Abstract
Background
The ventral intermediate nucleus (VIM) is the target of choice for Essential Tremor (ET) deep brain stimulation (DBS). Renewed interest in caudal zona incerta (cZI) stimulation for tremor control has recently emerged and some groups believe this approach may address long‐term reduction of benefit seen with VIM‐DBS.
Objectives
To compare clinical outcomes and DBS programming in the long‐term between VIM and cZI neurostimulation in ET‐DBS patients.
Materials and Methods
A retrospective review of 53 DBS leads from 47 patients was performed. Patients were classified into VIM or cZI groups according to the location of the activated DBS contact. Demographics, DBS settings, and Tremor Rating Scale scores were compared between groups at baseline and yearly follow‐up to 4 years after DBS. Student t‐tests and analysis of variance (ANOVA) were used to compare variables between groups.
Results
Relative to baseline, an improvement in ON‐DBS tremor scores was observed in both groups from 6 months to 4 years post‐DBS (p < 0.05). Although improvement was still significant at 4 years, scores from month 6 to 2 years were comparable between groups but at 3 and 4 years post‐DBS the outcome was better in the VIM group (p < 0.01). Stimulation settings were similar across groups, although we found a lower voltage in the VIM group at 3 years post‐DBS.
Conclusions
More ventral DBS contacts in the cZI region do improve tremor, however, VIM‐DBS provided better long‐term outcomes. Randomized controlled trials comparing cZI vs VIM targets should confirm these results.
Keywords: cerebellar pathways, intermediate nucleus, posterior subthalamic area, tremor, ventral zona incerta
Essential Tremor (ET) is the most common adult‐onset movement disorder, impairing approximately 10 million people in the United States.1, 2 Current evidence‐based guidelines recommend medical therapies as the first‐line treatment option.3 However, nearly 50% of patients will have a suboptimal response to medical management or will experience medication side effects that may lead to discontinuation of therapy.4 Thalamic ventralis intermedius nucleus (VIM) deep brain stimulation (DBS) received approval from the US Food and Drug Administration in 1997 and is well established as a safe and effective treatment for medically refractory tremor.5, 6, 7
Despite good patient selection and significant initial tremor suppression achieved with VIM‐DBS, around 40‐70% of patients will experience a worsening of tremor over time.8, 9 The reasons for this declining efficacy remain debated, but most cases have been attributed to disease progression and/or tolerance to the neuromodulatory therapy.10, 11 Recent brain imaging evidence suggests that worsening of tremors over time may be related to microstructural and degenerative changes in the dentate nucleus and superior cerebellar peduncle.12
Neuromodulation with VIM‐DBS for ET has been demonstrated to be effective by multiple studies.13, 14 Although the optimal target location for thalamic stimulation is considered to be the anterior margin of the VIM nucleus (antero‐posterior plane),15 stimulation below the intercommissural line appears more efficient, but equally effective as stimulation above it within the nucleus.16 Additionally, several groups have reported that targets located 2–3 mm below the inferior part of the thalamus provide profound reduction in tremor amplitude.9, 17
Given the expected worsening of tremors in ET‐DBS patients, it is important to consider other potential stimulation targets within the same dysfunctional tremor network. With initial efficacy in tremor suppression demonstrated in the 1960s and early 1970s,18 the subthalamic area has recently reemerged as an additional (and potentially more effective) target for tremor suppression in ET that could also influence the observed tremor progression.19, 20 Mostly referred to as caudal zona incerta (cZI), the terminology for this region varies in nomenclature and may also include the posterior subthalamic area (PSA) or prelemniscal radiations.19, 21, 22
The goal of this study was to compare long‐term tremor control between VIM and cZI‐region DBS. Establishing differences between the two targets and determining possible prevention of tremor worsening might help to improve clinical outcomes in ET‐DBS patients and facilitate a better understanding of the affected circuitries in this complex disease.23, 24
Methods
The University of Florida (UF) Institutional Review Board (IRB) approved the study (Protocol No. 201400613). Written informed consent from study participants was obtained for research purposes under the UF INFORM database protocol.
Study Design, Setting, and Participants
An observational study of ET patients was conducted at the UF‐Health Center for Movement Disorders and Neurorestoration. We queried subjects with a clinical diagnosis of ET in our DBS database. Diagnosis of ET was made by a movement disorder, fellowship‐trained specialist using international consensus criteria.25 Electronic records from 96 patients were reviewed for patients who had consistent stimulation provided from a single contact in either the VIM or cZI throughout follow‐up. Application of these criteria led to 47 patients (53 leads) being included in the final analysis.
Study Sources/variables
Data were extracted from each patient's electronic medical record and from the UF INFORM system, a movement disorders clinical‐research database. Basic demographics and clinical variables were recorded. We divided estimated disease duration into 10‐year epochs: zero was defined as ≤10 years, 1 as 11‐20 years, and so on, with a score of 5 defined as ≥51 years. For patients with bilateral implants (N = 6), each lead was separately included in the analysis since we examined unilateral tremor scores. DBS settings—voltage, pulse width, and frequency—were obtained from follow‐up programming visits. Attempts to optimize DBS settings for efficacy were made by the clinician programmer at each visit to achieve maximal tremor control without side effects.
Study Measurements
We divided the cohort into VIM or cZI stimulation groups according to the location of the deepest activated contact on the DBS lead recorded on each of the clinical programming visits. XYZ coordinates were measured from each DBS lead using a standard protocol that used postoperative CT scan fused to a high resolution, pre‐operatively acquired targeting MRI with 1 mm isometric voxel images from both gadolinium enhanced T1 and Fast Gray Matter Acquisition T1 Inversion Recovery.26 These scans were subsequently fused to a 3‐D morphable atlas using the Schaltenbrand‐Bailey Sudhyadhom technique.27 The stimulation groups were classified as: VIM—activated contacts at or above the anterior commissure‐posterior commissure (AC‐PC) line; and cZI—activated contacts below the AC‐PC line. Figure 1 depicts the studied targets among groups. Figure S1 provides a 3D view of an example ViM and ZI contact.
Figure 1.
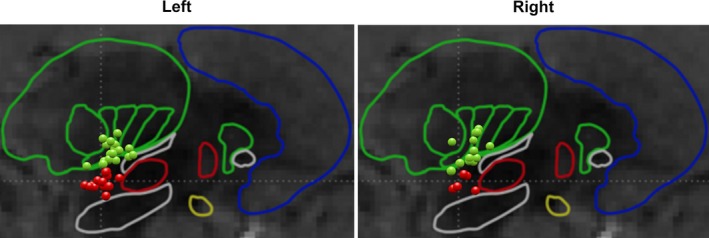
Representation of the active contacts in the targeting brain zones in the left and right hemispheres. Outlined regions represent the atlas used for targeting. Green dots indicate VIM contacts and red dots indicate cZI contacts.
The Fahn‐Tolosa‐Marin Tremor Rating Scale (TRS)28 was used for clinical evaluations. DBS effects were assessed only by scores of the contralateral upper extremity (CUE) with respect to the implanted lead, obtained at baseline, 6 months post DBS implantation, and at subsequent annual follow‐ups. The TRS rates the severity of tremor by body part from zero (none) to 4 (severe). Upper extremity tremor and hand function scores ipsilateral and contralateral to the implanted hemisphere were used as clinical outcomes. TRS item number 5 (right) or 6 (left) score, which evaluates upper extremity resting, postural, and action/intention tremor was used to obtain a tremor score (maximum possible score 12), while the items assessing motor tasks/functions (TRS items number 11‐14) were added to obtain a total hand function score (maximum possible score 16). We compared TRS postural and action/intention tremor scores separately between targets.
Surgical Planning and Procedure
Surgical procedure details have been published previously.29 Briefly, a Cosman‐Roberts‐Wells (CRW) stereotactic frame was applied under local anesthesia and a stereotactic head CT scan was then obtained and fused to a pre‐operative MRI. Starting from default VIM target coordinates (X = ±14.5; Y = ‐7; Z = ‐2), targeting was carried out using our UF modified Schaltenbrand‐Bailey atlas.27 During the surgical procedure, detailed single pass microelectrode recording was used to locate the anterior border of the ventralis caudalis (Vc) sensory nucleus. Afterwards, DBS Medtronic Leads Model 3387 (Medtronic) was implanted and macrostimulation testing was performed to evaluate stimulation‐induced side effects. Leads were targeted 2 mm below the ventral border of the thalamus and 2 mm anterior to the border of the Vc nucleus. Neurostimulators were placed 4 weeks later and activated during their first clinical DBS programming visit.
Statistical Analyses
Univariate descriptive analyses were used to report demographic and clinical characteristics. T‐test was used to compare age at surgery, clinical TRS scores, and lead XYZ coordinates between groups. Chi‐square test was used to compare disease duration (treated as a categorical variable; see above), family history of ET, medication status, and gender. Separate repeated measures ANOVA were used to assess changes in summed TRS tremor scores (considered continuous variables) for CUE tremor and hand function. Time and ON/OFF stimulation were used as within‐subject factors and target group was used as a between‐subjects factor for ANOVAs. All significant effects were followed up using Bonferroni‐corrected post hoc tests. Mann‐Kendall tests were used to identify monotonic trends in clinical outcomes and DBS settings across time. Statistical analyses were performed using the statistical software R with alpha levels set at 0.05.
Results
Study Population
The cohort included data from 53 leads (47 patients). Fifty‐seven percent of the cohort was male, with a mean age at surgery of 64.8 ± 10.8 years, and a mean disease duration score of 2.6 ± 1.8. The cohort had baseline mean scores as follows: TRS motor score of 38.6 ± 10.6, daily functioning score of 16.8 ± 5.5, and a total TRS score of 55.1 ± 15.1. Table 1 compares demographic and clinical characteristics between the studied groups. Significant differences were observed for gender and medication use, where the cZI group had a significantly higher number of male patients (p < 0.05) and a higher number of patients using medications (p < 0.01). No other baseline significant differences were observed.
Table 1.
Demographics, clinical characteristics, and DBS active contact coordinates in relationship to the midcommisural point
| Total N = 53 | VIM N = 33 | cZI N = 20 | P‐value | |
|---|---|---|---|---|
| Male, no. (%) | 30 (56.6) | 15 (45.5) | 15 (75) | 0.067b |
| Age at surgery, yrs. (SD) | 64.8 (10.8) | 65.2 (9.1) | 64.2 (13.3) | 0.74a |
| Disease duration score ≤3, no. (%) | 37 (69.8) | 23 (69.7) | 14 (70) | 0.98b |
| Family history of tremors, no. (%)c | 21 (40.4) | 12 (37.5) | 9 (45) | 0.59b |
| On medications, no. (%) | 30 (56.6) | 14 (42.4) | 16 (80) | 0.007b |
| Baseline TRS | ||||
| Parts A and B, (SD) | 38.6 (10.6) | 38.7 (11.5) | 38.3 (9.3) | 0.89a |
| Part C, (SD)c | 16.8 (5.5) | 17.2 (5.6) | 16.2 (5.4) | 0.58a |
| Total, (SD) | 55.1 (15.1) | 55.4 (13.1) | 48.8 (15.6) | 0.35a |
| Contact coordinates | ||||
| X (SD) | 13.9 (1.7) | 14.4 (1.4) | 13.2 (1.9) | 0.015a |
| Y (SD) | −4.6 (1.6) | −4.1 (1.5) | −5.4 (1.4) | 0.002a |
| Z (SD) | 1.0 (2.5) | 2.7 (1.5) | −1.7 (0.9) | <0.001a |
Abbreviations: VIM, ventral intermediate nucleus; cZI, caudal zona incerta; TRS, Fahn‐Tolosa‐Marin Tremor Rating Scale.
T‐test
Chi‐Square Test
n = 52
Tremor and Hand Function Scores
Figure 2 shows ON stimulation and OFF stimulation tremor scores in the VIM group (Fig. 2A) and cZI group (Fig. 2E). Baseline contralateral tremor scores revealed no significant differences between VIM (4.7 ± 1.68) and cZI (5.2 ± 1.99; p = 0.38). A three‐way ANOVA revealed an overall main effect of DBS stimulation (ON vs. OFF, p < 10‐10) and group (p < 0.001) on tremor scores. There was not a main effect of follow‐up time (p = 0.26). Post‐hoc analysis demonstrated that 6‐month, 1‐year, and 2‐year follow‐up tremor scores did not differ between groups for both OFF (p = 0.90, p = 0.71, and p = 0.16, respectively) and ON stimulation (p = 0.43, p = 0.75, and p = 0.58, respectively). While we also found no significant difference between tremor scores for OFF stimulation at 3 years follow‐up (p = 0.45), there was a significantly reduced ON stimulation tremor score in the VIM group (0.63 ± 0.68) compared to the cZI group (1.92 ± 0.95) at 3 years follow‐up (p < 0.001). There was also a difference between VIM and cZI tremor scores at 4 years follow‐up in both OFF stimulation (p < 0.01) and ON stimulation (p < 0.05).
Figure 2.
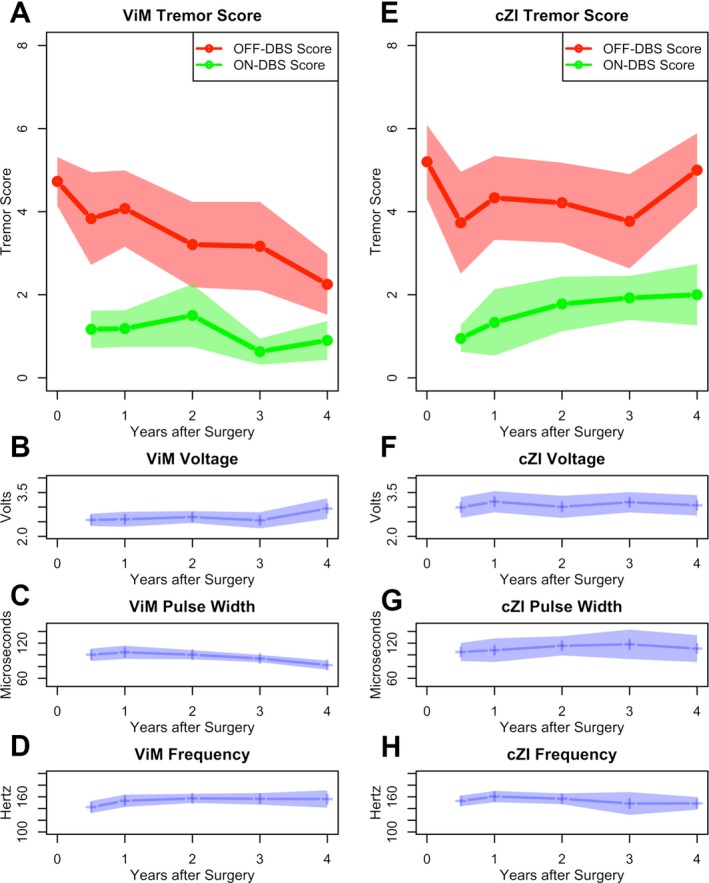
Comparison of ON stimulation (green) and OFF stimulation (red) tremor scores and DBS voltage, pulse width, and frequency settings in the VIM (A‐D) vs. cZI (E‐H) groups. A time point of 0 years after surgery refers to baseline data pre‐DBS.
Results were similar across groups with respect to tremor scores relative to baseline. At 6‐month follow‐up ON stimulation, tremor scores improved by 65‐83% (95% confidence interval [CI]) in the VIM group and 71‐87% in the cZI group (p = 0.47). ON stimulation tremor improvement was generally maintained at year 1 (VIM 59‐83% vs. cZI 35‐96%, p = 0.71) and year 2 (VIM 50‐87% vs. cZI 40‐79%, p = 0.54), but differed at year 3 (VIM 79‐94% vs. cZI 38‐73%, p < 0.05) and year 4 (VIM 68‐92% vs. cZI 33‐76%, p < 0.05).
Similar to tremor scores, an ANOVA revealed that hand function depended on stimulation being ON/OFF (p < 10‐6), group (p < 10‐6), and not follow‐up time (p = 0.45). Also similar to tremor scores, hand function only significantly differed for OFF stimulation at 3 years (p < 0.01). All other OFF and ON stimulation comparisons showed similar hand function scores. VIM ON stimulation showed 51‐73% (95% CI) improvement at 6 months, 40‐65% improvement at 1 year, 36‐79% improvement at 2 years, 42‐78% improvement at 3 years, and 42‐78% improvement at 4 years. The cZI ON stimulation showed 39‐66%, 30‐65%, 34‐61%, 20‐57%, and 27‐63% improvement at 6 months, 1 year, 2 years, 3 years, and 4 years, respectively. There were no differences across groups.
Last, we tested for monotonic trends in tremor scores over time following DBS. In contrast to the VIM cohort, ZI tremor scores tended to increase (tau = 0.26, p < 0.01) ON stimulation. There were no significant trends in hand function scores across time.
Postural and Action/intention Tremor Scores
Postural tremor scores and action/intention tremor scores improved after surgery relative to baseline in both groups (postural tremor improvement; VIM: 92 ± 17% cZI: 91 ± 25%; action/intention tremor improvement; VIM: 60 ± 34%, cZI: 57 ± 31%). There were no significant differences in absolute postural tremor scores or percent change in postural tremor scores relative to baseline between VIM and cZI at any follow‐up time points. In contrast, while there were also no differences across groups in absolute action/intention scores at 6 months, 1‐year, and 2‐year follow‐up, we found lower action/intention scores in the VIM group compared to the cZI group at 3‐year (0.68 ± 0.75 vs. 1.38 ± 0.87, p < 0.05) and 4‐year (0.70 ± 0.48, vs. 1.33 ± 0.65, p < 0.05) follow‐up. Relative to baseline, action/intention tremor scores marginally improved more at 3 years in the VIM group (54‐89% improvement) compared to the ZI group (2‐71%, p = 0.068). Similarly, action/intention tremor scores significantly improved more at 4 years in the VIM group (57‐87%) compared to the ZI group (1‐66%, p < 0.05).
DBS Settings
Figure 2 shows DBS settings in the VIM group (Fig. 2B‐D) and cZI group (Fig. 2F‐H). Overall, stimulation settings were similar across groups. However, average voltage use was significantly lower in the VIM group (2.55 ± 0.60 volts) compared to the cZI group at 3‐year follow‐up (3.17 ± 0.67 volts; p < 0.001). Stimulation settings did not trend over time with the exception of decreasing VIM pulse width (tau = −0.14, p < 0.05).
Discussion
We conducted a longitudinal retrospective study using an ET‐DBS cohort to compare clinical scores and DBS settings between the VIM and cZI regions. In our study, both regions showed improved tremor and hand function scores at 6 months post DBS surgery. Both targets maintained tremor and hand function improvement at 4‐year follow‐up, supporting a clinical benefit for ET regardless of the region of the activated contact. However, the data suggested a potential long‐term advantage for applying stimulation to the VIM region due to a gradual worsening in tremor scores over time in patients stimulated chronically in the cZI region.
Three studies have reported clinical improvement from cZI DBS at 1‐year follow‐up,21, 30, 31, 32, 33 which is similar to the reported obtained benefit with VIM‐DBS.34 Additionally, three case series reported maintenance of tremor suppression at a mean of 1.5 to 6‐year follow‐up when targeting the cZI.14, 19, 20 Our results are consistent with these studies, revealing a maintenance of improved tremor scores at 4 years post‐surgery. Based on these data, the targeted areas are effective in suppressing symptoms in the long‐term. A summary of these studies, including anatomical coordinates of implanted leads, has been provided in Table S1.
Three additional studies have compared the clinical effects of VIM verses cZI area in ET‐DBS patients. A study from Barbe et al. of 21 patients followed for 3 months post‐DBS concluded that stimulating the zone below the intercommisural line (sub‐ICL, Z: −1.4 ± 1.2) was more efficient, but equally effective, when compared to the VIM target (Z: 1.04 ± 1.2).16 A separate prospective study by Blomstedt et al. compared 68 patients targeting the VIM verses PSA (cZI) and revealed an improvement in TRS scores when stimulating both targets. Although the PSA group showed a greater improvement in tremor and hand function scores (89% vs. 70%), a difference in follow‐up could have affected results.35 In a third study by Sandvik et al., 36 patients with VIM verses PSA (cZI) were tested revealing greater improvement in tremor and hand function scores in the PSA group. As in the previous study, follow‐up periods were different between groups.36 While our study, however, revealed improvements in clinical scores in both targets, a significant difference was observed at 3‐year and 4‐year follow‐up, when the VIM group improved more than the cZI group, suggesting that VIM‐DBS may have maintained a better long‐term tremor control than the cZI target. We note that the cZI stimulation settings used in this study may be different from previously reported settings because our cohort had higher baseline tremor severity scores compared to prior studies.37
We analyzed the changes in scores for postural and action/intentional tremors over time. Both types of tremors significantly improved at 6 months post‐DBS. Our results agree with a similar finding from Herzog et al. demonstrating that the highest percentage improvement in the deceleration and the target periods was found in contacts below the AC‐PC line.38 However, it should be noted that the study combined ET and multiple sclerosis patients and did not include long‐term follow‐up. Additionally, in this study we observed that action/intentional tremor score significantly worsened in the cZI group when compared to the VIM group at the 3‐year and 4‐year follow‐up.
It has been suggested that VIM‐DBS suppresses tremor by disruption of pathological afferent oscillatory activity, thereby preventing further efferent propagation of this abnormal signal.39 In contrast, the PSA/cZI/Raprl area may be more prone to spreading current than would be expected in more classical structures such as the VIM.40 A recent study reported the production of ataxic symptoms in 7 ET patients when stimulating below the intercommisural line with what the authors defined as a “supratherapeutic” voltage, suggesting that the enlarged electrical field affected efferent fibers of the red nucleus, resulting in a disturbance of the cerebellar outflow system.41 Stimulating in the cZI/subthalamic area with supratherapeutic levels is likely not the only factor contributing to the action/intentional ataxic‐like tremor seen in ET.42 We might also consider that the activated deeper contacts might not be consistently located within the cZI, and that the tremor effect may have been due to the proximity to axons or to the STN.43 Ataxia and dysmetria could be an observed side effect when stimulating in the subthalamic area at therapeutic levels.44 An ataxic‐like tremor is also expected with disease progression due to cerebellar nuclei degeneration.12
Several important limitations of the present study need to be considered. The issue of a retrospective design and that the distribution among groups was not uniform could have biased the results. We did not prospectively determine which patients would be included in each group. Furthermore, all patients in this study were implanted with the intention of VIM stimulation. Thus, the cZI was not specifically targeted in the cZI group presented here. Specific targeting of the cZI region could result in different lead trajectories. Another consideration is that coordinates of the chronically active contact were obtained using local software and these may slightly vary when compared to other fusion software modules. User defined AC‐PC coordinates and subsequent anatomic coordinates may also vary. In addition, inaccuracies of atlas deformation and superimposition should be considered.45 Utilizing the Z‐axis for grouping patients is not the best method for targeting the cZI, as this target also depends on lead trajectory. Functional imaging studies, MRI tractography, or volumetric modeling of the stimulated field could improve targeting, while measuring quantitative tremor scores could also lead to more uniform data. The correlation between a model of volumetric stimulation measurements with clinical scores using more objective tools could be considered for future studies.
Conclusions
Deeper or more ventral contacts that are activated in the cZI region below the classical VIM target do improve tremor, however, VIM‐DBS provided better long‐term outcomes. Our study supports the continued use of the VIM as the standard target for tremor suppression and suggest that in some patients cZI may be an option. Because it is relatively easy for neurosurgeons to leave the deep ventral DBS contact in cZI even if not activated, this can provide options for select patients with suboptimal tremor control. Additional studies comparing anatomical targeting of VIM and cZI in a randomized controlled clinical trial will be the next step to confirm our results and differences between the two targets.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution C. Review and Critique; 3. Manuscript: A. Writing of the First Draft B. Review and Critique.
MSO: 1A, 1B, 2C, 3B
R.S.E.: 1A, 1B, 1C, 2A, 2B, 3A
D.M.R.: 1A, 1B, 1C, 2A, 3A, 3B
J.W.: 1C
L.A.: 1C, 2A, 2C
B.A.: 1C, 3A
J.G.: 1C, 3A
A.R.B.: 1C, 3A
J.D.H.: 1C, 3B
J.N.C.: 1C
C.W.H.: 2C, 3B
A.R.Z.: 3B
A.G.: 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest: This work was supported by the National Parkinson Foundation (1UH3NS095553‐01A1), Tyler's Hope, Bachmann‐Strauss Foundation, University of Florida Foundation, and the UF INFORM database.
Financial disclosures from previous 12 months: RSE, JW, LA, ARZ, JNC, JG, BA, ARB, AWS, EM, and JDH have no financial disclosures to report. CWH receives grant support from the University of Florida Clinical and Translational Research Institute, which is supported in part by NIH award KL2 TR001429. He has served as a research committee member for the Michael J. Fox Foundation and as a speaker for the National Parkinson Foundation, the Parkinson's Disease Foundation, and the Davis Phinney Foundation. CWH has participated in CME and educational activities on movement disorders sponsored by Allergan, Ipsen, Mertz Pharmaceuticals, Peerview Online, and QuantiaMD. DMR serves as a consultant for the National Parkinson Foundation and has received honoraria for educational activities from Teva, UCB, Boehringer‐Ingelheim, and the International Parkinson and Movement Disorders Society. KDF has received research grant support from Medtronic, St. Jude (now Abbott), Boston Scientific, Neuropace, and Functional Neuromodulation. He has also received fellowship support from Medtronic. He has not received any personal renumeration from any industrial source in the past 12 months. AG receives grant support from the NIH and NSF. The University of Florida and not AG receives device donations from Medtronic, LLC. MSO serves as a consultant for the National Parkinson Foundation and has received research grants from NIH, NPF, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, the Bachmann‐Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation. MSO's DBS research is supported by: R01 NR014852. MSO has previously received honoraria, but in the past > 60 months has received no support from industry. MSO has received royalties for publications with Demos, Manson, Amazon, Smashwords, Books4Patients, and Cambridge (movement disorders books). MSO is an associate editor for New England Journal of Medicine Journal Watch Neurology. MSO has participated in CME and educational activities on movement disorders (in the last 36) months sponsored by PeerView, Prime, QuantiaMD, WebMD, MedNet, Henry Stewart, and by Vanderbilt University. The institution and not MSO receives grants from Medtronic, AbbVie, Allergan, and ANS/St. Jude, and the Principal Investigator (PI) has no financial interest in these grants. MSO has participated as a site PI and/or co‐investigator for several NIH, foundation, and industry sponsored trials over the years, but has not received honoraria.
Supporting information
Figure S1. An example lead is shown in three‐dimensional space for a representative VIM (green) and cZI (red) patient. Leads are superimposed onto a standard MRI. See Data S1 for further details.
Table S1. Essential Tremor DBS studies reporting clinical effects targeting the subthalamic region (cZI, PSA, or subthalamic area).
Data S1. Methods.
Acknowledgements
We would like to acknowledge the support of the National Parkinson Foundation, Tyler's Hope, and the Bachmann‐Strauss Foundations. Also we would like to acknowledge the UF Foundation and the UF INFORM database.
Supporting information may be found in the online version of this article.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Elias WJ, Shah BB Tremor. JAMA 2014;311:948–954. [DOI] [PubMed] [Google Scholar]
- 2. Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord 2010;25:534–541. [DOI] [PubMed] [Google Scholar]
- 3. Zesiewicz TA, Elble RJ, Louis ED, et al. Evidence‐based guideline update: treatment of essential tremor: report of the Quality Standards subcommittee of the American Academy of Neurology. Neurology 2011;77:1752–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koller WC, Vetere‐Overfield B. Acute and chronic effects of propranolol and primidone in essential tremor. Neurology 1989;39:1587–1588. [DOI] [PubMed] [Google Scholar]
- 5. Miocinovic S, Somayajula S, Chitnis S, Vitek JL. History, applications, and mechanisms of deep brain stimulation. JAMA Neurol 2013;70:163–171. [DOI] [PubMed] [Google Scholar]
- 6. Baizabal‐Carvallo JF, Kagnoff MN, Jimenez‐Shahed J, Fekete R, Jankovic J. The safety and efficacy of thalamic deep brain stimulation in essential tremor: 10 years and beyond. J Neurol Neurosurg Psychiatry 2014;85:567–572. [DOI] [PubMed] [Google Scholar]
- 7. Dick JP. Multicentre European study of thalamic stimulation in essential tremor. J Neurol Neurosurg Psychiatry 2003;74:1362–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pilitsis JG, Metman LV, Toleikis JR, Hughes LE, Sani SB, Bakay RA. Factors involved in long‐term efficacy of deep brain stimulation of the thalamus for essential tremor. J Neurosurg 2008;109:640–646. [DOI] [PubMed] [Google Scholar]
- 9. Papavassiliou E, Rau G, Heath S, et al. Thalamic deep brain stimulation for essential tremor: relation of lead location to outcome. Neurosurgery 2004;54:1120–1129; discussion 1129–1130. [DOI] [PubMed] [Google Scholar]
- 10. Favilla CG, Ullman D, Wagle Shukla A, Foote KD, Jacobson CE IV, Okun MS. Worsening essential tremor following deep brain stimulation: disease progression versus tolerance. Brain 2012;135:1455–1462. [DOI] [PubMed] [Google Scholar]
- 11. Barbe MT, Liebhart L, Runge M, et al. Deep brain stimulation in the nucleus ventralis intermedius in patients with essential tremor: habituation of tremor suppression. J Neurol 2011;258:434–439. [DOI] [PubMed] [Google Scholar]
- 12. Nicoletti D, Manners F, Novellino F, et al. Quattrone. Diffusion tensor MRI changes in cerebellar structures of patients with familial essential tremor. Neurology 2010;74:988–994. [DOI] [PubMed] [Google Scholar]
- 13. Kitagawa M, Murata J, Kikuchi S, Sawamura Y, Saito H, Sasaki H, Tashiro K. Deep brain stimulation of subthalamic area for severe proximal tremor. Neurology 2000;55:114–116. [DOI] [PubMed] [Google Scholar]
- 14. Murata J‐i, Kitagawa M, Uesugi H, et al. Electrical stimulation of the posterior subthalamic area for the treatment of intractable proximal tremor. J Neurosurg 2003;99:708–715. [DOI] [PubMed] [Google Scholar]
- 15. Ramirez‐Zamora A, Smith H, Kumar V, Prusik J, Phookan S, Pilitsis JG. Evolving concepts in posterior subthalamic area deep brain stimulation for treatment of tremor: surgical neuroanatomy and practical considerations. Stereotact Funct Neurosurg 2016;94:283–297. [DOI] [PubMed] [Google Scholar]
- 16. Barbe MT, Liebhart L, Runge M, et al. Deep brain stimulation of the ventral intermediate nucleus in patients with essential tremor: stimulation below intercommissural line is more efficient but equally effective as stimulation above. Exp Neurol 2011;230:131–137. [DOI] [PubMed] [Google Scholar]
- 17. Velasco F, Jiménez F, Luisa Pérez M, Carrillo‐Ruiz JoséD, Velasco AL, Ceballos J, Velasco M. Electrical stimulation of the prelemniscal radiation in the treatment of Parkinson's disease: an old target revised with new techniques. Neurosurgery 2001;49:293–306; discussion 306‐298. [DOI] [PubMed] [Google Scholar]
- 18. Velasco FC, Molina‐Negro P, Bertrand C, Hardy J. Further definition of the subthalamic target for arrest of tremor. J Neurosurg 1972;36:184–191. [DOI] [PubMed] [Google Scholar]
- 19. Fytagoridis A, Sandvik U, Astrom M, Bergenheim T, Blomstedt P. Long term follow‐up of deep brain stimulation of the caudal zona incerta for essential tremor. J Neurol Neurosurg Psychiatry 2012;83:258–262. [DOI] [PubMed] [Google Scholar]
- 20. Plaha P, Javed S, Agombar D, O'Farrell G, Khan S, Whone A, Gill S. Bilateral caudal zona incerta nucleus stimulation for essential tremor: outcome and quality of life. J Neurol Neurosurg Psychiatry 2011;82:899–904. [DOI] [PubMed] [Google Scholar]
- 21. Sandvik U, Hariz GM, Blomstedt P. Quality of life following DBS in the caudal zona incerta in patients with essential tremor. Acta Neurochir (Wien) 2012;154:495–499. [DOI] [PubMed] [Google Scholar]
- 22. Blomstedt P, Fytagoridis A, Astrom M, Linder J, Forsgren L, Hariz MI. Unilateral caudal zona incerta deep brain stimulation for Parkinsonian tremor. Parkinsonism Relat Disord 2012;18:1062–1066. [DOI] [PubMed] [Google Scholar]
- 23. Hariz GM, Blomstedt P, Koskinen LO. Long‐term effect of deep brain stimulation for essential tremor on activities of daily living and health‐related quality of life. Acta Neurol Scand 2008;118:387–394. [DOI] [PubMed] [Google Scholar]
- 24. Nazzaro JM, Pahwa R, Lyons KE. Long‐term benefits in quality of life after unilateral thalamic deep brain stimulation for essential tremor. J Neurosurg 2012;117:156–161. [DOI] [PubMed] [Google Scholar]
- 25. Bain P, Brin M, Deuschl G, et al. Criteria for the diagnosis of essential tremor. Neurology 2000;54:S7. [PubMed] [Google Scholar]
- 26. Sudhyadhom A, Haq IU, Foote KD, Okun MS, Bova FJ. A high resolution and high contrast MRI for differentiation of subcortical structures for DBS targeting: the Fast Gray Matter Acquisition T1 Inversion Recovery (FGATIR). NeuroImage 2009;47(Suppl 2):T44–T52. [DOI] [PubMed] [Google Scholar]
- 27. Sudhyadhom A, Okun MS, Foote KD, Rahman M, Bova FJ. A three‐dimensional deformable brain atlas for DBS targeting. I. Methodology for atlas creation and artifact reduction. Open Neuroimag J 2012;6:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fahn S, Tolosa E, Concepcion M. In: Jankovic J, Tolosa E, eds. Parkinson's disease and movement disorders, 2nd ed Baltimore: Williams & Wilkins; 1993:271–280. [Google Scholar]
- 29. Morishita T, Foote KD, Wu SS, et al. Brain penetration effects of microelectrodes and deep brain stimulation leads in ventral intermediate nucleus stimulation for essential tremor. J Neurosurg 2010;112:491–496. [DOI] [PubMed] [Google Scholar]
- 30. Plaha P, Patel NK, Gill SS. Stimulation of the subthalamic region for essential tremor. J Neurosurg 2004;101:48–54. [DOI] [PubMed] [Google Scholar]
- 31. Hamel W, Herzog J, Kopper F, et al. Deep brain stimulation in the subthalamic area is more effective than nucleus ventralis intermedius stimulation for bilateral intention tremor. Acta Neurochir (Wien) 2007;149:749–758; discussion 758. [DOI] [PubMed] [Google Scholar]
- 32. Plaha P, Khan S, Gill SS. Bilateral stimulation of the caudal zona incerta nucleus for tremor control. J Neurol Neurosurg Psychiatry 2008;79:504–513. [DOI] [PubMed] [Google Scholar]
- 33. Blomstedt P, Sandvik U, Tisch S. Deep brain stimulation in the posterior subthalamic area in the treatment of essential tremor. Mov Disord 2010;25:1350–1356. [DOI] [PubMed] [Google Scholar]
- 34. Hariz MI, Krack P, Alesch F, et al. Multicentre European study of thalamic stimulation for parkinsonian tremor: a 6 year follow‐up. J Neurol Neurosurg Psychiatry 2008;79:694–699. [DOI] [PubMed] [Google Scholar]
- 35. Blomstedt P, Sandvik U, Hariz MI, Fytagoridis A, Forsgren L, Hariz G‐M, Koskinen L‐OD. Influence of age, gender and severity of tremor on outcome after thalamic and subthalamic DBS for essential tremor. Parkinsonism Relat Disord 2011;17:617–620. [DOI] [PubMed] [Google Scholar]
- 36. Sandvik U, Koskinen LO, Lundquist A, Blomstedt P. Thalamic and subthalamic deep brain stimulation for essential tremor: where is the optimal target? Neurosurgery 2012;70:840–845; discussion 845‐846. [DOI] [PubMed] [Google Scholar]
- 37. Caire F, Ranoux D, Guehl D, Burbaud P, Cuny E. A systematic review of studies on anatomical position of electrode contacts used for chronic subthalamic stimulation in Parkinson's disease. Acta Neurochir (Wien) 2013;155:1647–1654; discussion 1654. [DOI] [PubMed] [Google Scholar]
- 38. Herzog J, Hamel W, Wenzelburger R, et al. Kinematic analysis of thalamic versus subthalamic neurostimulation in postural and intention tremor. Brain 2007;130:1608–1625. [DOI] [PubMed] [Google Scholar]
- 39. Anderson TR, Hu B, Iremonger K, Kiss ZH. Selective attenuation of afferent synaptic transmission as a mechanism of thalamic deep brain stimulation‐induced tremor arrest. J Neurosci 2006;26:841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johnson MD, Miocinovic S, McIntyre CC, Vitek JL. Mechanisms and targets of deep brain stimulation in movement disorders. Neurotherapeutics 2008;5:294–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Groppa S, Herzog J, Falk D, Riedel C, Deuschl G, Volkmann J. Physiological and anatomical decomposition of subthalamic neurostimulation effects in essential tremor. Brain 2014;137:109–121. [DOI] [PubMed] [Google Scholar]
- 42. Elble RJ. Origins of tremor. Lancet 2000;355:1113–1114. [DOI] [PubMed] [Google Scholar]
- 43. Johnson LA, Xu W, Baker KB, Zhang J, Vitek JL. Modulation of motor cortex neuronal activity and motor behavior during subthalamic nucleus stimulation in the normal primate. J Neurophysiol 2015;113:2549–2554.jn 00997 02014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fytagoridis A, Astrom M, Wardell K, Blomstedt P. Stimulation‐induced side effects in the posterior subthalamic area: distribution, characteristics and visualization. Clin Neurol Neurosurg 2013;115:65–71. [DOI] [PubMed] [Google Scholar]
- 45. O'Gorman RL, Jarosz JM, Samuel M, Clough C, Selway RP, Ashkan K. CT/MR image fusion in the postoperative assessment of electrodes implanted for deep brain stimulation. Stereotact Funct Neurosurg 2009;87:205–210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. An example lead is shown in three‐dimensional space for a representative VIM (green) and cZI (red) patient. Leads are superimposed onto a standard MRI. See Data S1 for further details.
Table S1. Essential Tremor DBS studies reporting clinical effects targeting the subthalamic region (cZI, PSA, or subthalamic area).
Data S1. Methods.


