective randomized Phase II and III trials of patients with cancer, 2) random assignment of patients to treatment with regorafenib or control, and 3) available data on AEs associated with regorafenib. Studies were excluded when there were multiple publications of the same trial. The bias risk of the included studies was evaluated with the Jadad scoring system,16 which includes randomization, double blinding, and withdrawals.
Data extraction and clinical endpoint
The primary endpoint of this meta-analysis was all-grade and high-grade (grade ≥3) AEs associated with regorafenib. AEs were recorded according to the version of the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) in each trial. In this meta-analysis, all trials utilized CTCAE version 3.0, 4.0, or 4.03 criteria. Data extraction was performed independently by two investigators (XY and YY), and any discrepancy was resolved through a consensus by a discussion with a third investigator (CS). The following information was extracted from each study: first author’s name, publication year, trial phase, underlying malignancy, number of patients enrolled, patient characteristics, treatment regimen, median treatment duration, types of AEs, and numbers of all-grade and high-grade AEs.
Statistical analysis
All statistical analyses were performed using version 2 of the comprehensive meta-analysis program (Biostat, Englewood, NJ, USA) and Review manager 5.3. For the calculation of incidence rate (IR), the number of patients with all- and high-grade AEs and the total number of patients being treated with regorafenib were determined from each trial. The proportions of patients with all- and high-grade AEs and 95% CIs were derived. The relative risk (RR) of AE and 95% CIs were also computed for patients assigned to regorafenib vs those assigned to the control treatment in the same trial.
The heterogeneity of the included trials was assessed with Cochran’s Q statistic. The assumption of heterogeneity was considered invalid for a P-value <0.1. For the meta-analysis, a fixed or random effects model was considered depending on the heterogeneity among trials. A random effects model was used when the P-value was <0.1; otherwise, a fixed effects model was selected. Potential publication bias was evaluated using funnel plots (plots of study results against precision) and with Begg’s and Egger’s tests.17,18 A two-tailed P-value <0.05 was regarded as statistically significant.
Results
Search results
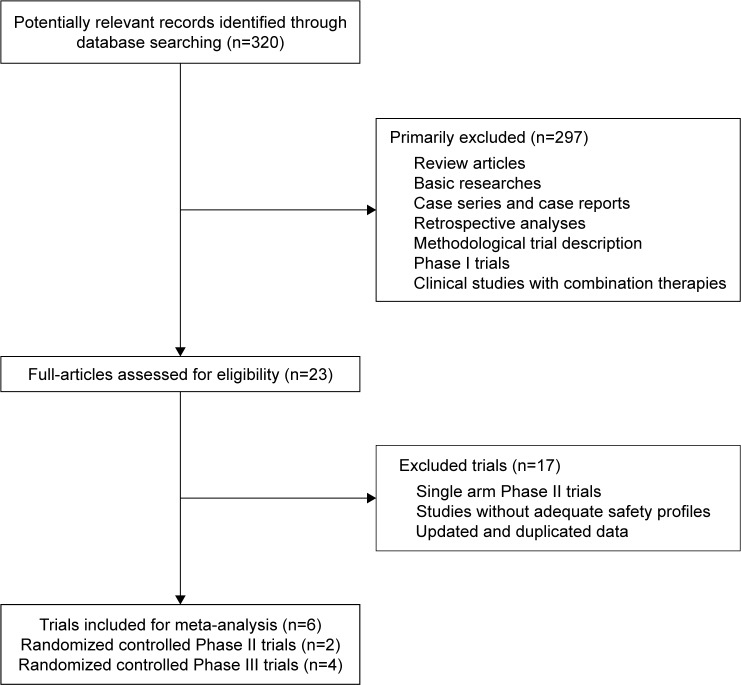
A total of 320 relevant studies were identified according to our search strategy. After initial screening by title and abstract, 23 relevant trials were selected for full-text review. In the review, 17 studies were excluded from the final analysis, and the reasons for exclusion are shown in Figure 1. Finally, six RCTs, including four Phase III and two Phase II trials, were included for statistical analysis. The characteristics of the included studies are summarized in Table 1.
Figure 1.
Selection of randomized controlled trials included in the meta-analysis.
Table 1.
Baseline characteristics of randomized controlled trials included in the meta-analysis
| Author | Year | Trial design | Jadad scale | Malignancy | Treatment arm | Sample size | Median age (range), years | Treatment duration, median (range) | ECOG PS 0–1 (%) | NCI-CTCAE version |
|---|---|---|---|---|---|---|---|---|---|---|
| Grothey et al3 | 2013 | III | 5 | Metastatic CRC | Regorafenib | 505 | 61 (54–67) | 1.7 months (1.4–3.7) | 100 | 3.0 |
| Placebo | 255 | 61 (54–68) | 1.6 months (1.3–1.7) | |||||||
| Demetri et al4 | 2013 | III | 5 | Advanced GIST | Regorafenib | 133 | 60 (51–67) | 22.9 weeks (9.3–28.6) | 100 | 4.0 |
| Placebo | 66 | 61 (48–66) | 7.0 weeks (5.1–11.3) | |||||||
| Li et al6 | 2015 | III | 5 | Metastatic CRC | Regorafenib | 136 | 57.5 (50–66) | 2.4 months (1.6–5.3) | 100 | 4.0 |
| Placebo | 68 | 55.5 (48.5–62) | 1.6 months (1.1–1.6) | |||||||
| Bruix et al5 | 2017 | III | 5 | Advanced HCC | Regorafenib | 379 | 64 (54–71) | 3.6 months (1.6–7.6) | 100 | 4.03 |
| Placebo | 194 | 62 (55–68) | 1.9 months (1.4–3.9) | |||||||
| Pavlakis et al8 | 2016 | II | 5 | Advanced GC | Regorafenib | 97 | 63 (33–81) | 1.8 months (1.4–2.0) | 100 | 4.0 |
| Placebo | 50 | 62 (32–85) | 0.9 months (0.9–1.0) | |||||||
| Mir et al7 | 2016 | II | 5 | Advanced liposarcoma | Regorafenib | 20 | 57 (24–76) | 1.6 months (0.7–2.4) | 98 | 4.03 |
| Placebo | 23 | 65 (22–80) | 1.9 months (1.6–4.8) | |||||||
| Advanced leiomyosarcoma | Regorafenib | 28 | 60 (37–74) | 3.9 months (2.0–8.7) | 100 | |||||
| Placebo | 28 | 60 (30–76) | 2.3 months (1.6–4.6) | |||||||
| Advanced synovial sarcomas | Regorafenib | 13 | 46 (21–73) | 3.4 months (0.7–6.7) | 100 | |||||
| Placebo | 14 | 35 (20–78) | 1.4 months (0.7–2.0) | |||||||
| Other advanced sarcomas | Regorafenib | 29 | 60 (26–81) | 3.5 months (0.6–10.2) | 100 | |||||
| Placebo | 27 | 55 (20–78) | 2.8 months (1.1–5.1) |
Abbreviations: CRC, colorectal cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; GC, gastric cancer; GIST, gastrointestinal stromal tumor; HCC, hepatocellular carcinoma; NCI-CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events.
Population characteristics
A total of 2,065 patients (regorafenib, n=1,340; control, n=725) from the six RCTs were included in the meta-analysis. The majority of patients had a baseline Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Underlying cancers were mCRC (two trials),3,6 metastatic or unresectable GIST (one trial),4 advanced HCC (one trial),5 advanced GC (one trial),8 and advanced soft tissue sarcoma (one trial).7 In the included trials, all patients received the best supportive care plus oral regorafenib (160 mg) or placebo once daily during weeks 1–3 of each 4-week cycle.
Description of AEs
In the six trials included in this meta-analysis, a total of 71 AEs were reported during the treatment of regorafenib. After the combination of synonyms and the exclusion of rare AEs, 16 all-grade AEs (HFSR, fatigue, diarrhea, hypertension, voice changes, anorexia, oral mucositis, rash or desquamation, hyperbilirubinemia, myalgia, increased aspartate aminotransferase [AST], hoarseness, nausea, increased alanine aminotransferase [ALT], weight loss, alopecia) and 15 high-grade AEs (HFSR, hypertension, fatigue, increased AST, hypophosphatemia, rash or desquamation, diarrhea, hyperbilirubinemia, increased ALT, lipase increase, anorexia, thrombocytopenia, anemia, oral mucositis, myalgia), including clinical AEs and laboratory abnormalities, were investigated for their association with regorafenib.
Incidence and RR of AEs
To evaluate the all-grade and high-grade AEs, data were included from all six RCTs comprising 2,065 patients. Tables 2 and 3 show the pooled IRs and RRs of the all-grade and high-grade AEs associated with regorafenib. The incidences of the all-grade and high-grade AEs were 95% and 54%, respectively. For any grade of AEs, HFSR, diarrhea, fatigue, hypertension, oral mucositis, and anorexia were the most frequent clinical AEs (54%, 33%, 32%, 31%, 28%, and 23%, respectively). The most common high-grade AEs were HFSR (16%), hypertension (13%), fatigue (6%), increased AST (6%), and hypophosphatemia (6%).
Table 2.
Incidence and RR of all-grade AEs resulting from regorafenib treatment
| AEs | No of studies | No of events/sample size | Incidence (95% CI) | I2 (%) | RR (95% CI) | P-value | ||
|---|---|---|---|---|---|---|---|---|
| Regorafenib | Placebo/control | Regorafenib | Placebo/control | |||||
| Any AE | 4 | 1,073/1,142 | 330/580 | 0.95 (0.92–0.97)a | 0.57 (0.48–0.65)a | 69 | 1.66 (1.45–1.91)a | <0.00001 |
| Clinical AE | ||||||||
| Hand–foot skin reaction | 5 | 638/1,231 | 45/672 | 0.54 (0.45–0.63)a | 0.07 (0.04–0.11)a | 54 | 7.42 (4.61–11.94) | <0.00001 |
| Fatigue | 4 | 421/1,142 | 131/580 | 0.32 (0.21–0.46)a | 0.21 (0.14–0.30)a | 0 | 1.63 (1.38–1.93) | <0.00001 |
| Diarrhea | 5 | 410/1,231 | 50/672 | 0.33 (0.27–0.40)a | 0.08 (0.06–0.10) | 0 | 4.51 (3.42–5.95) | <0.00001 |
| Hypertension | 5 | 353/1,231 | 48/672 | 0.31 (0.23–0.40)a | 0.08 (0.05–0.13)a | 0 | 4.12 (3.09–5.50) | <0.00001 |
| Voice changes | 2 | 161/632 | 16/319 | 0.19 (0.06–0.44)a | 0.05 (0.03–0.08) | 0 | 5.09 (3.10–8.34) | <0.00001 |
| Anorexia | 5 | 311/1,231 | 79/672 | 0.23 (0.16–0.32)a | 0.11 (0.06–0.18)a | 24 | 2.26 (1.80–2.84) | <0.00001 |
| Oral mucositis | 4 | 267/1,095 | 28/604 | 0.28 (0.16–0.44)a | 0.05 (0.03–0.10)a | 0 | 5.66 (3.87–8.29) | <0.00001 |
| Rash or desquamation | 3 | 166/768 | 13/387 | 0.17 (0.09–0.29)a | 0.04 (0.02–0.06) | 0 | 6.44 (3.72–11.17) | <0.00001 |
| Myalgia | 3 | 56/357 | 29/226 | 0.15 (0.05–0.37)a | 0.10 (0.03–0.31)a | 0 | 1.55 (1.04–2.32) | 0.03 |
| Hoarseness | 3 | 90/642 | 4/327 | 0.16 (0.09–0.27)a | 0.02 (0.004–0.08)a | 19 | 10.16 (4.00–25.78) | <0.00001 |
| Nausea | 3 | 133/1,006 | 47/512 | 0.13 (0.11–0.16) | 0.09 (0.07–0.12) | 0 | 1.44 (1.05–1.97) | 0.02 |
| Weight loss | 2 | 96/874 | 9/446 | 0.10 (0.05–0.19)a | 0.02 (0.01–0.04) | 0 | 5.43 (2.77–10.64) | <0.00001 |
| Alopecia | 2 | 67/632 | 2/319 | 0.13 (0.04–0.37)a | 0.008 (0.002–0.03) | 0 | 16.86 (4.17–68.14) | <0.0001 |
| Laboratory abnormalities | ||||||||
| Hyperbilirubinemia | 3 | 165/1,010 | 16/514 | 0.19 (0.09–0.37)a | 0.04 (0.02–0.08)a | 0 | 5.24 (3.19–8.63) | <0.00001 |
| Increased AST | 2 | 80/510 | 21/261 | 0.17 (0.09–0.30)a | 0.08 (0.05–0.12) | 0 | 1.94 (1.23–3.07) | 0.004 |
| Increased ALT | 2 | 61/510 | 13/261 | 0.14 (0.04–0.36)a | 0.05 (0.03–0.09) | 0 | 2.39 (1.34–4.25) | 0.003 |
Note:
The incidence or RR of AEs and 95% CI were calculated with a random effects model.
Abbreviations: AE/s, adverse event/s; ALT, alanine aminotransferase; AST, aspartate aminotransferase; RR, relative risk.
Table 3.
Incidence and RR of high-grade AEs resulting from regorafenib treatment
| AEs | No of studies | No of events/sample size | Incidence (95% CI) | I2 (%) | RR (95% CI) | P-value | ||
|---|---|---|---|---|---|---|---|---|
| Regorafenib | Placebo/control | Regorafenib | Placebo/control | |||||
| Any AE | 4 | 610/1,142 | 83/580 | 0.54 (0.50–0.57) | 0.15 (0.12–0.18) | 19 | 3.73 (3.04–4.59) | <0.00001 |
| Clinical AE | ||||||||
| Hand–foot skin reaction | 5 | 192/1,231 | 2/672 | 0.16 (0.14–0.18) | 0.005 (0.002–0.02) | 0 | 30.28 (10.51–87.22) | <0.00001 |
| Hypertension | 6 | 158/1,328 | 15/722 | 0.13 (0.09–0.19)a | 0.02 (0.01–0.04) | 0 | 5.85 (3.48–9.84) | <0.00001 |
| Fatigue | 4 | 79/1,142 | 20/580 | 0.06 (0.03–0.09)a | 0.04 (0.03–0.06) | 46 | 1.97 (1.23–3.16) | 0.005 |
| Rash or desquamation | 3 | 38/768 | 0/387 | 0.05 (0.04–0.07) | 0.005 (0.001–0.02) | 0 | 13.32 (2.62–67.68) | 0.002 |
| Diarrhea | 5 | 57/1,231 | 5/672 | 0.04 (0.02–0.07)a | 0.01 (0.01–0.03) | 19 | 5.55 (2.34–13.17) | <0.0001 |
| Anorexia | 6 | 36/1,328 | 14/722 | 0.03 (0.02–0.04) | 0.03 (0.02–0.05) | 0 | 1.41 (0.77–2.58) | 0.27 |
| Oral mucositis | 4 | 25/1,095 | 2/604 | 0.03 (0.02–0.04) | 0.006 (0.002–0.02) | 6 | 5.01 (1.54–16.28) | 0.007 |
| Myalgia | 3 | 7/357 | 3/226 | 0.02 (0.004–0.08)a | 0.02 (0.008–0.06) | 0 | 1.66 (0.50–5.45) | 0.41 |
| Laboratory abnormalities | ||||||||
| Increased AST | 3 | 36/607 | 10/311 | 0.06 (0.04–0.08) | 0.04 (0.02–0.08) | 59 | 2.95 (0.45–19.40) | 0.26 |
| Hypophosphatemia | 5 | 63/1,196 | 3/656 | 0.06 (0.04–0.09)a | 0.01 (0.002–0.02) | 0 | 9.56 (3.50–26.09) | <0.0001 |
| Hyperbilirubinemia | 3 | 44/1,010 | 7/514 | 0.05 (0.02–0.10)a | 0.02 (0.007–0.03) | 0 | 3.21 (1.46–7.06) | 0.004 |
| Increased ALT | 3 | 25/607 | 5/311 | 0.05 (0.02–0.11)a | 0.02 (0.005–0.08)a | 0 | 2.38 (0.96–5.89) | 0.06 |
| Lipase increase | 2 | 7/225 | 2/160 | 0.04 (0.02–0.07) | 0.01 (0.003–0.05) | 0 | 2.17 (0.42–11.10) | 0.35 |
| Thrombocytopenia | 4 | 27/1,099 | 1/606 | 0.03 (0.02–0.04) | 0.004 (0.001–0.02) | 0 | 6.28 (1.69–23.29) | 0.006 |
Note:
The incidence and RR of AEs and 95% CI were calculated with a random effects model.
Abbreviations: AE/s, adverse event/s; ALT, alanine aminotransferase; AST, aspartate aminotransferase; RR, relative risk.
To determine the specific contribution of regorafenib and exclude confounding factors, we determined the RR of AEs in patients assigned to regorafenib vs controls. The overall RR of all-grade AEs with regorafenib was 1.66 (95% CI, 1.45–1.91; P<0.00001) by using a random effects model. For any all-grade AE, the RR of the AEs with regorafenib was statistically >1 (all P-values <0.05). These findings suggest a significantly increased risk of AEs associated with regorafenib relative to the scenario in the control group. By using a fixed effects model, regorafenib group has a statistically significant increase in the risk of high-grade AEs which corresponded to an overall RR of 3.73 (95% CI, 3.04–4.59; P<0.00001). For clinical AEs, a statistically significant increase was found in the risk of high-grade AEs, including HFSR, hypertension, fatigue, rash or desquamation, diarrhea, and oral mucositis, with respect to the situation in the control. The RR of laboratory abnormalities in terms of high-grade AEs, including hypophosphatemia, hyperbilirubinemia, thrombocytopenia, and anemia, was markedly increased compared with that in the control group.
RR of AEs based on subgroup analyses
To explore the possible reasons that attribute to the heterogeneity between studies, we conducted the following subgroup analyses: prior MKI treatment vs no prior MKI treatment, colorectal cancer (CRC) vs other malignancies (non-CRC), as shown in Tables 4 and 5. In these included trials, the patients with advanced GIST and HCC received the treatment of other MKIs, such as sunitinib or sorafenib, before regorafenib. To determine whether prior MKI exposure influenced the RR of AEs with regorafenib, we performed a subgroup analysis based on prior MKI treatment. The risk of all-grade anorexia among patients with prior MKI treatment was significantly increased with an RR of 3.46 (95% CI, 2.13–5.63). For patients without prior MKI treatment, the RR of all-grade anorexia was 1.90 (95% CI, 1.47–2.46). There was a significantly increased risk of all-grade anorexia for patients with prior MKI treatment (P=0.03). However, for patients with prior MKI treatment, the RR of increased AST was 0.98 (95% CI, 0.47–2.07) in terms of high-grade AEs and the RR among patients without prior MKI treatment was 9.22 (95% CI, 1.25–68.19). There was a statistically significant increase in the risk of high-grade increased AST for patients without prior MKI treatment (P=0.04). To determine whether the tumor type influenced the RR of AEs associated with regorafenib, we performed a subgroup analysis of CRC (two trials) vs all other malignancies. However, no significant differences were found when comparing the RR of all-grade and high-grade AEs in patients with CRC or non-CRC.
Table 4.
RR of all-grade AEs associated with regorafenib based on the subgroup analysis
| AEs | Prior MKI exposure (95% CI) | Tumor type (95% CI) | ||||
|---|---|---|---|---|---|---|
| Yes (2 trials) | No (3 trials) | P-value | CRC (2 trials) | Non-CRC (4 trials) | P-value | |
| Any AE | 1.61 (1.30–2.00)a | 1.76 (1.27–2.45)a | 0.65 | 1.76 (1.27–2.45)a | 1.61 (1.30–2.00)a | 0.65 |
| Clinical AE | ||||||
| Hand–foot skin reaction | 5.78 (3.07–10.87)a | 11.77 (4.27–32.47)a | 0.24 | 8.92 (3.43–23.18)a | 7.25 (3.25–16.16)a | 0.74 |
| Fatigue | 1.50 (1.15–1.95) | 1.73 (1.40–2.14) | 0.40 | 1.73 (1.40–2.14) | 1.50 (1.15–1.95) | 0.40 |
| Diarrhea | 4.34 (2.83–6.66) | 4.64 (3.23–6.68) | 0.81 | 4.24 (2.82–6.39) | 4.76 (3.26–6.93) | 0.69 |
| Hypertension | 3.84 (2.49–5.93) | 4.34 (2.96–6.37) | 0.68 | 4.77 (2.99–7.61) | 3.70 (2.57–5.31) | 0.40 |
| Voice changes | 3.50 (0.82–14.95) | 5.31 (3.14–9.00) | 0.60 | 5.31 (3.14–9.00) | 3.50 (0.82–14.95) | 0.60 |
| Anorexia | 3.46 (2.13–5.63) | 1.90 (1.47–2.46) | 0.03 | 1.95 (1.43–2.65) | 2.67 (1.89–3.77) | 0.18 |
| Oral mucositis | 4.67 (2.49–8.76) | 6.30 (3.90–10.17) | 0.46 | 7.65 (3.96–14.76) | 4.59 (2.89–7.29) | 0.21 |
| Rash or desquamation | 6.00 (1.46–24.62) | 6.53 (3.59–11.86) | 0.91 | 6.53 (3.59–11.86) | 6.00 (1.46–24.62) | 0.91 |
| Myalgia | 1.50 (0.63–3.60) | 1.57 (1.00–2.47) | 0.93 | 7.55 (0.44–130.35) | 1.42 (0.95–2.13) | 0.26 |
| Hoarseness | 9.20 (3.20–26.46) | 13.50 (1.87–97.25) | 0.74 | 13.50 (1.87–97.25) | 9.20 (3.20–26.46) | 0.74 |
| Nausea | 1.64 (1.00–2.68) | 1.30 (0.86–1.96) | 0.48 | 1.30 (0.86–1.96) | 1.64 (1.00–2.68) | 0.48 |
| Weight loss | 4.64 (1.43–15.12) | 5.82 (2.56–13.22) | 0.76 | 5.82 (2.56–13.22) | 4.64 (1.43–15.12) | 0.76 |
| Alopecia | 15.50 (2.16–111.07) | 18.22 (2.51–132.10) | 0.91 | 18.22 (2.51–132.10) | 15.50 (2.16–111.07) | 0.91 |
| Laboratory abnormalities | ||||||
| Hyperbilirubinemia | 5.16 (2.42–11.00) | 5.31 (2.74–10.28) | 0.96 | 5.31 (2.74–10.28) | 5.16 (2.42–11.00) | 0.96 |
| Increased AST | 1.65 (0.95–2.87) | 2.67 (1.17–6.07) | 0.34 | 2.67 (1.17–6.07) | 1.65 (0.95–2.87) | 0.34 |
| Increased ALT | 1.87 (0.87–4.01) | 3.20 (1.31–7.84) | 0.37 | 3.20 (1.31–7.84) | 1.87 (0.87–4.01) | 0.37 |
Note:
The RR of AEs and 95% CI were calculated with a random effects model.
Abbreviations: AE/s, adverse event/s; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRC, colorectal cancer; MKI, multikinase inhibitor; RR, relative risk.
Table 5.
RR of high-grade AEs associated with regorafenib based on the subgroup analysis
| AEs | Prior MKI exposure (95% CI) | Tumor type (95% CI) | ||||
|---|---|---|---|---|---|---|
| Yes (2 trials) | No (4 trials) | P-value | CRC (2 trials) | Non-CRC (4 trials) | P-value | |
| Any AE | 3.58 (2.64–4.87) | 3.86 (2.92–5.11) | 0.73 | 3.86 (2.92–5.11) | 3.58 (2.64–4.87) | 0.73 |
| Clinical AE | ||||||
| Hand–foot skin reaction | 25.07 (5.01–125.38) | 34.44 (8.43–140.69) | 0.77 | 35.55 (7.13–177.11) | 26.05 (6.43–105.56) | 0.77 |
| Hypertension | 5.11 (2.51–10.40) | 6.77 (3.16–14.51) | 0.60 | 6.42 (2.35–17.59) | 5.63 (3.08–10.31) | 0.83 |
| Fatigue | 4.04 (1.34–12.16) | 1.55 (0.91–2.63) | 0.12 | 1.55 (0.91–2.63) | 4.04 (1.34–12.16) | 0.12 |
| Rash or desquamation | 3.53 (0.18–67.28) | 18.22 (2.51–132.21) | 0.36 | 18.22 (2.51–132.21) | 3.53 (0.18–67.28) | 0.36 |
| Diarrhea | 8.69 (1.16–64.80) | 4.86 (1.86–12.65) | 0.61 | 6.23 (1.94–20.06) | 4.73 (1.32–17.00) | 0.76 |
| Anorexia | 10.86 (0.64–184.42) | 1.06 (0.56–2.01) | 0.12 | 1.18 (0.51–2.74) | 1.67 (0.70–4.00) | 0.57 |
| Oral mucositis | 1.53 (0.31–7.51) | 12.99 (1.65–102.14) | 0.11 | 15.72 (0.94–261.60) | 2.74 (0.74–10.13) | 0.27 |
| Myalgia | 1.51 (0.06–36.60) | 1.68 (0.47–6.08) | 0.95 | 1.51 (0.06–36.61) | 1.68 (0.47–6.08) | 0.95 |
| Laboratory abnormalities | ||||||
| Increased AST | 0.98 (0.47–2.07) | 9.22 (1.25–68.19) | 0.04 | 8.56 (0.50–146.17) | 1.40 (0.70–2.80) | 0.22 |
| Hypophosphatemia | 9.29 (1.25–69.06) | 9.66 (3.03–30.78) | 0.97 | 9.60 (1.87–49.28) | 9.53 (2.68–33.89) | 0.99 |
| Hyperbilirubinemia | 3.23 (1.14–9.13) | 3.19 (0.95–10.68) | 0.99 | 3.19 (0.95–10.68) | 3.23 (1.14–9.13) | 0.99 |
| Increased ALT | 2.06 (0.44–9.63) | 2.55 (0.83–7.88) | 0.83 | 9.57 (0.57–162.00) | 1.65 (0.62–4.41) | 0.25 |
| Lipase increase | NA | 2.17 (0.42–11.10) | NA | 3.00 (0.37–24.42) | 1.03 (0.07–16.27) | 0.55 |
| Thrombocytopenia | 8.79 (0.51–151.57) | 5.61 (1.28–24.61) | 0.78 | 6.23 (1.19–32.72) | 6.36 (0.75–54.06) | 0.99 |
| Anemia | 3.10 (0.38–25.54) | 6.70 (1.50–29.96) | 0.56 | 8.60 (1.15–64.34) | 3.54 (0.78–16.04) | 0.49 |
Abbreviations: AE/s, adverse event/s; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRC, colorectal cancer; MKI, multikinase inhibitor; NA, not applicable; RR, relative risk.
Quality of studies
The trials included in the meta-analysis were assessed in terms of study quality by using the seven-point Jadad ranking system. All trials were randomized, double blinded, and placebo controlled. AEs, as defined by the CTCAE version 3.0, 4.0, or 4.03 criteria, were the primary endpoint of this meta-analysis and were reported in all included studies. The follow-up time was adequate for each trial. The Jadad scores for each trial are listed in Table 1, and the mean score was 5, indicating that the overall study quality is fair.
Publication bias
No evidence of publication bias was detected for incidence or RR of AEs (all grade and high grade) by the Egger’s test (incidence: Egger’s test, P=0.46; RR: Egger’s test, P=0.51).
Discussion
To the best of our knowledge, this study is the first to conduct a meta-analysis to systematically investigate the incidence and RR of AEs associated with regorafenib in patients with advanced solid tumors. Regorafenib has been proven efficacious in prolonging the progression-free survival and overall survival of patients with advanced solid tumors, including mCRC, GIST, HCC, GC, and soft tissue sarcoma. However, the application of regorafenib has also resulted in a series of unique side effect profiles, including HFSR, stomatitis, diarrhea, hypertension, liver abnormalities, and fatigue.10,19,20 Although Phase II and III clinical trials have suggested that regorafenib is generally tolerable, and most of its AEs are manageable. Up to 95% of patients may suffer from regorafenib-related AEs.14 Given that these AEs are relatively common, it is important that clinicians are able to recognize and manage these AEs to reduce the impact of AEs on patients. This meta-analysis showed the AE profile of regorafenib in a broad cancer population. Our meta-analysis demonstrated a 1.66-fold increase in the risk of all-grade AEs in patients with advanced solid tumors receiving regorafenib compared with the control; the most common AEs are HFSR (54%), diarrhea (33%), fatigue (32%), hypertension (31%), oral mucositis (28%), and anorexia (23%). A significantly increased RR of all-grade AEs with regorafenib was observed compared with the control group (all P-values <0.05). In addition, high-grade AEs were noted in 54% of the patients treated with regorafenib, with an RR of 3.73 (95% CI, 3.04–4.59, P<0.00001). HFSR (16%), hypertension (13%), fatigue (6%), increased AST (6%), and hypophosphatemia (6%) were the most frequently encountered events. These results are consistent with previous toxicity profiles across all related clinical trials.3–8
For any grade of AEs, alopecia achieved the highest RR of 16.86 (95% CI, 4.17–68.14, P<0.0001), followed by hoarseness (RR, 10.16 [95% CI, 4.00–25.78, P<0.00001]), HFSR (RR, 7.42 [95% CI, 4.61–11.94, P<0.00001]), rash or desquamation (RR, 6.48 [95% CI, 3.66–11.48, P<0.00001]), and oral mucositis (RR, 5.66 [95% CI, 3.87–8.29, P<0.00001]). For high-grade AEs, the study of HFSR events presented the highest RR of 30.28 (95% CI, 10.51–87.22, P<0.00001), followed by rash or desquamation (RR, 13.32 [95% CI, 2.62–67.68, P=0.002]), hypophosphatemia (RR, 9.56 [95% CI, 3.50–26.09, P<0.0001]), thrombocytopenia (RR, 6.28 [95% CI, 1.69–23.29, P=0.006]), and hypertension (RR, 5.85 [95% CI, 3.48–9.84, P<0.00001]). Despite the fact that these AEs are not life threatening, they may negatively influence the treatment adherence and quality of life of patients. Currently, no national or international guidelines are available for treating regorafenib-associated AEs. Hence, understanding the risk, appropriate preventive actions, and dose modifications are important for patients and clinicians to reduce the incidence of AEs and improve drug compliance.
Two recently published meta-analyses indicated a significantly increased risk of HFSR and hypertension associated with the use of regorafenib in solid tumors.21,22 By using data on patients treated with regorafenib in Phase II and III trials, Belum et al21 found that the incidence of HFSR is 60.5% at any grade and 20.4% at a high grade. The RR of high-grade HFSR is 41.99 (RR, 41.99, 95% CI, 5.88–299.93) for all cancer types. Wang et al22 reported that the incidence of all- and high-grade hypertension is 44.4% and 12.5%, respectively. The RR of high-grade hypertension with regorafenib is 8.39 (95% CI, 3.10–22.71). However, a decreased incidence and RR of HFSR (all-grade IR, 54%; high-grade IR, 16%; RR, 30.28) and hypertension (all-grade IR, 31%; high-grade IR, 13%; RR, 5.85) were observed in our study. The discrepancy can be attributed to the difference in the number of trials enrolled. In the study by Wang et al, a single-group Phase II trial of patients with RCC was included in the meta-analysis, and their results indicated that the incidence of regorafenib-related HFSR and hypertension was higher among RCC patients than among patients with other tumor types. For RCC, most patients experienced nephrectomy and renal dysfunction, resulting in increased baseline blood pressure (BP) and toxicity.12 Several previous studies have also suggested that the risk of regorafenib-related AEs varies among tumor types.15 To determine whether the tumor type influenced the RR of AEs associated with regorafenib, we performed a subgroup analysis of CRC (the most commonly occurring malignancy in two trials) vs all other malignancies. However, subgroup analysis showed that no significant differences were found when comparing the RR of all-grade and high-grade AEs in patients with CRC or non-CRC. In addition, prior exposure to other MKIs, such as sunitinib or sorafenib, may influence the incidence of AEs. As a result, we performed a subgroup analysis based on prior MKI exposure. According to the results of subgroup analysis, there was a significantly increased risk of all-grade anorexia for patients with prior MKI treatment when compared with those without prior MKI treatment (P=0.03). However, there was a statistically significant decrease in the RR of increased AST for patients with prior MKI treatment when compared with those without prior MKI treatment in terms of high-grade AEs (P=0.04). These findings indicate that prior MKI treatment is linked to an increased incidence of all-grade anorexia and a reduced incidence of high-grade increased AST. The reason underlying this symptom is not yet known. In addition, the RR of the remaining AEs between exposed and unexposed groups did not show significant difference, which suggests that the majority of toxicities associated with MKI are not cumulative and can be handled by supportive treatment and dose modification.
Our study presents several potential limitations. First, this meta-analysis was performed at the study level and not on the patient level. Hence, confounding variables were not incorporated into the analysis. Second, the trials included in the meta-analysis employed different versions of NCI-CTCAE to grade AE, which could contribute to variability in some AEs grading, such as hypertension and rash or desquamation. According to the CTCAE version 4.0 or 4.03, high-grade (grade ≥3) hypertension is characterized by systolic BP >160 mmHg or diastolic BP ≥100 mmHg. However, high-grade hypertension is defined as BP exceeding 150/100 mmHg and requires antihypertensive treatment or more intensive therapy based on the CTCAE version 3.0. Macules or papules covering ≥50% of body surface area (BSA) are defined as high-grade rash or desquamation according to the CTCAE version 3.0, whereas covering >30% are high grade according to the CTCAE version 4.0 or 4.03. Third, a further subgroup analysis based on each tumor type was not conducted in our study because only one trial in most types of malignancies is available. Fourth, our results were observed in the academic setting and may not absolutely reflect the prevailing clinical scenario.
In conclusion, regorafenib is a relatively safe and effective agent in the treatment of advanced solid tumors that have failed to respond adequately to all other standard therapies. This meta-analysis provided an adequate estimate of regorafenib-reduced AEs and revealed an increased RR in all- and high-grade AEs for patients treated with regorafenib. As a consequence, clinicians and patients should pay great attention to these regorafenib-related AEs. Proper education, monitoring, and management of AEs are important to reduce the incidence of AEs and improve drug compliance.
Ethics statement
This article does not contain any studies with human participants performed by any of the authors. Informed consent was obtained from each individual participant included in the study.
Acknowledgments
The authors gratefully acknowledge all the staff of the Department of Gastrointestinal Surgery and Pathology, West China Hospital, who generously provided assistance in the collection of data throughout the duration of the study. All authors disclosed no financial funding relevant to this publication. This study was funded by the National Natural Science Foundation of China (grant agreement number, 81572931).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Davis SL, Eckhardt SG, Messersmith WA, Jimeno A. The development of regorafenib and its current and potential future role in cancer therapy. Drugs Today. 2013;49(2):105–115. doi: 10.1358/dot.2013.49.2.1930525. [DOI] [PubMed] [Google Scholar]
- 2.Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129(1):245–255. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 3.Grothey A, Van Cutsem E, CORRECT Study Group et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 4.Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID). An international, multicentre, randomised, placebo-controlled, phase 3 trial. The Lancet. 2013;381(9863):295–302. doi: 10.1016/S0140-6736(12)61857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruix J, Qin S, RESORCE Investigators et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Qin S, CONCUR Investigators et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16(6):619–629. doi: 10.1016/S1470-2045(15)70156-7. [DOI] [PubMed] [Google Scholar]
- 7.Mir O, Brodowicz T, Italiano A, et al. Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016;17(12):1732–1742. doi: 10.1016/S1470-2045(16)30507-1. [DOI] [PubMed] [Google Scholar]
- 8.Pavlakis N, Sjoquist KM, Martin AJ, et al. Regorafenib for the Treatment of Advanced Gastric Cancer (INTEGRATE): A Multinational Placebo-Controlled Phase II Trial. J Clin Oncol. 2016;34(23):2728–2735. doi: 10.1200/JCO.2015.65.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hellmann MD, Sturm I, Trnkova ZJ, et al. Preliminary Safety, Pharmacokinetics, and Efficacy of Regorafenib, Cisplatin, and Pemetrexed in Patients With Advanced Nonsquamous Non-Small-Cell Lung Cancers. Clin Lung Cancer. 2015;16(6):514–522. doi: 10.1016/j.cllc.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones RL, Bendell JC, Smith DC, et al. A phase I open-label trial evaluating the cardiovascular safety of regorafenib in patients with advanced cancer. Cancer Chemother Pharmacol. 2015;76(4):777–784. doi: 10.1007/s00280-015-2827-3. [DOI] [PubMed] [Google Scholar]
- 11.Vasista A, Martin A, Pavlakis N, et al. Accuracy and prognostic significance of oncologists’ estimates and scenarios for survival time in a randomized phase 2 trial of regorafenib in advanced gastric cancer[J] Asia-Pacific Journal of Clinical Oncology. 2017;13:47–48. [Google Scholar]
- 12.Zaki K, Aslam S, Eisen T, Regorafenib ET. Regorafenib (BAY 73-4506): stromal and oncogenic multikinase inhibitor with potential activity in renal cell carcinoma. Curr Oncol Rep. 2013;15(2):91–97. doi: 10.1007/s11912-013-0292-x. [DOI] [PubMed] [Google Scholar]
- 13.Kirchner H, Strumberg D, Bahl A, Overkamp F. Patient-based strategy for systemic treatment of metastatic renal cell carcinoma. Expert Rev Anticancer Ther. 2010;10(4):585–596. doi: 10.1586/era.10.25. [DOI] [PubMed] [Google Scholar]
- 14.de Wit M, Boers-Doets CB, Saettini A, et al. Prevention and management of adverse events related to regorafenib. Support Care Cancer. 2014;22(3):837–846. doi: 10.1007/s00520-013-2085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mclellan B, Ciardiello F, Lacouture ME, Segaert S, van Cutsem E. Regorafenib-associated hand-foot skin reaction: practical advice on diagnosis, prevention, and management. Ann Oncol. 2015;26(10):2017–2026. doi: 10.1093/annonc/mdv244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 19.Han G, Qin S, Song T, et al. Efficacy and safety of regorafenib (REG) versus placebo (PBO) in Chinese patients with hepatocellular carcinoma (HCC) progressing on sorafenib (SOR): Subgroup analysis of the international, randomized phase 3 RESORCE trial[J] Hepatology International. 2017;11(1 Supplement 1):S9–S10. [Google Scholar]
- 20.Salazar Bravo M, Gutierrez Zuniga L, Rodriguez Goicoechea M, et al. Effectiveness of regorafenib in the treatment of metastatic colorectal cancer in selected patients[J] European Journal of Hospital Pharmacy. 2016;23:A103. [Google Scholar]
- 21.Belum VR, Wu S, Lacouture ME. Risk of hand-foot skin reaction with the novel multikinase inhibitor regorafenib: a meta-analysis. Invest New Drugs. 2013;31(4):1078–1086. doi: 10.1007/s10637-013-9977-0. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Xu J, Nie W, et al. Risk of hypertension with regorafenib in cancer patients: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2014;70(2):225–231. doi: 10.1007/s00228-013-1598-1. [DOI] [PubMed] [Google Scholar]