Abstract
Background
Providing high‐quality care for patients with Parkinson's disease (PD) involves addressing both motor and nonmotor features. We describe the implementation and evaluation of a 2‐day, interdisciplinary Comprehensive Care Clinic (CCC) for patients with PD.
Methods
Patients who attended the CCC between January 2010 and July 2013 were matched by sex and age with patients who were evaluated in specialist care during the same time frame. Provider documentation of the American Academy of Neurology (AAN) quality measures for PD and Unified Parkinson's Disease Rating Scale (UPDRS) motor scores were compared between specialist and CCC visits at baseline and at 12 months.
Results
Ninety‐five patients participated in the CCC (60% men; 75% white; mean age, 68 years; age range, 38–97 years). Of these, 29 patients were matched to specialist care patients based on the availability of 12‐month follow‐up data. Both groups were similar with respect to race, marital status, years with PD, and baseline UPDRS motor scores. On average, patients who received CCC care met 10 of 10 AAN quality measures, whereas those who received specialist care met only 5 of 10 quality measures (P < 0.001) over 12 months. At 12‐months, there were no significant differences in UPDRS motor scores between the groups (P = 0.5).
Conclusions
According to the AAN quality measures, the CCC provided higher quality care than the gold standard of specialty care. A randomized controlled trial of the CCC model is warranted to determine its impact on patient‐centered outcomes and to assess whether the standard model of care should be altered.
Keywords: multidisciplinary care, nonmotor, Parkinson's disease, quality of care
Over the last 2 decades, it has become increasingly clear that Parkinson's disease (PD) is more than just a motor disorder or a dopaminergic disease.1 Nonmotor features, including psychiatric disorders, cognitive decline, sleep disorders, and dysautonomia,2 are more closely correlated with quality of life than classical motor symptoms.3, 4 Furthermore, worse quality of life is associated with significant caregiver strain5 and more frequent hospitalizations.6 Guidelines for therapy of nonmotor features have been published,7, 8 and validated questionnaires have been developed.2, 9 In addition, quality measures for the care of PD highlight the need to address both motor and nonmotor conditions associated with the disease.10, 11, 12 Despite the general focus of quality measures on nonmotor features, these symptoms are still frequently unrecognized, under‐diagnosed, and untreated.13, 14, 15
The current mode of health care delivery in the United States, which occurs through neurology clinics with referral to other subspecialists, results in fragmented, uncoordinated, inconvenient, and often less than optimal care.16 Optimal care for PD should bring together the necessary multidisciplinary expertise in a coordinated and timely manner. Recent studies demonstrate the effectiveness of multidisciplinary care for PD in health systems in Canada and the Netherlands.17, 18 To enhance health care delivery and quality for patients with PD and improve the identification and treatment of nonmotor symptoms, a university‐based movement disorder program implemented an interdisciplinary approach to PD care termed the Comprehensive Care Clinic (CCC). Here, we describe program implementation and a comparison of the quality of PD care received in the CCC versus specialist care.
Patients and Methods
The CCC was established in a university‐based movement disorder program, including 16 movement disorder neurologists and 2 nurse practitioners, that accommodates over 11,000 visits annually and, in 2015, provided care for over 2200 patients with PD, who represent approximately 50% of the total clinic population. Specialist care for PD in the movement disorder clinical program, as in others, involves the referral to other specialists and evaluation of motor and nonmotor symptoms at the discretion of the clinic neurologist. A major goal of the movement disorder program was to develop a patient‐centered comprehensive care program that crossed department boundaries and brought interdisciplinary care to the individual living with PD. We hypothesized that this would improve outcomes in patient care and quality of life by making care more patient‐centered, coordinated, and timely.
Development of the PD CCC Team
Discussions on the development of the CCC for PD began in earnest in 2005 with the development of a written document prepared by a small committee consisting of clinicians from multiple disciplines. The key programs (rehabilitation medicine, psychiatry, sleep medicine, geriatric medicine, neurology, and neuropsychology) were located in the same complex. After further programmatic adjustments, a pilot program involving 5 patients was completed in January and February of 2010. Over the ensuing 2 months, with input from an advisory patient and caregiver couple committed to the development of such a program, the 5 participating patients, and the clinic team, we made the final 2 changes, including: (1) adjusting the neuropsychology battery and (2) adding a geriatrician with special interest in autonomic dysfunction.
PD CCC Implementation
The CCC was initiated in April 2010. During the 2‐day evaluation, the patient and caregiver meet with providers in the following specialties: sleep medicine, psychiatry, geriatrics, neuropsychology, speech and language therapy, occupational therapy, physical therapy, social services, and the movement disorder program. Sleep studies and laboratory and imaging evaluations are ordered, if needed. All potential patient referrals come through the program nurse coordinator. Patients may self‐refer or may be referred by their primary care clinician or neurologist. Once a PD diagnosis is confirmed by medical records review and an appointment date is determined, each member of the CCC team receives electronic notification. Each department then schedules the patient in a specifically selected time slot and retains insurance approval. During the final appointment of the 2‐day visit, the movement disorder neurologist develops a comprehensive care plan in discussion with the patient and caregiver/family. This report is also sent to the referring physician and the primary care physician.
All services are provided to each patient, i.e., there is no opt‐out option if a patient does not feel that a particular discipline's evaluation is needed. Because of the high prevalence of nonmotor symptoms throughout the disease course, and because nonmotor symptoms may be overlooked or denied by the patient and family, the team recommends that all patients and families have an evaluation with each discipline from both a diagnostic perspective and a patient education and empowerment perspective. Most services are covered under private insurance or Medicare/Medicaid (federally funded US health insurance programs); however, patients have paid out‐of‐pocket expenses to be seen by every discipline. Total out‐of‐pocket expenses for the entire 2‐day evaluation could reach approximately $2500 (not including additional tests, such as polysomnography or magnetic resonance imaging), depending on the assessments ordered as part of the evaluation.
Program Evaluation and Statistical Analysis
A retrospective chart review and descriptive statistics were used to characterize the first 95 participants (January 2010 to July 2013) who were evaluated in the CCC.
Standard Protocol Approval
To perform the matched‐pairs analysis, we received approval from the Emory University Institutional Review Board to conduct a retrospective chart review and were granted a waiver of consent and complete Health Insurance Portability and Affordability Act waiver. Participants were included in the matched analysis if they were seen in the PD CCC between January 2010 and July 2013 and had a 12 ± 2 month follow‐up evaluation in the clinic (n = 29 participants). Control patients were randomly selected from the health system corporate data warehouse based on a specialist clinic encounter carried out between January 2010 and July 2013 (n = 2794 patients) and were matched with CCC patients by sex and age (within 2 years). All patients had a clinical diagnosis of PD, as defined with conventional criteria by a neurologist who had additional specialty training in movement disorders. Patients who were seen in specialist care clinic by the movement disorder neurologist who staffs the CCC were excluded from the pool of patients who were available for matching. Patients were also excluded from analysis if they did not have a follow‐up visit in the university‐based movement disorder clinic at 12 months (±2 months). During the matching process, the first control participant who met all inclusion criteria on an alphabetical list was assigned. Of the 2794 potential controls, 342 patient records were reviewed to achieve a total of 29 matched pairs. For CCC patients, the follow‐up visit was a regularly scheduled visit with their movement disorders neurologist in the university‐based clinic and did not involve the multidisciplinary team.
Chart review and data collection were conducted by a research associate who was not involved in the CCC. The research associate evaluated provider notes to determine documentation of an assessment for each of the American Academy of Neurology (AAN) quality measures for PD based on the 10 indicators of the 2009 recommendations, which were available at the time of the study.11 The AAN quality measures include the following (at least annually): (1) PD diagnosis review, (2) assessment of psychiatric disorders or disturbances, (3) assessment of cognitive dysfunction, (4) query about autonomic symptoms, (5) query about sleep disturbance, (6) query about falls at every appointment, (7) discussion of rehabilitation therapy options, (8) counseling regarding PD‐related safety issues, (9) query about motor complications, and (10) a review of PD medical and surgical treatments. Motor scores for the Unified Parkinson's Disease Rating Scale (UPDRS) were collected for each patient. Individual item scores were imputed based on the average of the completed fields if 2 or fewer single‐item scores were missing; otherwise, these were considered missing data. Descriptive statistics were used to summarize the baseline clinical characteristics of each group. Assessment of change over time in UPDRS scores was evaluated using a repeated‐measures analysis of variance with group (CCC patients vs. controls) as the between‐subjects factor, and time (baseline vs. follow‐up) as the within‐subjects factor. The difference in the average number of AAN quality measures assessed between groups was evaluated with a standard t test. Analyses were conducted using the SAS 9.4 statistical package (SAS Institute, Cary, NC).
Results
Characteristics of the first 95 participants to complete the CCC are detailed in Table 1. Participants generally reflected demographic trends for PD, with 60% men, a mean age of 68 years, 90% were taking levodopa at the initial CCC visit, and the average duration of PD was 7.6 years. We observed that nonmotor features were quite common. Overall, 33% of patients were considered cognitively normal after the neuropsychological assessment, and 26% were diagnosed with dementia. More than one‐half were diagnosed with depression, and only 20% had no evidence of a psychiatric condition. Ninety‐eight percent reported a sleep condition, with insomnia and excessive daytime sleepiness being the most prominent. Nearly two‐thirds had constipation, and more than 40% reported bothersome nocturia (getting up at night to void). Rehabilitation therapy was frequently recommended, and only 13% of CCC participants did not receive a recommendation to consider physical, occupational, or speech therapy.
Table 1.
Baseline characteristics (n = 95) of participants who completed the Parkinson's disease Comprehensive Care Clinic (January 2010 to July 2013)
| Characteristic | No. (%) or mean ± SD |
|---|---|
| All patients | 95 (100) |
| Sex | |
| Men | 57 (60.0) |
| Women | 38 (40.0) |
| Race | |
| Caucasian or white | 71 (74.7) |
| African‐American or black | 12 (12.6) |
| Unreported or unavailable | 12 (12.6) |
| Marital status | |
| Single | 24 (25.3) |
| Married | 67 (70.5) |
| Unreported or unavailable | 4 (4.2) |
| Medications at CCC visit | |
| l‐dopa | 86 (90.5) |
| Dopamine agonist | 30 (31.6) |
| COMT inhibitor | 13 (13.7) |
| MAO B blocker | 13 (13.7) |
| Amantadine | 6 (6.3) |
| Acetylcholinesterase inhibitor | 12 (12.6) |
| On none of these | 3 (3.2) |
| Age, y | 68.28 [38–97], n = 95 |
| Duration of PD, y | 7.63 [0–22], n = 91 |
| UPDRS‐III score | 23.84 [3–72], n = 82 |
| MOCA score | 22.85 [3–30], n = 72 |
SD, standard deviation; l‐dopa, levodopa; COMT, catechol‐O‐methyl transferase; MAO, monoamine oxidase B; PD, Parkinson's disease; UPDRS‐III, Unified Parkinson's Disease Rating Scale, motor part; MOCA, Montreal Cognitive Assessment.
The matched analysis included a smaller number of CCC participants (n = 29) who had a follow‐up appointment in the same university‐based clinic 12 ± 2 months after the CCC evaluation index date. These participants were representative of the larger CCC cohort with regard to demographics (Table 2) and were matched by age (within 2 years) and sex with patients who were seen during the same index period in specialist care and also had a 12 ± 2 months follow‐up visit.
Table 2.
Baseline characteristics of participants included in the matched analysis (n = 58)
| Characteristic | Mean ± SD or no. (%) | P valuea | |
|---|---|---|---|
| Specialist care, n = 29 | PD CCC, n = 29 | ||
| Age at evaluation, y | 66.2 ± 8.6 | 67 ± 9.1 | 0.7 |
| Men | 17 (58.6) | 17 (58.6) | 1.0 |
| Race/ethnicity | 0.4 | ||
| White | 23 (79.3) | 21 (72.4) | |
| Black | 1 (3.4) | 4 (13.8) | |
| Other or unreported | 5 (17.2) | 4 (13.8) | |
| Marital status | 0.3 | ||
| Married | 21 (72.4) | 24 (82.8) | |
| Single/widowed/divorced | 8 (27.6) | 5 (17.2) | |
| Time since PD diagnosis, y | 8.4 ± 7.2 | 6.3 ± 6.1 | 0.2 |
| DBS present | 5 (17.2) | 3 (10.3) | 0.4 |
| UPDRS‐III score | 23.5 ± 13.2 | 21.0 ± 11.7 | 0.4 |
PD, Parkinson's disease; CCC, Comprehensive Care Clinic SD, standard deviation; DBS, deep‐brain stimulation; UPDRS‐III, Unified Parkinson's Disease Rating Scale, motor part.
P values were determined with the t test or the χ2 test, as appropriate.
On average, patients who were seen in the CCC program received an assessment for all 10 AAN quality measures (mean ± standard deviation [SD], 9.9 ± 0.3 quality measures), whereas patients who were seen in specialist care received an assessment for 5 of the quality measures (mean ± SD, 4.5 ± 1.4 quality measures; P < 0.001 for the between group‐difference) (Fig. 1). Because some of the quality measures are recommended only annually, an evaluation of chart documentation from a 12‐month follow‐up visit was conducted. At 12 months, on average, patients in specialist care received an assessment of the same 5 quality measures (confirmation of diagnosis, review of medical and surgical treatment options, mood, cognition, motor complications, and falls were the most frequently performed) (Fig. 2).
Figure 1.
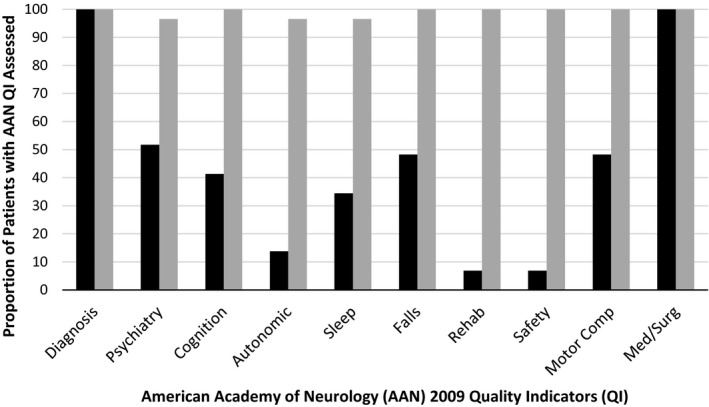
American Academy of Neurology quality‐improvement indicators for specialist care compared with Parkinson's disease interdisciplinary Comprehensive Care Clinic (PD CCC) care at the index visit. Black bars indicate specialist; gray bars, PD CCC.
Figure 2.
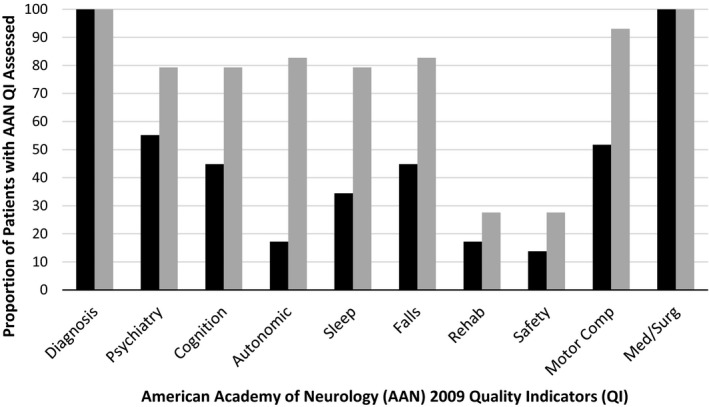
American Academy of Neurology quality‐improvement indicators for specialist care compared with Parkinson's disease interdisciplinary Comprehensive Care Clinic (PD CCC) care at the 12‐month follow‐up visit. Black bars indicate specialist; gray bars, PD CCC.
With regard to treatment recommendations for motor and selected nonmotor symptoms, CCC patients were more likely to be advised to adjust PD medications (19 of 29 CCC patients vs. 10 of 29 controls; P = 0.05) during the baseline visit. There was no significant difference between the groups in adjustments to PD medications based on a review of the 12‐month visit notes (10 of 29 CCC patients vs. 7 of 29 controls; P = 0.2). At the time of the baseline visit, there was no difference in the proportion of patients who reported an existing prescription for an antidepressant (14 of 29 CCC patients vs. 13 of 29 controls; P = 0.7) or an acetylcholinesterase inhibitor (4 of 29 in both groups). However, at the conclusion of the visit, CCC patients were more likely to receive a recommendation to adjust antidepressant medications (13 of 29 CCC patients vs. 1 of 29 controls; P < 0.0001) and acetylcholinesterase medications (9 of 29 CCC patients vs. 1 of 29 controls; P < 0.0001). There was no difference between the groups in the proportion that received a recommendation with regard to antidepressant or acetylcholinesterase therapy at 12 months when patients in both groups saw only a movement disorders provider. However, there was a trend for more patients who had been seen in the CCC to report a prescription for an acetylcholinesterase inhibitor 12 months later (9 of 29 CCC patients vs. 4 of 29 controls; P = 0.07).
A repeated‐measures analysis of variance revealed a significant main effect of time (F[1,46] = 4.18; P = 0.047) with UPDRS scores collapsed across groups at baseline (mean ± SD, 22.17 ± 12.34) worse than the scores at follow‐up (mean ± SD, 19.58 ± 11.32). However, there was no significant interaction of group and time (F[1,46] = 0.46; P = 0.50), indicating that the improvement in scores was comparable from baseline to follow‐up for both groups (CCC group: mean ± SD, from 21.04 ± 11.67 to 17.69 ± 9.32; control group: mean ± SD, from 23.5 ± 13.24 to 21.82 ± 13.18). The UPDRS evaluation was limited secondary to incomplete documentation of UPDRS scores in some provider notes in the specialist clinic. Although 26 of 29 CCC participants (90%) had documentation of both baseline and follow‐up UPDRS scores, 22 of 29 specialist patients (75%) had complete documentation.
Discussion
Compared with standard movement disorder specialist care, these preliminary data suggest that the CCC approach provided higher quality care, as assessed by the AAN quality measures for PD. Although many factors may contribute to less than optimal care for PD in movement disorder clinics, dysfunctional health care system models that disrupt care coordination between the provider and the patient/family are a major underlying cause.16, 19 PD is a complex neurodegenerative condition that encompasses a broad range of motor, autonomic, and neuropsychiatric symptoms. Visits with a movement disorder neurologist appear to focus primarily on medication and surgical treatment options, with fewer providers assessing motor complications, sleep, cognition, or mood. Autonomic symptoms were largely ignored despite research showing that bowel and bladder symptoms are common and correlate more highly with quality of life as PD progresses than motor symptoms.3
With advances in the treatment of PD motor symptoms, there is greater recognition of the burden of nonmotor features as PD progresses. Quality measures for PD care reflect the impact of nonmotor symptoms like cognitive decline, mood disturbance, impulse‐control disorder, sleep dysfunction, and autonomic symptoms.10, 11, 12 These measures encourage clinicians who are caring for patients with PD to incorporate assessment and initial management of motor and nonmotor symptoms with the goal of improving overall patient care and quality of life. Despite the availability of these measures and the emphasis on nonmotor symptoms in recent literature, adherence to guidelines and assessment of nonmotor symptoms remains low. Specifically, current models of care that rely on a single provider to address all of the motor and nonmotor symptoms of PD are unlikely to achieve high‐quality care according to quality metrics for PD care.10, 12 Several trials have documented the benefits of multidisciplinary care for PD, particularly for improving quality of life and nonmotor symptom burden.20 Our experience reported here describes a successful interdisciplinary assessment clinic for PD in a fee‐for‐service health system in the United States.
The goal of the PD CCC is to provide a comprehensive assessment and initial treatment plan for individuals with PD to address both motor and nonmotor symptoms of the disease. The CCC model has greater similarity to the Dutch model of PD care, because it involves several clinical disciplines (sleep medicine, geriatric psychiatry, geriatric medicine, neuropsychology) in addition to a movement disorder neurologist and rehabilitation therapists.18 However, in that model, the patients complete a questionnaire, and then their visit is tailored to their apparent needs. What has been shown is that few of those patients see a psychiatrist or sleep specialist. This begs the issue of under‐recognition of these features by both patients and physicians. Our experience, in which all patients see all specialties, confirms that all these problems are common and often require intervention. Hence, the fixed model seems to be a better approach. In addition, the entire clinical team meets at least once monthly to discuss previous treatment plans, avoiding any conflicting recommendations, and we learn from each other about the approach to management of specific symptoms in patients with PD.
Some might argue that an infrastructure to prompt screening for each of the quality measures, such as a brief questionnaire at triage, could improve quality of care and appropriately target interventions. However, screening alone does not enhance coordination of care among multiple providers or reduce the burden for patients and families of scheduling and attending multiple separate visits separated by months. Furthermore, in this scenario, practitioners often do not communicate, and patients often get conflicting recommendations. An advantage of the structure of the PD CCC is that every patient is given the opportunity to discuss these symptoms—even symptoms that may be perceived as embarrassing—with a provider who has expertise in managing nonmotor symptoms in PD. These practitioners communicate their findings and recommendations to each other, avoiding the conflicts that can arise.
On average, participants who were seen in the CCC were in the middle stage of PD and had an average disease duration of 7.6 years and an average UPDRS motor score of 23.8. However, the program is currently open to persons at all stages. Early stage patients and their families have found the program beneficial, because it helps them to understand early or prodromal nonmotor symptoms that might not have been attributed to PD and could be addressed with targeted interventions.
There are limitations to this evaluation. These preliminary results are from 1 US‐based institution and may not be generalizable outside of the US health care system. Despite this, these results are consistent with those reported by both the Canadian and Dutch programs. Most CCC participants have health insurance (either through private or federal health insurance). The availability of coverage could impact access to health care resources and health outcomes; however, because the control participants are from the same clinic setting, we expect the impact of health insurance coverage to be balanced between the groups in this analysis. Our results are retrospective and derived from a convenience sample based on CCC attendees who had follow‐up evaluations at 12 ± 2 months; however, the demographic distribution of the cohort mirrors that of the overall CCC population. The matching appears to be appropriate, because our specialist care patients are similar with respect to PD disease duration and severity. Although all clinical providers received specialty training in movement disorders, including assessment of PD motor symptom severity using the UPDRS, missing data in specialist clinician notes limited a comparison between groups of UPDRS scores. Finally, the difference in quality‐improvement metrics between the 2 groups could be impacted by the practice pattern of the CCC neurologist, who has become familiar with incorporating the quality‐improvement measures into daily practice.
Conclusions
Growing evidence supports an interdisciplinary model of care for PD that involves both a movement disorder neurologist and a team of providers who can address both motor and nonmotor symptoms of the disease.20 The CCC model provides a comprehensive 2‐day assessment and the development of an interdisciplinary treatment plan for individuals with PD. Our results provide preliminary evidence of higher quality care according to the AAN quality measures for the CCC model compared with specialist care alone. The CCC could serve as a viable complement to usual specialist visits for providing high‐quality, patient‐centered care for patients with PD. Additional evidence is needed through a randomized trial to evaluate this model of care compared with usual specialist care in order to influence policymakers and payment models. The cost effectiveness of such a program also deserves assessment to determine whether a comprehensive assessment leads to reduced health care spending later in the disease course.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
C.P.V.: 1A, 1B, 1C, 2A, 3A, 3B
L.P.P.: 1B, 3B
A.E.V.: 1B, 3B
F.C.G.: 1A, 2A, 3B
L.M.T.: 2A, 3B
A.P.H.: 2A, 3B
S.A.F.: 1A, 2A, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest: This study was funded by the Dan and Merrie Boone Foundation. Lindsay P. Prizer received support from the Dan and Merrie Boone Foundation for portions of this work. Stewart A. Factor is supported by the Sartain Lanier Family Foundation, National Institute of Neurological Disorders and Strokez (1 U10 NS077366‐01). The remaining authors report no conflicts of interest relevant to this work.
Financial Disclosures for the previous 12 months: Lynn Marie Trotti reports funds to her institution from Jazz Pharmaceuticals and Balance Therapeutics outside the scope of this work. Stewart A. Factor reports honoraria from Neurocrine, Lundbeck, Auspex/Teva, Avanir, Cynapsus, Adamas, and UCB; grants from Ipsen, Allergan, Medtronics, Auspex, US World Meds, Pharm‐Olam, Cynapsus Therapeutics, Solstice, the CHDI Foundation, the Michael J. Fox Foundation, and the National Institutes of Health; and royalties from Demos, Blackwell Futura (for textbooks), and UpToDate. The remaining authors report no sources of funding and no conflicts of interest.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson disease. Neurobiol Aging 2003;24:197–211. [DOI] [PubMed] [Google Scholar]
- 2. Martinez‐Martin P, Schapira AH, Stocchi F, et al. Prevalence of nonmotor symptoms in Parkinson's disease in an international setting; study using nonmotor symptoms questionnaire in 545 patients. Mov Disord 2007;22:1623–1629. [DOI] [PubMed] [Google Scholar]
- 3. Gallagher DA, Lees AJ, Schrag A. What are the most important nonmotor symptoms in patients with Parkinson's disease and are we missing them? Mov Disord 2010;25:2493–2500. [DOI] [PubMed] [Google Scholar]
- 4. Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson's disease? J Neurol Neurosurg Psychiatry 2000;69:308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oguh O, Kwasny M, Carter J, Stell B, Simuni T. Caregiver strain in Parkinson's disease: National Parkinson Foundation Quality Initiative Study. Parkinsonism Relat Disord 2013;19:975–979. [DOI] [PubMed] [Google Scholar]
- 6. Hassan A, Wu SS, Schmidt P, et al. High rates and the risk factors for emergency room visits and hospitalization in Parkinson's disease. Parkinsonism Relat Disord 2013;19:949–954. [DOI] [PubMed] [Google Scholar]
- 7. Zesiewicz TA, Sullivan KL, Arnulf I, et al. Practice parameter: treatment of nonmotor symptoms of Parkinson disease: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2010;74:924–931. [DOI] [PubMed] [Google Scholar]
- 8. Seppi K, Weintraub D, Coelho M, et al. The movement disorder society evidence‐based medicine review update: treatments for the non‐motor symptoms of parkinson's disease. Mov Disord 2011;26(Suppl 3):S42–S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Högl B, Arnulf I, Comella C, et al. Scales to assess sleep impairment in Parkinson's disease: critique and recommendations. Mov Disord 2010;25:2704–2716. [DOI] [PubMed] [Google Scholar]
- 10. Factor SA, Bennett AJ, Hohler AD, Wang D, Miyasaki JM. Quality improvement in neurology: Parkinson disease update quality measurement set: executive summary. Neurology 2016;86:2278–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng EM, Tonn S, Swain‐Eng R, et al. Quality improvement in neurology: AAN Parkinson disease quality measures: Report of the Quality Measurement and Reporting Subcommittee of the American Academy of Neurology. Neurology 2010;75:2021–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stewart DA. NICE guideline for Parkinson's disease. Age Ageing 2007;36:240–242. [DOI] [PubMed] [Google Scholar]
- 13. Shulman LM, Taback RL, Rabinstein AA, Weiner WJ. Non‐recognition of depression and other non‐motor symptoms in Parkinson's disease. Parkinsonism Relat Disord 2002;8:193–197. [DOI] [PubMed] [Google Scholar]
- 14. Weintraub D, Moberg PJ, Duda JE, Katz IR, Stern MB. Recognition and treatment of depression in Parkinson's disease. J Geriatr Psych Neurol 2003;16:178–183. [DOI] [PubMed] [Google Scholar]
- 15. Swarztrauber K, Graf E, Cheng E. The quality of care delivered to Parkinson's disease patients in the US Pacific Northwest Veterans Health System. BMC Neurol 2006;6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boult C, Wieland G. Comprehensive primary care for older patients with multiple chronic conditions: “nobody rushes you through”. JAMA 2010;304:1936–1943. [DOI] [PubMed] [Google Scholar]
- 17. van der Marck MA, Bloem BR, Borm GF, Overeem S, Munneke M, Guttman M. Effectiveness of multidisciplinary care for Parkinson's disease: a randomized, controlled trial. Mov Disord 2013;28:605–611. [DOI] [PubMed] [Google Scholar]
- 18. van der Marck MA, Munneke M, Mulleners W, et al. Integrated multidisciplinary care in Parkinson's disease: a non‐randomised, controlled trial (IMPACT). Lancet Neurol 2013;12:947–956. [DOI] [PubMed] [Google Scholar]
- 19. van der Eijk M, Nijhuis FAP, Faber MJ, Bloem BR. Moving from physician‐centered care towards patient‐centered care for Parkinson's disease patients. Parkinsonism Relat Disord 2013;19:923–927. [DOI] [PubMed] [Google Scholar]
- 20. van der Marck MA, Bloem BR. How to organize multispecialty care for patients with Parkinson's disease. Parkinsonism Relat Disord 2014;20(Suppl 1):S167–S173. [DOI] [PubMed] [Google Scholar]


