Abstract
Background
Mild cognitive impairment in Parkinson's disease (PD‐MCI) is associated with diminished norepinephrine from the locus coeruleus to the prefrontal cortex. Atomoxetine is a specific norepinephrine reuptake inhibitor that has been approved by the US Food and Drug Administration to treat attention deficit hyperactivity disorder in adults. The authors hypothesized that atomoxetine would improve attention and executive functioning in patients with PD‐MCI.
Methods
Thirty participants who met Movement Disorder Society Task Force Level I criteria for PD‐MCI were enrolled in a randomized controlled trial of atomoxetine. Cognitive evaluations were performed at baseline and after 10 weeks of treatment or placebo. A safety visit was performed at Week 12. A global statistical test was used to examine treatment effects on standardized tests of attention, working memory, processing speed, and set shifting (primary outcome measure). Secondary outcomes included cognitive measures hypothesized to be insensitive to atomoxetine, the Conners Adult Attention Deficit Hyperactivity Disorder Rating Scale, and safety measures.
Results
Fifteen participants were randomized to each arm. Groups were similar on medical and demographic variables and baseline cognition. Three serious adverse events occurred; 2 on atomoxetine (syncope, isolated episode of atrial fibrillation) and 1 on placebo (atrial fibrillation). The global statistical test of primary outcome measures did not reveal a significant difference between groups. However, significant improvements were observed for atomoxetine but not placebo on subjective measures of attention and impulsivity (Conners Adult Attention Deficit Hyperactivity Disorder Rating Scale).
Conclusions
Atomoxetine treatment produced subjective, but not objective, improvements in PD‐MCI. Failure to detect objective differences may be due to insensitivity of cognitive tests or severity of cognitive deficits in the study participants.
Keywords: atomoxetine, mild cognitive impairment, Parkinson's disease
Mild cognitive impairment (MCI) is common in Parkinson's disease (PD) and may be a precursor of dementia, which can occur in up to 80% of patients with PD over the course of the disease.1 PD‐MCI is characterized by an insidious decline in cognitive abilities caused primarily by the underlying disease process. The cognitive decline may be reported by either the patient or an informant, or it may be observed by the clinician, and it should not interfere significantly with functional independence.2 Cognitive deficits in PD‐MCI often interfere with activities of daily living3 but lack effective treatments. In addition, the executive dysfunction and attention deficits caused by PD‐MCI also play a role in gait and balance problems in PD.4 Thus, patients who have PD with MCI reportedly have higher postural instability and gait disorder subscale scores than cognitively normal patients with PD.5
The early loss of norepinephrine (NE)‐locus coeruleus (LC) neurons in PD corresponds with the development of cognitive deficits.6, 7, 8, 9 These deficits are mirrored in animals with lesions or pharmacological manipulations of NE‐LC innervation of the prefrontal cortex (PFC),10, 11, 12, 13 indicating that loss of this projection causes cognitive flexibility and working memory problems and is a novel target for PD therapeutics.
Atomoxetine (ATM) is a specific NE reuptake inhibitor14 that has been approved by the US Food and Drug Administration to treat attention deficit/hyperactivity disorder (ADHD), a syndrome associated with impaired concentration, vigilance, set‐shifting, and other executive function deficits.15 In preclinical studies, ATM improved executive dysfunction produced by LC‐NE lesions in the PFC.11 Jankovic16 observed a trend for improved gait and balance with ATM treatment in 5 patients with PD who had freezing phenomenon. Weintraub et al.17 conducted a double‐blind, placebo‐controlled study to evaluate the effect of ATM on depression in 55 patients with PD. Although it was not efficacious for depression, improvements in cognition (measured with the Mini‐Mental State Examination [MMSE]) and daytime sleepiness were reported. Finally, Marsh et al.18 conducted an open‐label pilot study using ATM to treat executive dysfunction in 12 patients with PD. In that study, clinically significant subjective improvements were observed in 75% of patients, supporting further study of ATM in treating executive dysfunction in PD.
We hypothesized that treatment with ATM would improve aspects of executive functioning related to NE‐LC function in patients with PD‐MCI, including attention, set‐shifting, information processing speed, and working memory. Furthermore, we examined whether potential improvement with ATM was specific to executive function. As such, analyses were planned to contrast potential changes on the primary outcome measures with other aspects of cognition that are disrupted in PD but are unlikely to be responsive to ATM; these included language, visual spatial functions, abstraction, and social reasoning.
Patients and Methods
Trial Design
The study followed a 12‐week, single‐site, double‐blind, placebo‐controlled, parallel‐group design. Participants were randomly allocated at a 1:1 ratio to either receive an ATM target dose of 80 mg or matching placebo daily. At the screening visit, potential candidates were evaluated with the Montreal Cognitive Assessment (MoCA).19, 20 Participants were evaluated at baseline and in Week 10 with a battery of neuropsychological tests (Table 1). Treatment started with a daily dose of 40 mg ATM or placebo. The study drug dose was increased to 80 mg after 2 weeks. Dose adjustments were not permitted. Study drug was discontinued after the Week‐10 visit, and patients were assessed at a final safety visit in Week 12 (Fig. 1). Adherence to assigned treatment was monitored through participant interviews and pill counts. All personnel directly involved in the conduct of the study remained unaware of the treatment assignment until all data had been collected for analysis. The Institutional Review Board of the Medical University of South Carolina approved this study.
Table 1.
Neuropsychological test battery
| Test | Domain |
|---|---|
| Primary outcome measures | |
| Paced Auditory Serial Addition Test (PASAT) | Attention/information processing |
| Neuropsychological Assessment Battery (NAB): Numbers and letters | Sustained attention/focused attention, divided attention |
| Delis‐Kaplan Executive Function System (D‐KEFS): Color‐Word Interference | Information processing speed/inhibition/set‐switching |
| Delis‐Kaplan Executive Function System (D‐KEFS): Trail‐Making | Information processing speed/set‐switching |
| Wechsler Adult Intelligence Scale (WAIS‐IV): Digit span | Focused attention/working memory |
| Secondary outcome measures | |
| Boston Naming Test (BNT) | Expressive language/confrontation naming |
| Judgment of Line Orientation (JOLO) | Visuospatial perception |
| Wechsler Adult Intelligence Scale (WAIS‐IV): Similarities | Abstract reasoning/conceptualization |
| Delis‐Kaplan Executive Function System (D‐KEFS): Proverbs | Abstract verbal reasoning |
| Neuropsychological Assessment Battery (NAB): Judgment | Social reasoning/Safety |
| Self‐report | |
| Connors Adult ADHD Rating Scale, short form, self‐report | Attention/hyperactivity/emotional instability |
| Geriatric Depression Scale | Depression |
| Geriatric Anxiety Inventory | Anxiety |
ADHD, attention deficit/hyperactivity disorder.
Figure 1.
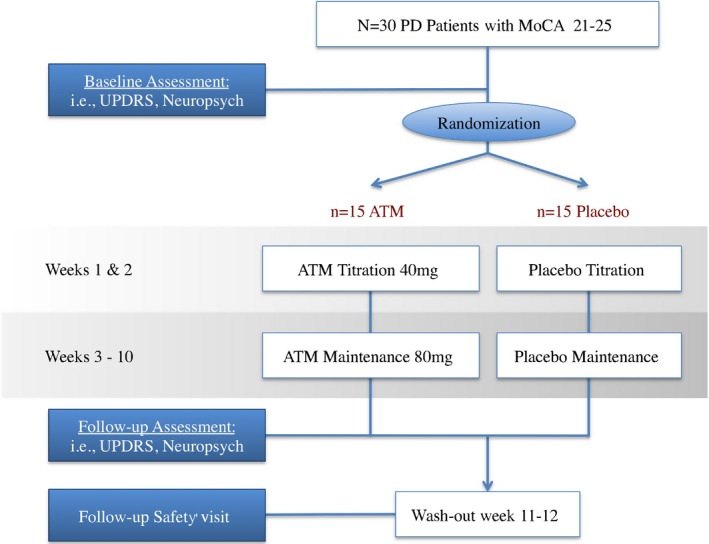
Study overview (PD, Parkinson's disease; MoCA, Montreal Cognitive Assessment; UPDRS, Unified Parkinson's Disease Rating Scale; PD; ATM, atomoxetine).
Participants
Study participants were men and women between ages 35 and 75 years who had a confirmed diagnosis of idiopathic PD according to UK Parkinson's Disease Society Brain Bank criteria21 and had received stable, concomitant medication for 60 days. Further inclusion criteria were a Level I diagnosis of PD‐MCI according to Movement Disorders Society criteria2 (operationally defined as having a MoCA score of 21–25). Exclusion criteria were a diagnosis of secondary or atypical parkinsonism, PD dementia, psychosis, pregnancy, suicidal ideations, serious cardiac abnormalities, narrow angle glaucoma, or pheochromocytoma; a history of bipolar disorder; elevated liver function tests; previous deep‐brain stimulation or other brain surgery; and current treatment with memantine, anticholinergics, monoamine oxidase inhibitors, neuroleptics, and acetylcholinesterase inhibitors.
Outcomes
The primary outcome measure was a composite score based on a battery of standardized executive function tests (Table 1), including the number of correct answers on the Paced Auditory Serial Addition Test22 (3‐second interstimulus interval), digit span from the Wechsler Adult Intelligence Scale (WAIS‐IV),23 efficiency scores from the Neuropsychological Assessment Battery (NAB)24 parts A and D, and completion times for the following subtests from the Delis‐Kaplan Executive Function System (D‐KEFS)25: Color‐Word Inhibition, Color‐Word Inhibition/Switching, and Trail Making Number/Letter Switching. To determine whether a possible benefit from ATM in patients with PD was specific to executive functioning, the following standardized cognitive tests were used as a secondary outcome: the Boston Naming Test,26 D‐KEFS Proverbs,25 WAIS‐IV Similarities,23 Judgment of Line Orientation,27 and NAB Judgment.24 Raw scores from all tests were corrected for demographics according to procedures described in test manuals; scaled scores were subsequently transformed to Z‐scores. For descriptive purposes, an individual's score was considered to be “impaired” if it fell at or below the second percentile relative to the score in the standardization sample.28 Behavioral and functional measures in this study included the Geriatric Depression Scale (GDS),29 the Geriatric Anxiety Inventory (GAI),30 and a PD‐specific quality‐of‐life measure (the PDQ‐39).31 To detect subjective changes in attention and executive functioning that might not be apparent in the neuropsychological test measures, the Conners Adult ADHD Rating Scale, short form, self‐report (CAARS),32 was also administered. Scores from the CAARS were corrected for age and sex according to the test manual. To detect any possible changes caused by ATM in the global severity of the participants' PD symptoms, the Parts I through IV of the United Parkinson's Disease Rating Scale (UPDRS) were administered. Nonmotor symptoms were monitored with the Non‐Motor Symptom Scale (NMSS),33 and suicidality was measured using the Columbia‐Suicide Severity Rating Scale.34 The Udvalg for Kliniske Undersogelser side‐effect rating scale35 was used as a systematic indicator of possible side effects of ATM. The monitoring of data was done by an independent Data and Safety Monitoring Committee.
Randomization
The Investigational Drug Services Pharmacy at the Medical University of South Carolina built and maintained a blind for this study. The randomization schedule was designed to yield an expected assignment ratio of 1:1 for ATM and placebo.
Statistical Methods
For the primary comparison between ATM and placebo, O'Brien's Global Statistical Test36 (GST) was used to analyze changes from baseline to 10 weeks for the set of neuropsychological measures included in the primary efficacy outcome. The GST is a novel approach to testing multiple outcomes. This approach allowed us to assess the battery of clinically relevant outcomes, rather than arbitrarily specifying a single primary outcome. A global treatment effect (GTE) equal to zero implies no treatment effect, a positive GTE implies that the treatment is beneficial, and a negative GTE implies that the treatment is detrimental.37
The secondary analyses compared ATM versus placebo on the neuropsychological tests that we hypothesized would be insensitive to ATM (Table 1). Once again, a GST was used to assess whether there was a treatment effect on these secondary outcome measures. To ensure that no worsening of PD symptoms was caused by to ATM treatment, the mean changes from baseline in scores on the UPDRS and the PDQ‐39, as well as other safety measures described above, were compared in the ATM group versus the placebo group.
Sample Size
The clinically meaningful treatment difference was defined as a 30% improvement between the placebo and ATM treatment groups on the primary outcome measure after 10 weeks of ATM versus placebo therapy. Using prior estimates of the mean and standard deviation (SD) for each item of the primary outcome measure composite, the GTE was computed, which corresponded to a 30% improvement in each of the scores. Thus, the clinically meaningful treatment difference, given in terms of the GTE, was 0.59. To achieve 80% power to reject the null hypothesis that the GTE is zero when the true GTE is 0.59 (two‐sided test with α = 0.05), 24 participants were required in total, with 12 in each group. The total sample size was inflated from 24 to 30 patients using the noncompliance inflation factor described by Friedman et al.,38, 39, 40 for potential missing data or noncompliance to treatment over the 12‐week study.
Results
Recruitment and Participant Flow
Participants were recruited from a tertiary care movement disorders clinic as well as an online recruitment portal (Fox Trial Finder). Thirty individuals enrolled in the study (15 in the ATM group and 15 in the placebo group), and 5 prematurely withdrew because of side effects (4 in the ATM group and 1 in the placebo group). Complete follow‐up was available for 25 of the 30 participants (83%). The average (±SD) participant age was 68 ± 8 years, and patients had an average 15 ± 3 years of education. Most participants were men (73%) and were non‐Hispanic Caucasians. The average (±SD) scores were 46 ± 15 on the Total UPDRS, 40 ± 22 on the NMSS Total, and 27 ± 15 on the PDQ‐39 Summary Index.
Baseline Data
Baseline results for neuropsychological assessments are displayed in Table 2. Participants in both groups were similar at baseline, although mean values for 5 of the primary outcome measures were nominally higher in the placebo group than in the ATM group. The frequency of impaired performance per measure was roughly equivalent across groups and was greater for the primary versus secondary outcomes. No statistically significant differences at baseline were detected (two‐sample t test).
Table 2.
Neuropsychological test battery: Baseline performance, percent of individuals scoring in the impaired range at baseline, and mean change from baseline to Week 10a
| Test | Atomoxetine (N = 15) | Placebo (N = 15) | P value for 2‐sample t test of baseline means | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline score: Mean ± SD | Percent impaired at baselineb | Change in score | Baseline score: Mean ± SD | Percent impaired at baselineb | Change in score | ||||
| Mean ± SD | 95% CI | Mean ± SD | 95% CI | ||||||
| Primary outcome measures | |||||||||
| PASAT | −1.58 ± 1.37 | 44 | −0.96 ± 1.96 | −2.05, 0.12 | −1.86 ± 1.28 | 68 | 0.42 ± 1.22 | −0.26, 1.09 | 0.58 |
| NAB: Part A | −1.04 ± 1.15 | 33 | −0.71 ± 1.05 | −1.29, −0.13 | −0.92 ± 1.48 | 20 | −0.23 ± 1.07 | −0.82, 0.36 | 0.81 |
| NAB: Part D | −1.25 ± 1.12 | 20 | −0.35 ± 1.31 | −1.08, 0.38 | −1.12 ± 1.13 | 20 | −0.11 ± 0.75 | −0.53, 0.31 | 0.76 |
| D‐KEFS: Inhibition Time | −0.86 ± 1.15 | 14 | −0.77 ± 1.4 | −1.54, 0.01 | −0.18 ± 0.87 | 7 | −0.3 ± 1.17 | −0.95, 0.35 | 0.08 |
| D‐KEFS: Inhibition‐Switching Time | −0.86 ± 1.57 | 36 | −0.87 ± 1.63 | −1.77, 0.04 | −0.71 ± 1.26 | 13 | −0.2 ± 1.63 | −1.11, 0.71 | 0.78 |
| D‐KEFS: Number‐Letter Switching Time | −1.14 ± 1.65 | 43 | −0.9 ± 1.33 | −1.64, −0.16 | −0.64 ± 1.63 | 27 | −0.67 ± 1.43 | −1.46, 0.13 | 0.42 |
| WAIS‐IV: Digit span | 0.36 ± 0.96 | 0 | −0.17 ± 0.8 | −0.61, 0.28 | 0.27 ± 0.58 | 0 | 0 ± 0.67 | −0.37, 0.37 | 0.76 |
| Secondary outcome measures | |||||||||
| WAIS‐IV: Similarities | −0.11 ± 0.96 | 0 | −0.2 ± 0.87 | −0.68, 0.28 | 0.11 ± 1.04 | 0 | −0.57 ± 1.23 | −1.25, 0.12 | 0.55 |
| BNT | 0.39 ± 1.22 | 0 | 0.23 ± 0.88 | −0.26, 0.72 | −0.25 ± 1.11 | 7 | 0.36 ± 1.32 | −0.37, 1.09 | 0.15 |
| JOLO | −0.20 ± 1.07 | 0 | −0.3 ± 1.13 | −0.93, 0.33 | −0.02 ± 0.78 | 0 | −0.23 ± 1.1 | −0.84, 0.38 | 0.61 |
| D‐KEFS: Proverbs | −0.36 ± 0.99 | 0 | −0.57 ± 1.1 | −1.18, 0.04 | 0.09 ± 1.07 | 0 | −0.13 ± 1.63 | −1.04, 0.77 | 0.25 |
| NAB: Judgement | −0.08 ± 1.37 | 7 | 0.05 ± 1.54 | −0.8, 0.9 | 0.27 ± 1.07 | 0 | −0.37 ± 1.51 | −1.21, 0.47 | 0.45 |
SD, standard deviation; CI, confidence interval; PASAT, Paced Auditory Serial Addition Test; NAB, Neuropsychological Assessment Battery; D‐KEFS, Delis‐Kaplan Executive Function System; WAIS‐IV, Wechsler Adult Intelligence Scale; BNT, Boston Naming Test; JOLO, Judgment of Line Orientation.
Neuropsychological test batteries are reported as Z‐scores (corrected for demographics based on normative samples described in the test manuals). Higher scores indicate better performance.
Percent impaired indicates the percentage of participants with baseline scores at or below the second percentile relative to the standardization sample (Z ≤ −2.0537).
Outcomes
The primary analysis is presented in Table 2. Missing data for five patients (four in the ATM group and one in the placebo group) at 10 weeks were imputed using the worst observed score for that treatment group. In the 30 enrolled participants, the mean (95% confidence interval [CI]) of the summed ranks was 117.5 (95% CI: 98.51–136.40) for the placebo group and 99.5 (95% CI: 73.10–126.00) for the ATM group. Higher summed ranks (range: 10–172.5) indicate better outcomes. The GST did not yield statistically significant group differences (t[28] = −1.18, P = 0.25; two sided). Based on the primary analysis, there is no evidence that the ATM group differed from the placebo group in the primary outcome measure. Using only the 25 participants who completed 10 weeks of treatment, the GST yielded a value of t = −0.05 (degrees of freedom = 23; two‐sided P value = 0.96). The GST did not suggest group differences using only observed values (i.e., no imputation; t[23] = −0.05; P = 0.096; two‐tailed). Examination of change scores did not reveal any statistically significant differences on any of the individual outcome measures, including the primary and secondary cognitive tests, GDS, GAI, NMSS, and PDQ‐39. A follow‐up analysis using 90% reliable change index scores41 was performed to explore potential responders versus nonresponders. Examination of individual change data within the ATM group did not identify a pattern suggestive of “responders” or “nonresponders.”
Subjective changes in attention and cognition at Week 12 were evaluated using the CAARS. Trends favoring ATM over placebo were observed on subscales for inattention/memory problems (t[23] = 1.70; P = 0.10) and impulsivity/emotional lability (t[23] = 1.8; P = 0.08). The 95% CI for difference scores suggested statistically a significant improvement in the ATM group, as shown in Table 3; the magnitude of change corresponded to mean reductions from baseline of roughly 21% for inattention/memory problems and 18% for impulsivity/emotional lability. No significant changes from baseline were observed in the placebo group. For the ATM group, follow‐up analyses revealed that subjective changes from baseline were not redundant (the correlation between changes on subscales was not significant: Pearson's correlation [r] = 0.137; P = 0.67; and changes on CAARS subscales were not associated with changes on any neuropsychological measures).
Table 3.
Change from baseline for the Connors Adult Attention Deficit/Hyperactivity Disorder Rating Scale, short form (CAARS)
| CAARS item | Treatment groupa | |||||
|---|---|---|---|---|---|---|
| Atomoxetine | Placebo | |||||
| No. | Mean ± SD | 95% CI | No. | Mean ± SD | 95% CI | |
| Change from baseline to 2 weeks | ||||||
| Inattention | 14 | −0.6 ± 9.6 | −6.1, 5.0 | 14 | 0.8 ± 6.1 | −2.7, 4.3 |
| Hyperactivity | 14 | −1.4 ± 9.7 | −7.0, 4.2 | 14 | −1.3 ± 6.3 | −4.9, 2.4 |
| Impulsivity | 14 | −0.9 ± 11.9 | −7.8, 5.9 | 14 | −1.9 ± 6.9 | −5.9, 2.1 |
| Problems with self‐concept | 14 | 1.1 ± 10.6 | −5.0, 7.3 | 14 | 0.5 ± 6.5 | −3.3, 4.3 |
| ADHD index | 14 | −1.3 ± 11.0 | −7.7, 5.1 | 14 | −0.4 ± 4.5 | −3.0, 2.2 |
| Change from baseline to 10 weeks | ||||||
| Inattention | 11 | −5.7 ± 7.9 | −11.0, −0.4 | 14 | −1.6 ± 3.9 | −3.9, 0.6 |
| Hyperactivity | 11 | −2.4 ± 8.2 | −7.9, 3.1 | 14 | −1.1 ± 7.1 | −5.2, 3.0 |
| Impulsivity | 11 | −5.4 ± 7.4 | −10.4, −0.4 | 14 | −0.6 ± 5.7 | −3.9, 2.6 |
| Problems with self‐concept | 11 | −0.8 ± 9.9 | −7.5, 5.8 | 14 | −3.1 ± 8.2 | −7.9, 1.6 |
| ADHD index | 11 | −4.9 ± 9.7 | −11.4, 1.6 | 14 | −1.7 ± 7.7 | −6.2, 2.7 |
SD, standard deviation; CI, confidence interval; ADHD, attention deficit/hyperactivity disorder.
Values reflect changes in T‐scores (mean ± SD, 50 ± 10) after demographic correction according to the test manual.
Adverse Events and Tolerability
There were no signs indicating worsening PD severity during exposure to study drug as measured by the UPDRS and the NMSS. On the contrary, there was a large improvement in total UPDRS scores for both groups at 2 weeks; the mean ± SD change was −5.7 ± 8.8 in the ATM group and −5.6 ± 6.4 in the placebo group, although this effect diminished over time. Vital signs remained stable, and laboratory monitoring of serum chemistry levels and liver function did not reveal any clinically significant changes related to study drug. Monitoring with the Columbia‐Suicide Severity Rating Scale produced no evidence of the development of suicidal thoughts in any of the patients. Adverse events in the ATM group (Table 4) included: “jitteriness” (n = 3), reduced urine stream (n = 2), a brief episode of chest pain (n = 1), kidney stone (n = 1), nausea (n = 1), syncope (n = 1), and questionable atrial fibrillation (n = 1; the study physician read an electrocardiogram as atrial fibrillation, and the Emergency Department physician read the same electrocardiogram as an artifact). Adverse events in the placebo group were: “jitteriness” (n = 3), worsening memory (n = 3), hypertension (n = 1), atrial fibrillation (n = 1), freezing of gait (n = 1), erectile dysfunction (n = 1), insomnia (n = 1), and fatigue (n = 1). At the end of the study, participants were offered to continue ATM as part of their routine clinical care. Thirteen of 15 patients who had been assigned to ATM therapy during the study were interested in continuing ATM therapy. Of those, two patients were not candidates for continuation because of cardiac adverse events while on study drug, and four patients indicated that the cost of continuous ATM use would be prohibitive. In the end, 6 of 15 patients continued ATM outside of the clinical trial.
Table 4.
Adverse events
| Adverse event | No. of patients affected | |
|---|---|---|
| Atomoxetine | Placebo | |
| Jitteriness | 3 | 3 |
| Decreased memory | 0 | 3 |
| Decreased urine stream | 2 | 0 |
| Atrial fibrillation | 1a | 1 |
| Syncope | 1 | 0 |
| Chest pain | 1 | 0 |
| Insomnia | 0 | 1 |
| Freezing of gait | 0 | 1 |
| Erectile dysfunction | 0 | 1 |
| Kidney stone | 1 | 0 |
| Hypertension | 0 | 1 |
| Nausea | 1 | 0 |
An electrocardiogram was read as atrial fibrillation by 1 physician and as artifact by another.
Discussion
In this double‐blind, placebo‐controlled trial, treatment with ATM produced subjective, but not objective, improvements in executive functioning in patients with PD‐MCI. CAARS subscale scores for inattention and impulsivity were significantly improved in the ATM group compared with baseline, but there were no changes in the placebo group. ATM was generally well tolerated and did not worsen PD severity. Two serious adverse events occurred on ATM (syncope and a questionable episode of atrial fibrillation), and one occurred on placebo (atrial fibrillation).
One limitation of this study was that the sample size calculation assumed a moderately large effect on the targeted neuropsychological tests as a whole. It is possible that a larger sample size may have detected a more modest treatment effect; however, we saw no evidence to suggest an effect of ATM in any of the cognitive tests chosen from the primary comparison or in the other aspects of cognition that were not thought to be affected. It is unlikely that ATM affects other aspects of cognition that were not considered.
The improvement in behavioral measures on the CAARS with ATM is consistent with our initial hypothesis of improving attention, and set‐shifting abilities with the ATM intervention, related to its selective inhibition of presynaptic NE reuptake in the PFC. However, this was not reflected by improvements on conventional neuropsychological tests of executive function. This result is consistent with those from the open‐label study by Marsh et al. regarding the use of ATM for PD executive dysfunction.18 In that study, ratings on the CAARS and on the Frontal Systems Behavioral Scale improved, although participants generally were unimpaired on psychometric testing at baseline and during follow‐up. Of note, unlike patients in the study by Marsh and colleagues, our participants were markedly impaired at baseline, as measured on neuropsychological testing, even though they fulfilled Level I PD‐MCI criteria at screening. Many participants in our sample registered scores within the impaired range (at or below the second percentile) on primary outcome measures, as indicated in Table 2. Although it is not detailed in the table, 9 participants scored below the first percentile on the number‐letter switching condition of the D‐KEFS Trail Making test, 8 scored below the first percentile on D‐KEFS Inhibition Switching, and 16 scored below the first percentile impaired on the Paced Auditory Serial Addition Test. It is highly likely that this level of impairment impacted our results. We could speculate that a less severely impaired population might exhibit ATM treatment effects on objective cognition measures, along with the subjective effects demonstrated here. Indeed, the study by Weintraub et al. demonstrated that ATM improved cognition in a sample of patients who had PD without MCI using a screening instrument, the MMSE, as the outcome measure.17 As a point of reference, the mean baseline MMSE score reported in that sample was 28.1, which is equivalent to an MoCA score of 25 or 26 using the conversion table proposed by Lawton et al.,42 suggesting higher functioning than our sample, in which the mean MoCA score was 23.3, and the upper limit was aa score of 25. To that end, a study of ATM in patients with PD who do not fulfill PD‐MCI criteria or in PD‐MCI single‐domain (attention or executive functioning) participants might demonstrate correspondence between objective and subjective measures.
A methodological challenge in all randomized controlled trials involving cognitive outcome measures is identifying tests with suitable psychometric properties for detecting change.43 As detailed in the 2015 review by Goldman and Weintraub,44 most published clinical trials of cognitive interventions in PD use screening measures like the MMSE. Although scores on screening measures are readily interpretable to clinicians and researchers, such tools are not designed to measure functioning within specific cognitive domains. For cognitive screening and domain‐specific tests, practice effects and measurement error can obscure subtle improvements. This is particularly challenging in studies involving patients with PD‐MCI, because fluctuating motor and medication states produce noise, and Lewy body deposition is associated with vacillating mental acuity.45, 46 Such issues may explain the apparent lack of benefit associated with cognitive interventions for PD in other clinical trials using similar standardized neuropsychological tests, such as the open‐label ATM study by Marsh et al.18 Thus, alternative “surrogate” outcome measures linked to cognitive functioning in PD‐MCI with improved reliability might be useful. As such, facets of oculomotor functioning (e.g., ability to initiate and inhibit saccades), olfaction, or levels of neurotrophic factors might be considered in future research.
In conclusion, the current results suggest that patients with PD‐MCI who receive treatment with ATM appreciate a subjective sense of improved cognitive functioning that is not reflected with objective testing. Future research in patients with PD with less severe cognitive impairment and improved objective outcome measures are needed to determine the utility of ATM in this population.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
V.K.H.: 1A, 1B, 1C, 3A
A.D.: 1A, 1C
J.E.: 2A, 2B, 3B
T.T.: 1A, 1C, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest: This study was funded by the Michael J. Fox Foundation for Parkinson's Research. The authors report no conflicts of interest relevant to this work.
Financial Disclosures for the previous 12 months: All 4 authors received salary support from the Michael J. Fox Foundation for Parkinson's Research. In addition, Vanessa K. Hinson received grant support from the National Institutes of Health, the Barmore Fund, Biotie, and Acorda Therapeutics and consultant fees from Acadia and Lundbeck. Jordan Elm received research grants from the National Institutes of Health and Remedy Pharmaceuticals and consultant fees from Remedy Pharmaceuticals and Teva Pharmaceuticals. Travis Turner received grant support from the Department of Defense and Aker Biomarin.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh‐Sorensen P. Prevalence and characteristics of dementia in Parkinson's disease: an 8‐year prospective study. Arch Neurol 2003;60:387–392. [DOI] [PubMed] [Google Scholar]
- 2. Litvan L, Goldman JG, Troester A, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord 2012;27:349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosenthal E, Brennan L, Xie S, et al. Association between cognition and function in patients with Parkinson disease with and without dementia. Mov Disord 2010;25:1170–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yogev‐Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord 2008;23:329–342, quiz 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sollinger AB, Goldstein FC, Lah JJ, Levey AI, Factor SA. Mild cognitive impairment in Parkinson's disease: subtypes and motor characteristics. Parkinsonism Relat Disord 2010;16:177–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol 2006;73:19–38. [DOI] [PubMed] [Google Scholar]
- 7. Mavridis M, Degryse AD, Lategan AJ, Marien MR, Colpaert FC. Effects of locus coeruleus lesions on parkinsonian signs, striatal dopamine and substantia nigra cell loss after 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine in monkeys: a possible role for the locus coeruleus in the progression of Parkinson's disease. Neuroscience 1991;41(2–3):507–523. [DOI] [PubMed] [Google Scholar]
- 8. Gesi M, Soldani P, Giorgi FS, Santinami A, Bonaccorsi I, Fornai F. The role of the locus coeruleus in the development of Parkinson's disease. Neurosci Biobehav Rev 2000;24:655–668. [DOI] [PubMed] [Google Scholar]
- 9. Srinivasan J, Schmidt WJ. Potentiation of parkinsonian symptoms by depletion of locus coeruleus noradrenaline in 6‐hydroxydopamine‐induced partial degeneration of substantia nigra in rats. Eur J Neurosci 2003;17:2586–2592. [DOI] [PubMed] [Google Scholar]
- 10. Arnsten AF, Steere JC, Jentsch DJ, Li BM. Noradrenergic influences on prefrontal cortical cognitive function: opposing actions at postjunctional alpha 1 versus alpha 2‐adrenergic receptors. Adv Pharmacol 1998;42:764–767. [DOI] [PubMed] [Google Scholar]
- 11. Newman LA, Darling J, McGaughy J. Atomoxetine reverses attentional deficits produced by noradrenergic deafferentation of medial prefrontal cortex. Psychopharmacology 2008;200:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McGaughy J, Ross RS, Eichenbaum H. Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set‐shifting. Neuroscience 2008;153:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lapiz MD, Morilak DA. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience 2006;137:1039–1049. [DOI] [PubMed] [Google Scholar]
- 14. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 2002;27:699–711. [DOI] [PubMed] [Google Scholar]
- 15. Arnsten AF. Toward a new understanding of attention‐deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs 2009;23(Suppl 1):33–41. [DOI] [PubMed] [Google Scholar]
- 16. Jankovic J. Atomoxetine for freezing of gait in Parkinson disease. J Neurol Sci 2009;284:177–178. [DOI] [PubMed] [Google Scholar]
- 17. Weintraub D, Mavandadi S, Mamikonyan E, et al. Atomoxetine for depression and other neuropsychiatric symptoms in Parkinson's disease. Neurology 2010;75:448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marsh L, Biglan K, Gerstenhaber M, Williams JR. Atomoxetine for the treatment of executive dysfunction in Parkinson's disease: a pilot open‐label study. Mov Disord 2009;24:277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 20. Dalrymple‐Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well‐suited screen for cognitive impairment in Parkinson disease. Neurology 2010;75:1717–1725. [DOI] [PubMed] [Google Scholar]
- 21. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of Parkinson's disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gronwall DM. Paced auditory serial‐addition task: a measure of recovery from concussion. Percept Motor Skills 1977;44:367–373. [DOI] [PubMed] [Google Scholar]
- 23. Wechsler D. Manual for the Wechsler Adult Intelligence Scale‐IV. San Antonio, TX: The Psychological Corporation; 2008. [Google Scholar]
- 24. White T, Stern RA. Neuropsychological Assessment Battery: Psychometric and Technical Manual. Lutz, FL: Psychological Assessment Resources; 2003. [Google Scholar]
- 25. Delis DC, Kaplan E, Kramer JH. The Delis‐Kaplan Executive Function System (D‐KEFS): Examiner's Manual. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 26. Kaplan E, Goodglass H, Weintraub S. Boston Naming Test, 2nd ed Philadelphia, PA: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 27. Benton AL. Judgment of line orientation In: Benton AL, Hamsher KD, Varney NR, Spreen O, eds. Contributions to Neuropsychological Assessment. New York: Oxford University Press; 1983:44–54. [Google Scholar]
- 28. Spreen O, Strauss E, eds. A Compendium of Neuropsychological Tests, 2nd ed New York: Oxford University Press; 1998. [Google Scholar]
- 29. Adams KB, Matto HC, Sanders S. Confirmatory factor analysis of the Geriatric Depression Scale. Gerontologist 2004;44:818–826. [DOI] [PubMed] [Google Scholar]
- 30. Pachana N, Byrne G, Siddle H, Koloski N, Harley E, Arnold E. Development and validation of the Geriatric Anxiety Inventory. Int Psychogeriatr 2007;19:103–114. [DOI] [PubMed] [Google Scholar]
- 31. Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson's Disease Questionnaire (PDQ‐39): development and validation of a Parkinson's disease summary index score. Age Ageing 1997;26:353–357. [DOI] [PubMed] [Google Scholar]
- 32. Conners CK. Rating scales in attention‐deficit/hyperactivity disorder: use in assessment and treatment monitoring. J Clin Psychiatry 1998;59(Suppl 7):24–30. [PubMed] [Google Scholar]
- 33. Chaudhuri KL, Martinez‐Martin P, Brown RG, et al. The metric properties of a novel non‐motor symptoms scale for Parkinson's disease: results from an international pilot study. Mov Disord 2007;22:1901–1911. [DOI] [PubMed] [Google Scholar]
- 34. Posner K, Brown GK, Stanley B, et al. The Columbia‐Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 2011;168:1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU Side Effect Rating Scale: a new comprehensive rating scale for psychotropic drugs, and a cross‐sectional study of side effects in neuroleptic‐treated patients. Acta Psychiatr Scand Suppl 1987;334:1–100. [DOI] [PubMed] [Google Scholar]
- 36. O'Brien PC. Procedures for comparing samples with multiple endpoints. Biometrics 1984;40:1079–1087. [PubMed] [Google Scholar]
- 37. Huang P, Goetz CG, Woolson RF, et al. Using global statistical tests in long‐term Parkinson's disease clinical trials. Mov Disord 2009;24:1732–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Friedman L, Furberg C, DeMets DL. Fundamentals of Clinical Trials. Littleton, MA: John Wright‐PSG Inc.; 1981. [Google Scholar]
- 39. Friedman L, Furberg C, DeMets DL. Fundamentals of Clinical Trials, 2nd ed St. Louis, MO: Mosby‐Year Book, Inc.; 1985. [Google Scholar]
- 40. Friedman L, Furberg C, DeMets DL. Fundamentals of Clinical Trials, 3rd ed New York: Springer‐Verlag; 1996. [Google Scholar]
- 41. Chelune GJ, Naugle RI, Lüders H, Sedlak J, Awad IA. Individual change after epilepsy surgery: practice effects and base‐rate information. Neuropsychology 1993;7:41–52. [Google Scholar]
- 42. Lawton M, Kasten M, May MT, et al. Validation of conversion between mini–mental state examination and Montreal cognitive assessment. Mov Disord 2016;31:593–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eberling J, Vincent L, Goldman JG, et al. Therapeutic development paths for cognitive impairment in Parkinson's disease: report of a regulatory roundtable. J Parkinsons Dis 2014;4:585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goldman JG, Weintraub D. Advances in the treatment of cognitive impairment in Parkinson's disease. Mov Disord 2015;30:1471–1489. [DOI] [PubMed] [Google Scholar]
- 45. Brown RG, Marsden CD, Quinn N, Wyke MA. Alterations in cognitive performance and affect‐arousal state during fluctuations in motor function in Parkinson's disease. J Neurol Neurosurg Psychiatry 1984;47:454–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ballard CG, Aarsland D, McKeith I, O'Brien J, Gray A, Cormack F, Tovee M. Fluctuations in attention: PD dementia vs DLB with parkinsonism. Neurology 2002;59:1714–1720. [DOI] [PubMed] [Google Scholar]


