Abstract
A significant proportion of patients’ experience recurrence of atrial fibrillation (AF) despite pulmonary venous isolation (PVI), especially those with persistent AF. Isolation of the left atrial appendage (LAA) may reduce AF recurrence. The aim of this study was to assess the efficacy of LAA isolation in addition to PVI compared with PVI alone. We conducted a comprehensive search of electronic databases, up to April 21st, 2017, for all studies comparing the effect LAA electrical isolation or ligation in addition to PVI, as opposed to PVI alone, on the recurrence of atrial fibrillation after catheter ablation. We used random‐effects meta‐analysis models to summarize the studies. One RCT and four observational studies enrolling 781 patients were retained. Four studies assessed the added effect of LAA catheter ablation, and one study evaluated the effect of LAA ligation with the aim of LAA electrical isolation. Four studies exclusively enrolled patients with persistent atrial fibrillation and one study predominantly enrolled patients with persistent atrial fibrillation. Follow‐up ranged from 12 to 15 months. The addition of LAA isolation to PVI reduced AF recurrence compared with the latter alone (odds ratio (OR) = 0.19; 95% confidence intervals (CI) = 0.10‐0.37; P < 0.00001). Left atrial appendage isolation was also associated with a reduction in AF recurrence after repeat ablation (OR = 0.40; CI = 0.25‐0.65; P = 0.0003). The addition of LAA isolation to PVI was associated with a decrease in AF recurrence in patients with persistent AF. Further studies are needed to assess the effect on long‐term risk of stroke.
Keywords: atrial fibrillation, catheter ablation, left atrial appendage isolation, meta‐analysis, pulmonary vein isolation
1. INTRODUCTION
Catheter ablation is an established therapeutic modality for rhythm control of symptomatic atrial fibrillation (AF).1, 2, 3 Electrical isolation of the pulmonary veins (PVI) is effective in improving symptoms 4, 5 and is the cornerstone of AF catheter ablation.1, 2 Despite improvements in catheter ablative techniques, the AF recurrence rate remains relatively high.6, 7 This is especially true in patients with persistent AF where recurrence rates of 34% at 1 year and 52% at 2‐year follow‐up have been shown.8, 9 Strategies targeting other AF triggers have thus far proven to be of limited success.9, 10, 11, 12
The left atrial appendage (LAA) represents a potential arrhythmic substrate. Takahashi et al13 originally identified the LAA as a trigger and substrate for AF maintenance after pulmonary vein isolation (PVI), with several case reports and case series confirming this proarrhythmic role.14, 15, 16 Studies have demonstrated the feasibility of targeting the LAA during catheter ablation17, 18 and during surgical ablation19, 20 with current guidelines recommending closure of the LAA during surgical AF ablation.21 Ligation of the LAA by the LARIAT suture delivery device has also been shown to reduce LAA voltage and capture during pacing22 as well as AF burden.23 Similar to surgical ligation, LAA ligation may alter the ganglionated plexi of the autonomic nervous system in the epicardial fat which may reduce atrial fibrillation paroxysms.24 In addition, epicardial ligation of the LAA is associated with lower levels of neurohormonal activation and systemic blood pressure of compared to endocardial LAA closure.25 Mechanistically, ischemic necrosis of the LAA will develop leading to its electrical isolation.22 This contrasts with surgical isolation in which tissue ingrowth leads to encapsulation from adjacent structures, a more prolonged process.26 Catheter ablation of the LAA is undertaken by either radiofrequency or cryoablation and should be performed with caution given the thin wall of the LAA that may be prone to perforation.27 Ligation of the LAA has the advantage of decreasing thrombus formation in the LAA.28
The electrical isolation of the LAA by catheter ablation as well as percutaneous‐based LAA ligation represents potential options to improve freedom from AF recurrence. The aim of this study was to assess the efficacy and safety of LAA isolation, through catheter ablation or ligation. To this end, we performed a systematic review and meta‐analysis.
2. METHODS
2.1. Data sources and searches
This systematic review was performed according to the guidelines described in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) statement29 and the MOOSE checklist.30 We systematically searched, with no language restriction, PubMed, the Cochrane Library, Web of Science and Scopus and MEDLINE for articles published from inception through April 21st, 2017. We used permutations of the terms atrial fibrillation, left atrial appendage, catheter ablation and electrical isolation to identify potential studies for inclusion. Hand searching with cross‐references of retrieved publications, review articles and guidelines was also performed to ensure the inclusion of all relevant studies.
2.2. Study selection
Two authors (A.A.T. and A.D.) independently performed the initial screening of titles and abstracts to identify potentially relevant articles. Review articles, case reports, meeting abstracts and duplicates were excluded. In addition, articles assessing the surgical ligation of the LAA were also excluded as this topic has been relatively well studied and is beyond the scope of this study. The full text of selected articles was independently assessed by two authors (A.A.T. and A.D.) to determine relevance for inclusion. Conflicts were resolved by discussion between the assessors, and it was arranged that any disagreement is resolved by discussion with the senior author (V.E.).
Eligibility criteria for included studies were decided a priori. RCT and cohort studies were included if they fulfilled all the following criteria: (a) assessed the effect of LAA isolation or ligation; (b) included a comparator arm of PVI; and (c) reported AF‐free survival in both groups.
2.3. Data extraction
Two investigators (A.A.T. and A.D.) independently extracted information on study characteristics (author, study type and publication year), duration of follow‐up (mean, median or maximum number of follow‐up), sample size, sex, mean age, atrial fibrillation type and duration, left atrial size or diameter, diabetes mellitus, hypertension, coronary artery disease, and mean left ventricular ejection fraction (LVEF). Disagreements were resolved by consensus.
Outcomes of interest were:
Recurrence of atrial tachycardia/atrial fibrillation after initial ablation
Recurrence of atrial tachycardia/atrial fibrillation after repeat ablation
All reported periprocedural adverse events
Periprocedural and long‐term thromboembolic events
2.4. Quality assessment
We used the Newcastle‐Ottawa Scale to evaluate the quality of the included studies on three broad perspectives: selection of the study groups, the comparability of the groups, and the ascertainment of exposure and outcome of interest. Nine points were available to be awarded based on (a) representativeness of the exposed cohort, (b) selection of nonexposed cohort, (c) exposure ascertainment, (d) the absence of the outcome of interest at study onset, (e) study controls for left atrial size, (f) study controls for other factors, (g) assessment of outcome, (h) follow‐up long enough for outcomes to occur, and (h) outcome of controls adequacy. Studies were categorized as either: (a) high quality: seven to nine points; (b) fair quality: four to six points; or (c) poor quality: zero to three points.
2.5. Statistical analysis
We calculated odds ratios and 95% confidence intervals for all studies. The odds ratios were then pooled using a DerSimonian and Laird random‐effects model.31 Peto odds ratio was used if there were less than ten events in both groups. Publication bias was assessed visually using a funnel plot and quantified using Egger's test for small study effects. Heterogeneity among studies was examined with the I 2 test. Statistical analyses were carried out using StatsDirect version 3 (England: StatsDirect Ltd. 2013). We conducted an influence analysis with sequential exclusion of individual studies.
3. RESULTS
3.1. Study selection
The search strategy led to the retrieval of 3030 citations from electronic database and manual searches as shown in Figure S1. We reviewed 19 citations for full‐text articles; five full‐text articles were included in final analysis.27, 32, 33, 34, 35 Of the five included studies, one was a randomized clinical trials,32 three were prospective cohort studies,27, 33, 34 and one was a retrospective cohort study.35
3.2. Quality assessment
Four studies were classified as high quality, and one was classified as fair quality using the nine parameters of the Newcastle‐Ottawa scale (Table S1).
3.3. Baseline characteristics
Four studies compared LAA isolation in addition to PVI compared to PVI alone.27, 32, 34, 35 Nonpulmonary triggers, such as the posterior wall of the left atrium, the coronary sinus and the superior vena cava, were similarly ablated in both groups at the operators’ discretion. Three studies required further entry criteria in the form of firing from the LAA27 and at least one risk factor for stroke.33, 34, 36 One study also involved occlusion of the LAA in addition to PVI to reduce stroke risk.34 Lakkireddy et al33 examined the effect of LAA ligation in addition to PVI vs PVI alone, on AF recurrence. LAA isolation by catheter ablation was not performed in this study. Non‐PV triggers were also similarly ablated in both groups.
In the five included studies, 781 patients were enrolled. There was a total of 441 patients in the PVI + LAA isolation/ligation arm and 340 patients in the PVI alone arm. Baseline characteristics in all studies were comparable in both groups. Study and baseline patient characteristics in the individual studies are shown in Tables 1 and 2, respectively. Four studies included patients with predominantly LSPAF,27, 32, 33, 34 one study was conducted in predominantly persistent AF.35 The duration of follow‐up ranged from 12 to 15 months. The average duration of AF pre‐intervention ranged from 25 to 90 months in PVI + LAAI group and 24‐83 months in PVI‐alone group. Of the four studies that used LAA isolation, three were performed using radiofrequency ablation27, 32, 34 and one using cryoballoon ablation.35 In the study by Panniker et al,34 LAA isolation was followed by occlusion of the LAA using the WATCHMAN device.
Table 1.
Baseline study characteristics
| Study first author (y) | Type | Procedure | Comparator | Follow‐up (mo) | Primary endpoint | Mode of Follow‐up |
|---|---|---|---|---|---|---|
| Di Biase (2010) | Prospective cohort | LAAI + PVI | PVI | 15 | AF recurrence | Holter monitor and event recorder |
| Di Biase (2016) | Randomized clinical trial | LAAI + PVI | PVI | 12 | AF recurrence | Holter monitor and event recorder |
| Lakkireddy (2015) | Prospective cohort | LAA ligation (LARIAT) + PVI | PVI | 12 | AF recurrence | History, ECG and event recorder |
| Panikker (2016) | Prospective cohort | LAAI + LAA Occlusion (WATCHMAN) + PVI | PVI | 12 | Successful LAA electric isolation and occlusion. (AF recurrence was a secondary end point) | Holter monitor |
| Yorgun (2017) | Retrospective cohort | LAAI + PVI (CB) | PVI (CB) | 12 | AF recurrence | History, ECG and Holter monitor |
LAAI, left atrial appendage isolation; PVI, pulmonary vein isolation; AF, atrial fibrillation; ECG, electrocardiogram; CB, cryoballoon.
Table 2.
Baseline patient characteristics
| Study first author | Number | Age (y) | Male | Mean LVEF | AF type | AF duration | LA diameter/ Size | CAD (%) | HTN (%) | DM (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | |
| Di Biase (2010) | 167 | 43 | 64 | 61 | 73 | 74 | 59 | 58 |
PAF 13 PER 23 LSP 64 |
PAF 28 PER 23 LSP 49 |
90 | 83 | 43 | 41 | ‐ | ‐ | 47 | 40 | 8 | 7 |
| Di Biase (2016) | 85 | 88 | 64 | 64 | 75 | 73 | 54 | 55 | LSP 100 | LSP 100 | ‐ | ‐ | 48 | 48 | 20 | 19 | 58 | 60 | 17 | 18 |
| Lakkireddy (2015) | 69 | 69 | 67 | 67 | 48 | 48 | 53 | 53 | LSP 100 | LSP 100 | 52 | 52 | 50 | 48 | 20 | 12 | 51 | 54 | 20 | 18 |
| Panikker (2016) | 20 | 40 | 68 | 67 | 65 | 65 | ‐ | ‐ | PER/LSP 100 | PER/LSP 100 | 25 | 24 | 46 | 45 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Yorgun (2017) | 100 | 100 | 57 | 57 | 48 | 49 | 65 | 65 |
PER 90 LSP 10 |
PER 91 LSP 9 |
60 | 60 | 44 | 43 | 16 | 15 | 33 | 36 | 20 | 18 |
A = left atrial appendage isolation + pulmonary vein isolation.
B = pulmonary vein isolation alone.
LVEF, left ventricular ejection fraction; AF, atrial fibrillation; LA, left atrium; CAD, coronary artery disease; HTN, hypertension; DM, diabetes mellitus; PAF, paroxysmal atrial fibrillation; PER, persistent; LSP, long‐standing persistent.
3.4. Arrhythmia recurrence
The addition of LAA isolation to PVI reduced AF recurrence compared to the latter alone at 12‐ to 15‐month follow‐up (odds ratio (OR) = 0.23; 95% confidence intervals (CI) = 0.14‐0.38; P < 0.00001; Cochran Q = 7.54, P = 0.11; I 2 = 46%) as demonstrated in Figure 1. There was no difference in the results after influence analysis (Table S2). One study, which included patients with paroxysmal AF and was the only study with fair quality, caused the heterogeneity but there was no difference in the final results with its exclusion.27 Two studies provided data on AF recurrence after repeat catheter ablation.32, 33 The addition of LAA isolation was associated with a reduction in AF recurrence after repeat ablation (OR = 0.40; CI = 0.25‐0.65; P = 0.0003) (Figure 2). There was no asymmetry in the funnel plot to suggest publication bias, and Egger's test was not significant for publication bias (Figure S2).
Figure 1.
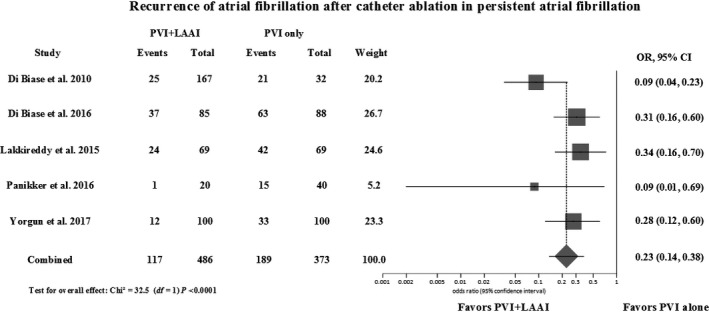
Forest plot of recurrence events of atrial fibrillation at 12‐ to 15‐mo follow‐up comparing PVI + LAA isolation/ligation vs PVI alone. PVI, pulmonary vein isolation; LAAI, left atrial appendage isolation; OR, odds ratio; CI, confidence interval
Figure 2.
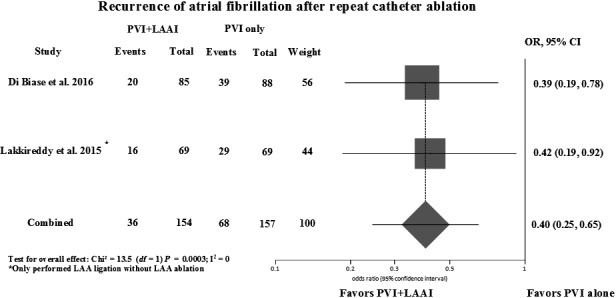
Forest plot of recurrence of AF comparing PVI + LAA isolation/ligation vs PVI alone after repeat procedures. PVI, pulmonary vein isolation; LAAI, left atrial appendage isolation; OR, odds ratio; CI, confidence interval
3.5. Complications
One periprocedural ischemic stroke event occurred in all LAA intervention groups compared to two periprocedural transient ischemic attacks and six periprocedural ischemic strokes in the PVI only group. LAA isolation with PVI was associated with less periprocedural cerebrovascular (transient ischemic attack and ischemic stroke) events than PVI alone (Peto: OR, 0.21; 95% CI, 0.06‐0.78; P = 0.02; I 2 = 0%; Figure 3). None of the included studies reported on long‐term cerebrovascular events. All reported adverse events are summarized in Table 3. The difference between the 2 groups was not statistically significant (Peto: OR, 0.39; 95% CI, 0.13‐1.10; P = 0.07; I 2 = 14%; Figure 3).
Figure 3.
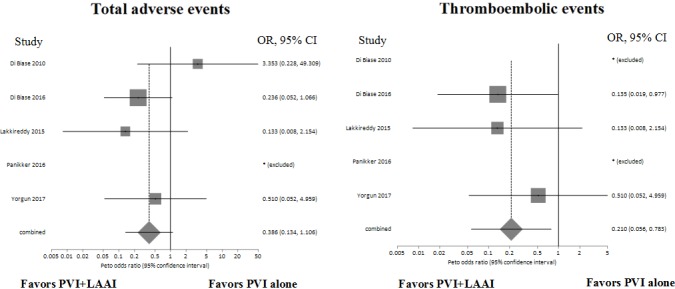
Forest plots of reported thromboembolic events and all reported adverse events in PVI + LAA isolation/LAA ligation vs PVI alone. PVI, pulmonary vein isolation; LAAI, left atrial appendage isolation; OR, odds ratio; CI, confidence interval
Table 3.
Adverse events
| Study first author | Reported adverse events | |
|---|---|---|
| PVI + LAAI | PVI alone | |
| Di Biase (2010) | 4 (1.8%) pericardial effusions requiring pericardiocentesis | No reported complications |
| Di Biase (2016) | 1 (%) pericardial effusions |
4 (4.5%) patients had ischemic stroke 1 (%) pericardial effusions 1 (%) gastrointestinal bleed |
| Lakkireddy (2015) | No reported complications | Two (3%) patients had a transient ischemic attack |
| Panikker (2016) | No major periprocedural complications | No major periprocedural complications |
| Yorgun (2017) | 1 (1%) patients had ischemic stroke | 2 (2%) patients had ischemic stroke |
LAAI, left atrial appendage isolation; PVI, pulmonary vein isolation.
4. DISCUSSION
We found that the addition of LAA isolation by catheter ablation or ligation to PVI is associated with an 80% reduction in the odds of AF recurrence in patients with persistent and LSPAF. This approach may help reduce AF recurrence after PVI in persistent AF.9, 10 Our analysis demonstrates a robust benefit as shown in the influence analysis despite relatively small sample sizes. All studies demonstrated more than 65% reduction in the odds of AF recurrence.27, 32, 33, 34, 35
Current guidelines give a class 2A recommendation for catheter ablation of persistent AF in patients who remain symptomatic despite antiarrhythmic drug therapy.2, 37 This meta‐analysis demonstrates a significant reduction in AF recurrence in patients with persistent AF who underwent LAA isolation in addition to PVI. In a worldwide survey, over 40% of patients undergoing ablation for persistent or LSPAF had lower success rates as compared to patients with paroxysmal AF (PAF).38 Given that at least 30% of patients with persistent AF have identifiable triggers in the LAA region,39 these LAA triggers may be an important source of recurrent AF in patients with persistent AF at index and repeat ablation procedures.17
The benefit of LAA ligation is in line with the benefits of LAA isolation seen in the other included studies and the overall meta‐analysis, indicating the effectiveness of this technique in isolating the LAA. Lakkireddy et al33 demonstrated a reduction in AF recurrence as well as a decreased need for repeat ablation in the LAA group. LAA ligation leads to an acute infarct of the tissue and eventual fibrosis of the LAA22 which results in the electrical isolation of the LAA from the rest of the left atrium. The aMAZE trial is an ongoing study to assess the incremental benefit of catheter‐based LAA ligation procedure as an adjunctive therapy to PVI for persistent or LSPAF (NCT02513797).
This meta‐analysis shows that the benefits attained with LAA isolation occur without an increase in acute complications. However, none of the studies reported the long‐term risk of thromboembolic events. The major concerns of the use of LAA ablation are increased thromboembolic risk, perforation, and phrenic nerve injury.40 LAA isolation may lead to the formation of thrombus in the LAA due to decreased contractility.18, 41 At odds with this hypothesis, Di Biase et al32 and Park et al18 showed that 50% of patients have flow velocity in the LAA that is within the normal range after LAA isolation. Di Biase et al27 also demonstrated an absence of LAA thrombus after distal LAA isolation on TEE after 3 and 6 months postisolation. Distal isolation is performed by circumferential ablation at the ostium of the LAA. Furthermore, a large proportion of these patients have an ongoing indication for anticoagulation due to their CHADS‐VASc score >2 regardless of having undergone LAA isolation.41 In contrast, Rillig et al found that 21% of patients developed an LAA thrombus after LAA ablation despite 90% of patients receiving oral anticoagulation at the time. However, LAA ablation in this study included electrical isolation of the LAA base using bidirectional block of both an anterior and mitral isthmus line, which may explain the increased stroke risk.42 On the other hand, all studies included in this meta‐analysis made use of distal LAA isolation. Anticoagulation should be continued after the procedure, although concomitant ligation of the LAA may provide the added benefit of preventing thrombus formation and reducing the need for anticoagulation.40 Given the theoretical increased risk of stroke due to decreased contraction of the LAA, transesophageal echocardiography is usually performed around 6 months after the ablation to rule out the presence of thrombus.6, 41 Reassuringly, there were no transient ischemic attacks or strokes in the LAA isolation groups, although long‐term follow‐up was limited.
Electrical isolation of the LAA may be technically challenging. Given the thin wall of the LAA, there is a risk of perforation with subsequent pericardial effusion which was seen in 1.8% of cases in the study by Di Biase et al27 Acute reconnections requiring further intraprocedural ablation are not uncommon occurring in 85% of patients in the study by Panikker et al34 However, in patients with arrhythmia recurrence requiring redo ablation, none showed recurrence of firing in the LAA.34
Caution must be taken in the interpretation of these results. Most of the studies included were observational, had a very limited number of participating centers, and had the procedure performed at the hands of very experienced operators. The findings of this study should therefore be considered hypothesis generating and highlight the need for multicenter, randomized studies with blinded adjudication of endpoints. Our results are consistent with an earlier meta‐analysis that included studies assessing surgical excision of the LAA, which represents a distinct therapeutic approach for a different patient population, as well as a study evaluating left atrial anterior wall ablation.43
4.1. Limitations
There are several potential limitations to our meta‐analysis. First, there is only one randomized study included, and therefore, the major effect seen is from observational studies with a variation in design. Second, the number of studies as well as the number of patients included in each of the studies is small and there were differences between baseline characteristics in the different studies, although characteristics were similar in comparative groups. Third, intraprocedural differences related to operators’ experience may have occurred between studies and centers. In addition, there was some uncertainty regarding the actual ablation approaches in some studies. Fourth, although no clear publication bias was demonstrable, the current tools for the assessment of publication bias are likely underpowered given the small number of studies. Finally, long‐term follow‐up, especially with regard to stroke risk, is needed to demonstrate the safety of this technique.
5. CONCLUSIONS
The addition of LAA isolation to PVI was associated with decreases in AF recurrence in patients with persistent AF. Large, multicenter randomized studies are needed to confirm this benefit. Future research is required to elucidate the impact of LAA isolation on long‐term risk of stroke.
CONFLICT OF INTERESTS
Dr Essebag has received honoraria from Abbott, Biosense Medical, Boston Scientific, and Medtronic.
Supporting information
AlTurki A, Huynh T, Dawas A, et al. Left atrial appendage isolation in atrial fibrillation catheter ablation: A meta‐analysis. J Arrhythmia. 2018;34:478–484. 10.1002/joa3.12095
Funding information
Dr. Essebag is the recipient of a Clinical Research Scholar Award from the Fonds de recherche du Québec‐Santé (FRQS).
REFERENCES
- 1. Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow‐up, definitions, endpoints, and research trial design. Europace. 2012;14(4):528–606. [DOI] [PubMed] [Google Scholar]
- 2. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1–76. [DOI] [PubMed] [Google Scholar]
- 3. Verma A, Cairns JA, Mitchell LB, et al. 2014 focused update of the Canadian cardiovascular society guidelines for the management of atrial fibrillation. Can J Cardiol. 2014;30(10):1114–30. [DOI] [PubMed] [Google Scholar]
- 4. Oral H, Knight BP, Tada H, et al. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation. 2002;105(9):1077–81. [DOI] [PubMed] [Google Scholar]
- 5. Ouyang F, Tilz R, Chun J, et al. Long‐term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5‐year follow‐up. Circulation. 2010;122(23):2368–77. [DOI] [PubMed] [Google Scholar]
- 6. Di Biase L, Natale A. Left atrial appendage after electrical isolation: to occlude or not to occlude, that is the question. Circ Arrhythm Electrophysiol. 2016;9(7):e004372. [DOI] [PubMed] [Google Scholar]
- 7. Gokoglan Y, Mohanty S, Gunes MF, et al. Pulmonary vein antrum isolation in patients with paroxysmal atrial fibrillation: more than a decade of follow‐up. Circ Arrhythm Electrophysiol. 2016;9(5):e003660. [DOI] [PubMed] [Google Scholar]
- 8. Wynn GJ, El‐Kadri M, Haq I, et al. Long‐term outcomes after ablation of persistent atrial fibrillation: an observational study over 6 years. Open Heart. 2016;3(2):e000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wynn GJ, Das M, Bonnett LJ, Panikker S, Wong T, Gupta D. Efficacy of catheter ablation for persistent atrial fibrillation: a systematic review and meta‐analysis of evidence from randomized and nonrandomized controlled trials. Circ Arrhythm Electrophysiol. 2014;7(5):841–52. [DOI] [PubMed] [Google Scholar]
- 10. Park J, Pak HN. Elimination of triggers without an additional substrate modification is not sufficient in patients with persistent atrial fibrillation. J Atr Fibrillation. 2015;7(5):1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372(19):1812–22. [DOI] [PubMed] [Google Scholar]
- 12. AlTurki A, Marshall HJ, Proietti R. Targeting nonpulmonary vein triggers during atrial fibrillation ablation: is the game worth the candle? Curr Opin Cardiol. 2018;33(1):50–7. [DOI] [PubMed] [Google Scholar]
- 13. Takahashi Y, Sanders P, Rotter M, Haissaguerre M. Disconnection of the left atrial appendage for elimination of foci maintaining atrial fibrillation. J Cardiovasc Electrophysiol. 2005;16(8):917–9. [DOI] [PubMed] [Google Scholar]
- 14. Yamada T, McElderry HT, Allison JS, Kay GN. Focal atrial tachycardia originating from the epicardial left atrial appendage. Heart Rhythm. 2008;5(5):766–7. [DOI] [PubMed] [Google Scholar]
- 15. Yamada T, Murakami Y, Yoshida Y, et al. Electrophysiologic and electrocardiographic characteristics and radiofrequency catheter ablation of focal atrial tachycardia originating from the left atrial appendage. Heart Rhythm. 2007;4(10):1284–91. [DOI] [PubMed] [Google Scholar]
- 16. Yang Q, Ma J, Zhang S, Hu JQ, Liao ZL. Focal atrial tachycardia originating from the distal portion of the left atrial appendage: characteristics and long‐term outcomes of radiofrequency ablation. Europace. 2012;14(2):254–60. [DOI] [PubMed] [Google Scholar]
- 17. Hocini M, Shah AJ, Nault I, et al. Localized reentry within the left atrial appendage: arrhythmogenic role in patients undergoing ablation of persistent atrial fibrillation. Heart Rhythm. 2011;8(12):1853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park HC, Lee D, Shim J, Choi JI, Kim YH. The clinical efficacy of left atrial appendage isolation caused by extensive left atrial anterior wall ablation in patients with atrial fibrillation. J Interv Card Electrophysiol. 2016;46(3):287–97. [DOI] [PubMed] [Google Scholar]
- 19. Tsai YC, Phan K, Munkholm‐Larsen S, Tian DH, La Meir M, Yan TD. Surgical left atrial appendage occlusion during cardiac surgery for patients with atrial fibrillation: a meta‐analysis. Eur J Cardiothorac Surg. 2015;47(5):847–54. [DOI] [PubMed] [Google Scholar]
- 20. Benussi S, Mazzone P, Maccabelli G, et al. Thoracoscopic appendage exclusion with an atriclip device as a solo treatment for focal atrial tachycardia. Circulation. 2011;123(14):1575–8. [DOI] [PubMed] [Google Scholar]
- 21. Macle L, Cairns J, Leblanc K, et al. 2016 focused update of the Canadian cardiovascular society guidelines for the management of atrial fibrillation. Can J Cardiol. 2016;32(10):1170–85. [DOI] [PubMed] [Google Scholar]
- 22. Han FT, Bartus K, Lakkireddy D, et al. The effects of LAA ligation on LAA electrical activity. Heart Rhythm. 2014;11(5):864–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Afzal MR, Kanmanthareddy A, Earnest M, et al. Impact of left atrial appendage exclusion using an epicardial ligation system (LARIAT) on atrial fibrillation burden in patients with cardiac implantable electronic devices. Heart Rhythm. 2015;12(1):52–9. [DOI] [PubMed] [Google Scholar]
- 24. Barta J, Brat R. Assessment of the effect of left atrial cryoablation enhanced by ganglionated plexi ablation in the treatment of atrial fibrillation in patients undergoing open heart surgery. J Cardiothorac Surg. 2017;12(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lakkireddy D, Turagam M, Afzal MR, et al. Left atrial appendage closure and systemic homeostasis: the LAA HOMEOSTASIS study. J Am Coll Cardiol. 2018;71(2):135–44. [DOI] [PubMed] [Google Scholar]
- 26. Fumoto H, Gillinov AM, Ootaki Y, et al. A novel device for left atrial appendage exclusion: the third‐generation atrial exclusion device. J Thorac Cardiovasc Surg. 2008;136(4):1019–27. [DOI] [PubMed] [Google Scholar]
- 27. Di Biase L, Burkhardt JD, Mohanty P, et al. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation. 2010;122(2):109–18. [DOI] [PubMed] [Google Scholar]
- 28. Proietti R, Joza J, Arensi A, et al. Novel nonpharmacologic approaches for stroke prevention in atrial fibrillation: results from clinical trials. Med Devices. 2015;8:103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med 2009;151(4):264–9. , W64. [DOI] [PubMed] [Google Scholar]
- 30. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. [DOI] [PubMed] [Google Scholar]
- 31. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 32. Di Biase L, Burkhardt JD, Mohanty P, et al. Left atrial appendage isolation in patients with longstanding persistent AF undergoing catheter ablation: BELIEF trial. J Am Coll Cardiol. 2016;68(18):1929–40. [DOI] [PubMed] [Google Scholar]
- 33. Lakkireddy D, Sridhar Mahankali A, Kanmanthareddy A, et al. Left atrial appendage ligation and ablation for persistent atrial fibrillation: the LAALA‐AF Registry. JACC Clin Electrophysiol. 2015;1(3):153–60. [DOI] [PubMed] [Google Scholar]
- 34. Panikker S, Jarman JW, Virmani R, et al. Left atrial appendage electrical isolation and concomitant device occlusion to treat persistent atrial fibrillation: a first‐in‐human safety, feasibility, and efficacy study. Circ Arrhythm Electrophysiol. 2016;9(7):e004652. [DOI] [PubMed] [Google Scholar]
- 35. Yorgun H, Canpolat U, Kocyigit D, Coteli C, Evranos B, Aytemir K. Left atrial appendage isolation in addition to pulmonary vein isolation in persistent atrial fibrillation: one‐year clinical outcome after cryoballoon‐based ablation. Europace. 2017;19(5):758–68. [DOI] [PubMed] [Google Scholar]
- 36. Romanov A, Pokushalov E, Artemenko S, et al. Does left atrial appendage closure improve the success of pulmonary vein isolation? Results of a randomized clinical trial. J Interv Card Electrophysiol. 2015;44(1):9–16. [DOI] [PubMed] [Google Scholar]
- 37. Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;33(21):2719–47. [DOI] [PubMed] [Google Scholar]
- 38. Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3(1):32–8. [DOI] [PubMed] [Google Scholar]
- 39. Romero J, Avendano R, Natale A, Di Biase L. Ablation of advanced subtypes of atrial fibrillation: highlighting the art of when and when not to perform additional ablation. Curr Cardiovasc Risk Rep. 2017;11(6):19. [Google Scholar]
- 40. Calkins H, Hindricks G, Cappato R, et al. Temporary removal: 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Di Biase L, Natale A. To, “isolate” or “not to isolate”, the left atrial appendage, “that is the question”. J Interv Card Electrophysiol. 2016;47(3):261–3. [DOI] [PubMed] [Google Scholar]
- 42. Rillig A, Tilz RR, Lin T, et al. Unexpectedly high incidence of stroke and left atrial appendage thrombus formation after electrical isolation of the left atrial appendage for the treatment of atrial tachyarrhythmias. Circ Arrhythm Electrophysiol. 2016;9(5):e003461. [DOI] [PubMed] [Google Scholar]
- 43. Friedman DJ, Black‐Maier EW, Barnett AS, et al. Left atrial appendage electrical isolation for treatment of recurrent atrial fibrillation: a meta‐analysis. JACC Clin Electrophysiol. 2018;4(1):112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


