Abstract
Background
The Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS‐UPDRS) is a newly developed tool to assess Parkinson's disease (PD). Changes in scores on the scale over the course of PD, including increasing disease duration and Hoehn and Yahr (HY) stages, have not been described. The objectives of this study were to analyze MDS‐UPDRS scores on Parts I through IV and their differences based on HY stage and disease duration in a large cohort of patients with PD.
Methods
For this cross‐sectional study, demographic data and MDS‐UPDRS scores were collected, including HY stage. Subscores on MDS‐UPDRS Parts I through IV were analyzed using 1‐way analyses of variance for each HY stage and in 5‐year increments of disease duration. Part III (motor assessment) scores were analyzed separately for on and off states.
Results
The mean age of the 3206 patients was 65.8 ± 10.6 years, 53.3% were men, the mean disease duration was 11.5 ± 4.6 years, and the median HY stage was 2 (range, 0–5); 2156 patients were examined in an on state and 987 were examined in an off state. Scores for all MDS‐UPDRS parts increased significantly through HY stages 1 through 5, with an average increase of 3.8, 7.7, 14.6, and 2.0 points consecutively for parts I through IV, respectively. For the 5‐year increments of disease duration, MDS‐UPDRS subscores increased by an average of 1.6, 3.3, 4.2, and 1.4 points consecutively for parts I through IV, respectively. This increase was significant only during the first 15 years of disease for all 4 parts, including part III scores evaluated in both on and off states.
Conclusions
MDS‐UPDRS scores for all 4 parts increase significantly with every HY stage and also with 5‐year increments of disease duration in the first 15 years of the disease.
Keywords: disease duration, Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS‐UPDRS), Hoehn and Yahr, Parkinson's disease, scale
Parkinson's disease (PD) is a progressive, neurodegenerative disorder characterized by a combination of motor and nonmotor manifestations, which evolve from very mild nonmotor manifestations in the premotor phase to advanced stages in which patients are severely disabled. The original Unified Parkinson's Disease Rating Scale (UPDRS) was initially developed to comprehensively assess the major symptoms of PD and to monitor PD‐related disability and impairment.1 Despite its frequent use for both research and clinical practice, a subsequent revision of the UPDRS by the Movement Disorder Society (MDS) Task Force on Rating Scales identified several shortcomings of the original scale.2 Based on this revision, the MDS sponsored a new Unified Parkinson's Disease Rating Scale (MDS‐UPDRS), which addresses the identified shortcomings of the original UPDRS.3 The MDS‐UPDRS specifically covers a greater number of PD manifestations, including nonmotor symptoms (NMS); better discriminates slight/mild manifestations of PD compared with the original UPDRS; resolves ambiguities and gives clear instructions for both raters and patients; and assesses all items in a uniform way. It has been demonstrated that the MDS‐UPDRS is valid, reliable, and sensitive to change3, 4, 5; it has been translated through a rigorous process into 14 languages, and versions in several of other languages are in the translation process.6
Despite several studies using the MDS‐UPDRS to date, it remains to be determined how the MDS‐UPDRS scores differ over different stages of the disease. The most widely used and accepted staging system for severity of PD is the Hoehn and Yahr (HY) scale.7 Despite some limitations, such as mixing impairment and disability, nonlinear character, and being more heavily weighted toward some aspects of the disease, such as postural instability and mobility problems, the HY scale has several strengths and is considered the reference standard for disability and impairment measures.8 It significantly correlates with both quality‐of‐life measures9 and studies of objective motor performance.10 Progressively higher HY stages correlate with neuroimaging studies of dopaminergic loss.11 Also, changes in a patient's HY stage carries prognostic significance and influences clinician‐based interventions.12
Thus, the objective of this study was to characterize the demographics of a large, international, multicenter, representative cohort of PD patients (the Quality of Life in Parkinson's Disease [QUALPD] study cohort) and to determine how MDS‐UPDRS scores differ across different stages of the disease based on HY stage as well as disease duration.
Patients and Methods
Design
This was an observational, cross‐sectional, multicenter, international study.
Patients
The study data set consists of patients who were included in the QUALPD study cohort. In this cohort, consecutive patients from 25 tertiary movement disorder centers from 15 countries, including Argentina, Austria, Chile, Colombia, Cuba, Ecuador, Estonia, France, Hungary, Mexico, Russia, Slovakia, Spain, the United Kingdom, and the United States, were enrolled. Only patients who were diagnosed with PD according to internationally recognized criteria13 and from countries that use an officially validated language version of the MDS‐UPDRS were eligible for inclusion into the study, with a minimal inclusion requirement of 100 patients per language data set. The majority of patients were included during the official validation studies of non‐English–language translations of the MDS‐UPDRS. In language versions for which required data were not collected during these MDS‐UPDRS validation studies (English, French, and German), a different patient cohort was identified and included in the final study data set.
The study was approved by the local ethics committees in all participating centers. All patients participated voluntarily and gave written informed consent. The investigation was performed according to the Declaration of Helsinki.
Measures
Sociodemographic data, including age, sex, and length of education, along with information on disease duration and antiparkinsonian medication, were collected from patients who were examined using the MDS‐UPDRS. The levodopa (l‐dopa) equivalent daily dosage (LEDD) was calculated using a previously published formula.14
The MDS‐UPDRS is a 4‐subscale, combined scale that comprehensively assesses the symptoms of PD and consists of: Part I, nonmotor experiences of daily living, including 13 items (6 semistructured interview items and 7 self‐reported items); Part II, motor experiences of daily living, including 13 self‐reported items; Part III, motor examination, including 18 items (33 scores); and Part IV, motor complications, including 6 items assessed in a semistructured inteview.3 All items are scored on a scale from 0 (normal) to 4 (severe), and total scores are obtained from the sum of the corresponding item scores. English, Estonian, French, German, Hungarian, Russian, Slovak, and Spanish language versions of the MDS‐UPDRS have been used in this study.4, 15, 16 All non‐English translations of the MDS‐UPDRS were officially validated, which means that, for each language translation, the confirmatory fit index of the final model for each section of MDS‐UPDRS must be ≥0.9 relative to the English version.6 The disease stage was assessed by the original HY scale, which was applied to gauge the course of disease over time.7
Statistical Analyses
Statistical analyses were performed using the statistical software program PASW SPSS version 21.0 for Windows (SPSS Inc., Chicago IL). First, the demographic and clinical characteristics of our study cohort were described. Patients were divided into subgroups based on disease duration in 5‐year increments (0–5, 6–10, 11–15, 16–20, 21–25, and >25 years), and they also were divided according to PD severity levels defined by MDS‐UPDRS cutoff scores, as previously published by Martinez‐Martin et al.17 The MDS‐UPDRS cutoff scores used to define mild/moderate and moderate/severe levels were as follows: Part I, 10/11 and 21/22, respectively; Part II, 12/13 and 29/30, respectively; Part III, 32/33 and 58/59, respectively; and Part IV, 4/5 and 12/13, respectively. The proportions of these disease‐duration groups and disease‐severity levels for each HY stage were calculated. Also, the median disease duration for each HY stage, the mean disease duration for each MDS‐UPDRS severity level, the number of positive NMS items on the MDS‐UPDRS for each HY stage and disease‐duration group, and mean MDS‐UPDRS scores for each year of disease duration were counted. Finally, subscores on MDS‐UPDRS Parts I through IV were analyzed using a 2‐way analysis of variance (least significant difference post‐hoc analysis) for each HY stage and for 5‐year increments of disease duration. Because some patients were rated in an on state and some were rated in an off state, separate analyses were performed for MDS‐UPDRS Part III scores obtained in on and off states. Conversely, scores on MDS‐UPDRS Parts I, II, and IV relate to the period of the past week and thus do not depend on the actual period of patient evaluation. Therefore, calculations for MDS‐UPDRS Parts I, II, and IV were performed for the whole patient sample regardless of the state in which they were examined.
Results
In total, 3206 patients with PD were included in the study. The mean age of patients was 65.8 ± 10.6 years; 53.3% were men; the mean disease duration was 11.5 ± 4.6 years; the median HY stage was 2 (interquartile range [25%–75%], Stages 2–3); 2156 patients (67.2%) had their motor status examined during the on state; 987 patients (30.8%) had their motor status measured during the off state; and, for 63 patients (2%), on/off state was not recorded. Baseline characteristics of the study sample are provided in Table 1.
Table 1.
Characteristics of the study sample
| Characteristic | Mean ± SD | Median [Range] | 95% CI |
|---|---|---|---|
| Sample size | 3206 | ||
| Age, y | 65.76 ± 10.60 | 67 [22–96] | 46–81 |
| Men:women, % | 53.28 | ||
| Length of education, y | 11.45 ± 4.61 | 12 [0–36] | 3–18 |
| Disease duration, y | 7.64 ± 5.75 | 6 [0–43] | 1–19 |
| Language, no. | |||
| English | 137 | ||
| Estonian | 266 | ||
| French | 411 | ||
| German | 100 | ||
| Hungarian | 547 | ||
| Russian | 122 | ||
| Slovak | 275 | ||
| Spanish | 1348 | ||
| Hoehn and Yahr scale | 2 [0–5] | 2–3a | |
| MDS‐UPDRS | |||
| Part I | 12.19 ± 7.23 | 11 [0–41] | 3–26 |
| Part II | 14.50 ± 9.64 | 13 [0–52] | 2–34 |
| Part III | 34.38 ± 18.39 | 31 [0–118] | 10–70 |
| Part IV | 3.53 ± 4.35 | 2 [0–22] | 0–12 |
| Rated in | |||
| on | 2156 (67.2) | ||
| off | 987 (30.8) | ||
| Missing | 63 (2.0) | ||
| LEDD | 617.31 ± 424.01 | 550 [0–4000] | 0–1357.9 |
CI, confidence interval; MDS‐UPDRS, Movement Disorder Society Unified Parkinson's Disease Rating Scale; LEDD, levodopa equivalence daily dosage.
This is the interquartile range (25%‐75%).
The mean LEDD of the study sample was 617.31 ± 424.01 mg/day. Regarding pharmacological therapy, 78.3% of patients received l‐dopa, 59.4% received dopamine agonists, 48.1% received both dopamine agonists and l‐dopa, 37.3% received other dopaminergic therapy (such as amantadine or monoclonal antibody inhibitors), and 5.4% did not receive any dopaminergic therapy. Concerning advanced PD therapies, 177 patients underwent a surgical procedure, most commonly bilateral subthalamic nucleus deep‐brain stimulation (141 patients), 24 patients were treated with l‐dopa/carbidopa intestinal gel pump therapy, and 14 patients received continuous apomorphine.
The majority of patients in HY stage 1 (71%) who were rated in the on state had the shortest disease duration of 0 to 5 years, and none of the patients in HY stage 1 had disease duration longer than 20 years (see Fig. 1). With increasing HY stage, the proportion of patients with the shortest disease duration (0–5 years) constantly decreased, whereas the proportion of patients with longer disease duration increased. Patients with disease duration greater than 10 years represented 4.1%, 17.5%, 30.4%, 49.5%, and 67.2% of those in HY stages 1 through 5, respectively. The proportions of patients with disease duration from 6 to 10 years were evenly represented in HY stages 1 through 5 (25.2%,; 28.7%, 32.7%, 30.4%, and 32.8%, respectively). The median disease duration for individual HY stages among patients who were evaluated in the on state was 4 years for Stage 1, 5 years for Stage 2, 7 years for Stage 3, 10 years for Stage 4, and 14 years for Stage 5. The mean disease duration for individual HY stages among patients who were evaluated in the on state was 4.5 years for Stage 1, 6.5 years for Stage 2, 8.5 years for Stage 3, 11.5 years for Stage 4, and 15 years for Stage 5.
Figure 1.
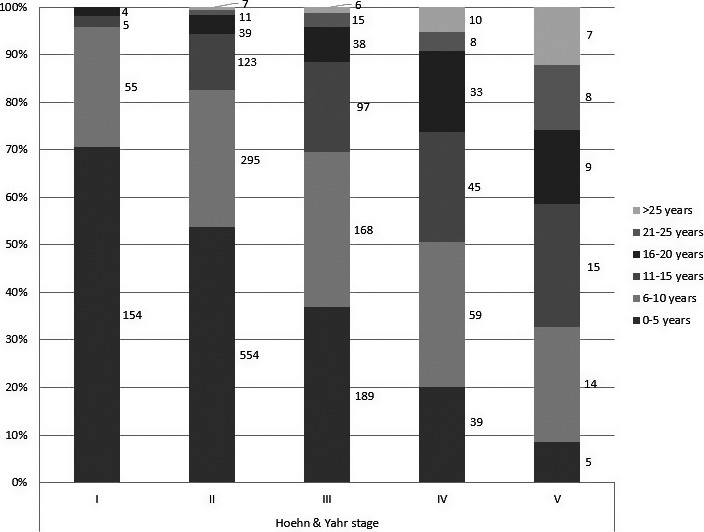
Relationship between Hoehn and Yahr stages and disease duration for patients rated in their on states.
Scores for all 4 MDS‐UPDRS parts increased significantly over HY stages 1 through 5, with an average increase of 3.8, 7.7, 14.6, and 2.0 points consecutively for MDS‐UPDRS Parts I through IV, respectively. There was a statistically significant difference between groups for all 4 parts of the MDS‐UPDRS, including separate analyses of Part III in on and off states, as determined by 1‐way analysis of variance. For Part I, F = 203.291 (P < 0.001); for Part II, F = 580.133 (P < 0.001); for Part III in the on state, F = 396.184 (P < 0.001); for Part III in the off state, F = 275.534 (P < 0.001); and, for Part IV, F = 184.419 (P < 0.001). Post‐hoc analyses showed statistically significant differences between all consecutive HY stages for all parts of the MDS‐UPDRS (see Table 2).
Table 2.
Differences in scores on the Movement Disorder Society Unified Parkinson's Disease Rating Scale based on Hoehn and Yahr stage
| MDS‐UPDRS | HY stage 1 | HY stage 2 | HY stage 3 | HY stage 4 | HY stage 5 |
|---|---|---|---|---|---|
| Part I | |||||
| No. of patients | 343 | 1617 | 754 | 304 | 89 |
| Mean ± SD | 7.5 ± 4.6 | 10.4 ± 5.9*** | 14.4 ± 6.7*** | 18.9 ± 7.8*** | 22.8 ± 9.3*** |
| Range | 0–26 | 0–35 | 0–40 | 1–39 | 2–41 |
| Part II | |||||
| No. of patients | 343 | 1613 | 754 | 303 | 88 |
| Mean ± SD | 6.5 ± 4.8 | 11.2 ± 6.6*** | 17.5 ± 7.4*** | 27.3 ± 8.8*** | 37.1 ± 8.3*** |
| Range | 0–25 | 0–44 | 1–45 | 1–49 | 10–52 |
| Part III on | |||||
| No. of patients | 226 | 1080 | 522 | 196 | 55 |
| Mean ± SD | 14.4 ± 7.8 | 28.8 ± 12.3*** | 40.5 ± 14.2*** | 58.0 ± 16.2*** | 72.2 ± 16.3*** |
| Range | 2–44 | 4–71 | 8–95 | 21–106 | 38–118 |
| Part III off | |||||
| No. of patients | 111 | 508 | 227 | 105 | 29 |
| Mean ± SD | 14.4 ± 7.0 | 29.4 ± 12.0*** | 42.7 ± 15.6*** | 61.1 ± 16.6*** | 73.7 ± 17.0*** |
| Range | 0–39 | 2–83 | 4–94 | 13–97 | 47–109 |
| Part IV | |||||
| No. of patients | 342 | 1616 | 756 | 305 | 89 |
| Mean ± SD | 0.8 ± 2.0 | 2.4 ± 3.2*** | 4.8 ± 4.4*** | 7.6 ± 5.5*** | 8.4 ± 6.4* |
| Range | 0–15 | 0–16 | 0–21 | 0–21 | 0–22 |
MDS‐UPDRS, Movement Disorder Society Unified Parkinson's Disease Rating Scale; HY, Hoehn and Yahr scale; SD, standard deviation.
*P < 0.05, ***P < 0.001 relating to differences between MDS‐UPDRS subscores in each consecutive HY stage.
For 5‐year increments in disease duration, MDS‐UPDRS subscores increased by an average of 1.6, 3.3, 4.2, and 1.4 points consecutively for Parts I through IV, respectively. There was a statistically significant difference between groups for all 4 parts of the MDS‐UPDRS, including separate analyses of Part III in on and off states, as determined by one‐way analysis of variance. For Part I, F = 50.312 (P < 0.001); for Part II, F = 142.544 (P < 0.001); for Part III in the on state, F = 39.481 (P < 0.001); for Part III in the off state, F = 23.056 (P < 0.001); and, for Part IV, F = 204.852 (P < 0.001). Post‐hoc analyses revealed statistically significant differences between consecutive disease duration groups in the first 15 years of the disease for all 4 MDS‐UPDRS parts; whereas Parts II, Part III in the off state, and Part IV increased significantly in the 15‐year to 20‐year interval; Part III in the on state increased significantly; and Part III in the off state decreased significantly in the 20‐year to 25‐year interval; and only Part II increased significantly in the ≥25‐year interval (see Table 3). Fig. S1 illustrates the mean MDS‐UPDRS scores for each year of disease duration.
Table 3.
Differences in scores on the Movement Disorder Society Unified Parkinson's Disease Rating Scale based on 5‐year increments of disease duration
| MDS‐UPDRS | 5‐Year increments of disease duration | |||||
|---|---|---|---|---|---|---|
| 0–5 Years | 6–10 Years | 10–15 Years | 16–20 Years | 21–25 Years | >25 Years | |
| Part I | ||||||
| No. of patients | 1368 | 919 | 474 | 191 | 74 | 46 |
| Mean ± SD | 10.4 ± 6.5 | 12.4 ± 7.2*** | 14.7 ± 7.4*** | 15.4 ± 7.7 | 16.0 ± 7.5 | 18.2 ± 7.5 |
| Range | 0–37 | 0–40 | 0–41 | 2–38 | 4–38 | 3–34 |
| Part II | ||||||
| No. of patients | 1368 | 918 | 473 | 190 | 71 | 46 |
| Mean ± SD | 10.8 ± 7.4 | 14.9 ± 9.0*** | 19.3 ± 9.4*** | 22.3 ± 11.5*** | 22.9 ± 11.6 | 27.5 ± 12.2* |
| Range | 0–48 | 0–52 | 0–51 | 0–49 | 2–47 | 3–50 |
| Part III | ||||||
| on | ||||||
| No. of patients | 948 | 611 | 289 | 124 | 44 | 30 |
| Mean ± SD | 29.8 ± 15.5 | 34.5 ± 18.3*** | 40.8 ± 18.0*** | 42.6 ± 19.0 | 49.2 ± 23.1* | 51.3 ± 23.9 |
| Range | 0–97 | 2–95 | 5–93 | 8–92 | 9–106 | 13–118 |
| off | ||||||
| No. of patients | 391 | 300 | 178 | 67 | 30 | 15 |
| Mean ± SD | 29.3 ± 16.0 | 35.9 ± 18.8*** | 40.5 ± 18.8** | 50.2 ± 22.9*** | 41.1 ± 18.3* | 49.4 ± 21.4 |
| Range | 0–91 | 2–95 | 7–109 | 6–89 | 9–74 | 14–97 |
| Part IV | ||||||
| No. of patients | 1369 | 918 | 475 | 192 | 74 | 46 |
| Mean ± SD | 1.4 ± 2.5 | 4.1 ± 4.2*** | 6.3 ± 4.8*** | 7.2 ± 5.1** | 7.2 ± 4.5 | 8.2 ± 5.1 |
| Range | 0–15 | 0–21 | 0–22 | 0–21 | 0–18 | 0–20 |
MDS‐UPDRS, Movement Disorder Society Unified Parkinson's Disease Rating Scale; SD, standard deviation.
*P < 0.05, **P < 0.01, ***P < 0.001 relating to differences between MDS‐UPDRS subscores in each consecutive disease duration group.
The frequency of mild PD severity levels, as defined by MDS‐UPDRS cutoff scores. gradually decreased from HY stage 1 to stage 5 for all MDS‐UPDRS parts, whereas the frequency of severe levels gradually increased (see Fig 2). Moderate severity levels were most prevalent in HY stage 3 for all MDS‐UPDRS parts. Severity levels correlated with HY stages mostly for MDS‐UPDRS Parts II and III. The mean disease duration for each MDS‐UPDRS severity level is shown in Table S1.
Figure 2.
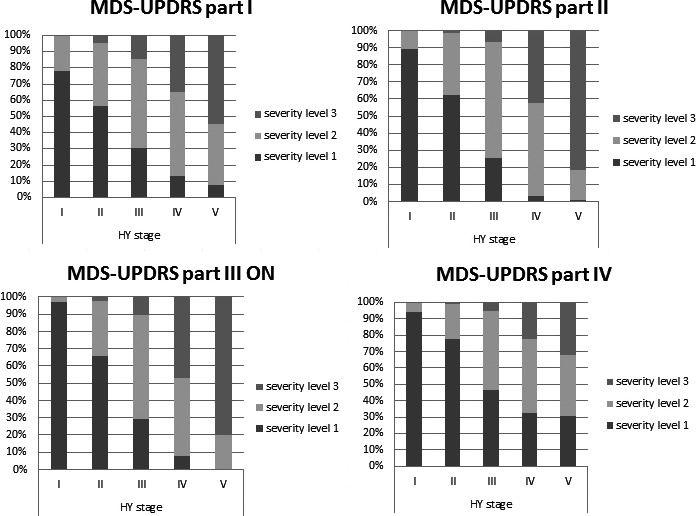
Relationship between Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS‐UPDRS) severity levels and Hoehn and Yahr (HY) stage.
The number of MDS‐UPDRS NMS items on which patients scored ≥1 point increased by 4.2 items between HY stages 1 and 5 (5.2 ± 2.9, 6.5 ± 2.8, 8.1 ± 2.7, 9.1 ± 2.7, and 9.4 ± 2.5 items in HY stages 1–5, respectively). For the disease duration groups, the number of NMS items on which patients scored ≥1 point differed by 2.2 items between the shortest (0–5 years) and longest (>25 years) disease duration groups (6.4 ± 2.9, 7.1 ± 2.9, 7.9 ± 2.7, 8.3 ± 2.8, 8.5 ± 2.5, and 8.6 ± 2.5 NMS items for each 5‐year increment of disease duration, respectively).
Discussion
The QUALPD study cohort is 1 of the largest PD populations studied to date. Similar to other previous studies,3 the most prevalent HY stages in this study cohort were Stages 2 and 3 (52.1% and 24.3%, respectively). Also in line with previous reports, by enrolling consecutive patients, only 2.7% of patients were in HY stage 5. This may be a consequence of decreased health care accessibility because of significant motor disability in these patients. Lower recruitment of patients in late advanced stages of the disease presents one of the challenges of descriptive studies; however the large sample recruited in our cohort allows further analyses for all HY stages.
Regarding disease duration, most of the included patients (74.6%) had a disease duration ≤10 years, and only 3.8% had a disease duration >20 years. As expected, disease duration increased from HY Stage 1 to Stage 5, and 4.1% of patients had a disease duration from >10 years in HY Stage 1 to 67.2% in Stage 5. We note that, in the original HY study cohort,7 patients were enrolled in the pre‐l‐dopa era; whereas 95% of our patients received dopaminergic therapy (mostly combined), and 7% had advanced pump placement or surgical therapy for PD. Compared with the original HY study, the median disease duration for individual HY stages of our patients examined in the on state was slightly increased only for stages 1 and 4 (with median disease duration 3, 6, 7, 9, and 14 years for HY stages 1–5, respectively, in the original HY publication). Compared with these values, the mean disease duration in our cohort was higher for every HY stage; however, the original HY study did not mention mean disease durations and thus it is not possible to directly compare these cohorts. Therefore, in contrast to some other studies suggesting that treatment prolongs latencies to successive HY stages by about 3 to 5 years,8 this finding was not reproduced in our large, multicenter cohort of patients with PD. This might call into question the efficacy of current medication in preventing the natural progression of the disease considering the HY staging system. However, the HY scale, especially from Stage 3 and above, is heavily weighted toward postural instability, which is in large part considered an l‐dopa–nonresponsive symptom.18 It has also been shown that, after 15 years of disease duration, the disability of patients is mainly driven by l‐dopa–nonresponsive symptoms, such as cognitive problems, falls, hallucinations, depression, swallowing, and urinary problems.19 Thus, the HY scale does not necessarily reflect therapy‐related improvements in many other aspects of the disease, especially nonmotor symptoms and motor fluctuations, which are not well captured by this staging system. Therefore, the development of comprehensive measures like the MDS‐UPDRS enables us to better understand and follow the progression of different aspects of PD. The MDS‐UPDRS covers 4 major areas of PD, including NMS, activities of daily living, motor examination, and motor complications, and enables clinicians to detect the presence and also the severity of studied symptoms. Subscores for individual parts of the scale give continuous scores, which are more sensitive to change as the disease progresses.
When analyzing the MDS‐UPDRS subscores in different stages of the disease, with each successive HY stage, all 4 MDS‐UPDRS subscores increase significantly, including MDS‐UPDRS Part III scores evaluated in both on and off states. On the other hand, when comparing the study cohort divided by 5‐year increments in disease duration, all MDS‐UPDRS subscores increased significantly only in the first 15 years of the disease, reaching a relative plateau afterward. A previous report defined the minimal clinically important difference (MCID) for worsening of the MDS‐UPDRS Part III score (motor examination) as 4.63 points.5 Differences in Part III scores with each successive HY stage are clearly higher than the defined MCID; however, when analyzing the differences between disease duration groups, increased Part III scores rated in the on state did not reach the MCID in intervals between 15 to 20 years and ≥25 years. This lower correlation of MDS‐UPDRS scores with disease duration may be a result of heterogeneous composition of disease duration groups considering PD severity levels due to different rates of disease progression. Various factors may affect the rate of disease progression, including age of onset, cognitive impairment, or PD subtype (tremor dominant vs. nontremor dominant and postural instability and gait disorder subtype).19 Another reason may be that the MDS‐UPDRS was developed to better capture the slight/mild manifestations of the disease compared with more severe symptoms; thus, it may be more sensitive to change, especially in the early stage rather than in late, advanced stages of the disease. Also, with increasing disease duration, overall burden of other comorbidities, age, and survival become significant factors; therefore, it is possible that patients who have a long disease duration may have a better prognosis compared with most other patients.
In another study, PD severity levels (mild, moderate, severe) were defined by MDS‐UPDRS cutoff values for all 4 parts.16 The proportion of these severity levels for all 4 MDS‐UPDRS parts were highly correlated with HY stage in our study. Because HY staging is driven mainly by motor features and disability, it is not surprising that these proportions fit most for MDS‐UPDRS Parts II and III. Also, because MDS‐UPDRS severity levels were not calculated on the basis of the HY stage, this may be confirmation that HY Stage 3 really is “moderate,” as usually considered.8
Although the HY scale does not capture NMS, with each HY stage increment, we observed not only significant increases in MDS‐UPDRS Part I scores but also an increase in the number of NMS items on which patients scored ≥1 point, with an increase of 4.2 items when comparing HY Stage 1 versus Stage 5. In the disease duration groups, MDS‐UPDRS Part I scores increased only in the first 15 years of the disease. The same was true for the number of NMS items on which patients scored ≥1 point, which differed by only 2.2 items between the shortest (0–5 years) and longest (>25 years) disease duration groups. Patients in the most severe MDS‐UPDRS Part I level had a shorter mean disease duration compared with those in the most severe level for other MDS‐UPDRS parts. NMS‐like constipation, hyposmia, fatigue, mood, and sleep‐related problems may be present more than 10 years before the onset of motor symptoms and, by the time of initial motor manifestations, may already present a significant burden to the patient.20, 21 Considering the relationship between the overall burden of NMS and disease duration, in a study by Chaudhuri et al.,22 patients were divided into 5 levels of NMS severity (no, mild, moderate, severe, very severe) based on the number of declared symptoms on an NMS questionnaire. Interestingly, those authors reported that patients in the very severe group had a mean disease duration that was even shorter (5.05 years) than that among patients in the moderate and severe groups (5.89 and 6.33 years, respectively). This suggests that significant NMS burden may be present very early in the disease course and that longer disease duration must not necessarily correlate with an increase in NMS burden.
Conclusions
Despite the cross‐sectional character of our study, which is its major limitation, the large sample size enables us to describe the characteristics and differences between PD populations over the course of the disease concerning HY stages and disease duration. Based on our results, PD severity levels, as defined by HY stages, reflect differences in all aspects of disease measured by the MDS‐UPDRS, despite being mostly driven by motor symptoms, especially postural instability and mobility. On the other hand, MDS‐UPDRS scores increase only in the first 15 years of the disease for all 4 parts and then reach a relative plateau. Prospective, longitudinal studies should be performed to confirm these results and provide more detailed information on the transition from 1 stage to another. The MDS‐UPDRS was designed to discriminate especially mild rather than severe symptoms of the disease. Therefore, it remains to be determined whether the original UPDRS, which better discriminates the severe and very severe symptoms, would be more suitable and discriminative for studies in late and advanced stages of PD.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
M.S.: 1A, 1B, 1C, 2A, 2B, 3A
P.M.M.: 1A, 1C, 2A, 2C, 3B
N.K.: 1A, 1C, 2C, 3B
M.R.V.: 1C, 2C, 3B
J.C.C.: 1C, 2C, 3B
P.T.: 1C, 2C, 3B
K.S.: 1C, 2C, 3B
O.L.: 1C, 2C, 3B
A.S.: 1C, 2C, 3B
T.F.: 1C, 2C, 3B
M.A.S.: 1C, 2C, 3B
T.A.: 1C, 2C, 3B
Z.A.: 1C, 2C, 3B
I.A.O.: 1C, 2C, 3B
E.B.: 1C, 2C, 3B
C.B.: 1C, 2C, 3B
A.B.Y.: 1C, 2C, 3B
A.C.A.: 1C, 2C, 3B
A.C.: 1C, 2C, 3B
F.C.: 1C, 2C, 3B
V.D.: 1C, 2C, 3B
D.A.G.: 1C, 2C, 3B
N.G.: 1C, 2C, 3B
Z.G.: 1C, 2C, 3B
O.G.: 1C, 2C, 3B
M.G.: 1C, 2C, 3B
V.H.: 1C, 2C, 3B
J.H.: 1C, 2C, 3B
L.K.E.: 1C, 2C, 3B
M.M.K.: 1C, 2C, 3B
G.M.: 1C, 2C, 3B
J.C.M.C.: 1C, 2C, 3B
A.M.R.: 1C, 2C, 3B
M.M.: 1C, 2C, 3B
H.P.M.: 1C, 2C, 3B
M.M.: 1C, 2C, 3B
C.M.: 1C, 2C, 3B
B.P.: 1C, 2C, 3B
W.P.: 1C, 2C, 3B
K.R.: 1C, 2C, 3B
E.R.: 1C, 2C, 3B
C.R.B.: 1C, 2C, 3B
C.S.: 1C, 2C, 3B
B.C.T.: 1C, 2C, 3B
P.V.: 1C, 2C, 3B
C.G.G.: 1AC, 2C, 3B
G.T.S.: 1A, 1C, 2A, 2C, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest: The funding for research leading to this study was provided by the Slovak Research and Development Agency under contract no. APVV‐14‐0415 and by the Slovak Scientific Grant Agency under contract no. VEGA 1/0024/14; by the Movement Disorder Society, which received partial support from UCB Spain; from the program “Investissements d'Avenir” ANR‐10‐IAIHU‐06; by Grant PUT1239 from the Estonian Research Council; by the Bolyai Scholarship of the Hungarian Academy of Sciences (OTKA PD103964) and the Hungarian Brain Research Program (Grant KTIA_13_NAP‐A‐II/10; government‐based funds); by Parkinson's UK (G‐0508); and by the Austrian Science Fund (FWF Project: KLI82‐B00). Christopher G. Goetz, Glenn T. Stebbins, Pablo Martinez‐Martin, and Anette Schrag developed the MDS‐UPDRS scale. Matej Skorvanek, Pablo Martinez‐Martin, Norbert Kovacs, Pille Taba, and Oleg Levin served as principal investigators in the translation phase of the MDS‐UPDRS in their respective languages. The authors report no sources of funding and no conflicts of interest relevant to this work.
Financial disclosures for the past 12 months: M.S. received grants from the Slovak Research and Development Agency and the Slovak Scientific Grant Agency as well as speaker's honoraria and compensation for consultations from AbbVie, Actavis, Egis, Krka, Lundbeck, Medtronic, TEVA, and UCB. J.C.C. received honoraria for participating to scientific advisory boards for BMS, Zambon, Pfizer, and AbbVie; a research grant from Ipsen; travel grants from AbbVie and Zambon; and owns stock in B&A Therapeutics. M.R.V. has received honoraria from Boehringer‐Ingelheim, Ever Neuro Pharma, UCB, Vanquish, Medtronic, and MedLearning; she has participated in advisory boards for UCB Latin America and Teva. T.F. has received honoraria for speaking at meetings sponsored by BIAL, Profile Pharma, Medtronic, Brittania, and AbbVie; he has received grants from the Michael J. Fox Foundation, European Union FP7, the John Black Charitable Foundation, and Brain Research Trust. Z.G. received grants from the Slovak Research and Development Agency and the Slovak Scientific Grant Agency as well as speaker's honoraria and compensation for consultations from Boehringer‐Ingelheim, Pfizer, Bayer, Novartis, and Lundbeck. V.H. received grants from the Slovak Research and Development Agency and the Slovak Scientific Grant Agency as well as speaker's honoraria and compensation for consultations from Abbvie, Medtronic, and UCB. M.K. has received speaker's honoraria from UCB and Zambon. J.C.M.C. has received honoraria for speaking at meetings sponsored by AbbVie, UCB, Allergan, Merz, Italfarmaco, BIAL, and KRKA. C.S. received grants from Adamas, Cynapsus, Allergan, Medtronic, the National Parkinson Foundation, and the National Institutes of Health and has received honoraria for panel membership from Neurocrine. P.V. received a grant from the Slovak Scientific Grant Agency as well as speaker's honoraria and compensation for consultations from Abbvie, Actavis, Krka, Lundbeck, Medtronic, Sanomed, Schwabe Slovakia, TEVA, and UCB. C.G.G. received honoraria for membership, consultancies, or advisory board service from Acadia, Addex, Avanir, Boston Scientific, Clevexel, Oxford Biomedica, Pfizer, WebMD; he is employed by Rush University Medical Center and has a contract with the International Parkinson and Movement Disorder Society for managing translation programs for the MDS‐UPDRS and the Unified Dystonia Rating Scale; he has received honoraria from Oregon Health and Science University and the University of Pennsylvania; royalties from Elsevier Publishers, Oxford University Press, Wolters Kluwer; and grants from the National Institutes of Health, the Michael J. Fox Founding, the Parkinson's Disease Foundation, and International Parkinson and Movement Disorder Society. The remaining authors report no sources of funding and no conflicts of interest.
Supporting information
Table S1. Mean disease durations for MDS‐UPDRS severity level group.
Figure S1. Mean MDS‐UPDRS scores for each year of disease duration.
Acknowledgements
We acknowledge Martina Sukenikova (Kosice, Slovakia) for her technical support during organization of this project.
Supporting information may be found in the online version of this article.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Fahn S, Elton RL; Members of the UPDRS Development Committee . Unified Parkinson's Disease Rating Scale In: Fahn S, Marsden CD, Calne DB, Lieberman A, eds. Recent Developments in Parkinson's Disease, vol 2 Florham Park, NJ: Macmillan Health Care Information; 1987:153–163. [Google Scholar]
- 2. Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease . The Unified Parkinson's Disease Rating Scale (UPDRS): status and recommendations. Mov Disord 2003;18:738–750. [DOI] [PubMed] [Google Scholar]
- 3. Goetz CG, Tilley BC, Schaftman SR, et al. Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–2170. [DOI] [PubMed] [Google Scholar]
- 4. Martinez‐Martin P, Rodriguez‐Blazquez C, Alvarez‐Sanches M, et al. Expanded and independent validation of the Movement Disorder Society‐Unified Parkinson's Disease Rating Scale (MDS‐UPDRS). J Neurol 2013;260:228–236. [DOI] [PubMed] [Google Scholar]
- 5. Horvath K, Aschermann Z, Acs P, et al. Minimal clinically important difference on the Motor Examination part of MDS‐UPDRS. Parkinsonism Related Disord 2015;21:1421–1426. [DOI] [PubMed] [Google Scholar]
- 6. Goetz CG, Stebbins GT, Wang L, LaPelle NR, Luo S, Tilley BC. The International Parkinson and Movement Disorder Society translation program for the MDS‐UPDRS and UDysRS. Mov Disord 2014;29:S185. [Google Scholar]
- 7. Hoehn MM, Yahr MD. Parkinsonism onset, progression and mortality. Neurology 1967;17:427–442. [DOI] [PubMed] [Google Scholar]
- 8. Goetz CG, Poewe W, Rascol O, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging system: status and recommendations. Mov Disord 2004;19:1020–1028. [DOI] [PubMed] [Google Scholar]
- 9. Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson's Disease Questionnaire (PDQ39): development and validation of a Parkinson's disease summary index score. Age Ageing 1997;26:353–357. [DOI] [PubMed] [Google Scholar]
- 10. Reynolds NC, Montgomery GK. Factor analysis of Parkinson's impairment. Arch Neurol 1987;44:1013–1016. [DOI] [PubMed] [Google Scholar]
- 11. Staffen W, Mair A, Unterrainer J, Trinka E, Ladurner G. Measuring the progression of idiopathic Parkinson's disease with 123I‐β‐CIT‐SPECT. J Neur Trans 2000;107:543–552. [DOI] [PubMed] [Google Scholar]
- 12. Poewe W, Wenning GK. The natural history of Parkinson's disease. Neurology 1996;47:146–152. [DOI] [PubMed] [Google Scholar]
- 13. Hughes AJ, Daniels SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25:2649–2653. [DOI] [PubMed] [Google Scholar]
- 15. Horváth K, Aschermann Z, Ács P, et al. Validation of the Hungarian MDS‐UPDRS: why do we need a new Parkinson scale? Ideggyogy Sz 2014;67:129–134. [PubMed] [Google Scholar]
- 16. Skorvanek M, Kosutzka Z, Valkovic P, et al. Validation of the Slovak version of the Movement Disorder Society‐Unified Parkinson's Disease Rating Scale (MDS‐UPDRS). Cesk Slov Neurol Neurochir 2013;76:463–468. [Google Scholar]
- 17. Martinez‐Martin P, Rodriguez‐Blazquez R, Alvarez M, et al. Parkinson's disease severity levels and MDS‐Unified Parkinson's Disease Rating Scale. Parkinsonism Relat Disord 2015;21:50–54. [DOI] [PubMed] [Google Scholar]
- 18. Galna B, Lord S, Burn DJ, Rochester L. Progression of gait dysfunction in incident Parkinson's disease: Impact of medication and phenotype. Mov Disord 2015;30:359–367. [DOI] [PubMed] [Google Scholar]
- 19. Hely MA, Morris JG, Reid WG, Trafficante R. Sydney multicentre study of Parkinson's disease: non‐l‐dopa‐responsive problems dominate at 15 years. Mov Disord 2005;20:190–199. [DOI] [PubMed] [Google Scholar]
- 20. Leonardi M, Raggi A, Pagani M, Carella F, Soliveri P, Albanese A, Romito L. Relationship between disability, quality of life and prevalence of nonmotor symptoms in Parkinson's disease. Parkinsonism Relat Disord 2012;18:35–39. [DOI] [PubMed] [Google Scholar]
- 21. Pont‐Sunyer C, Hotter A, Gaig C, et al. The onset of nonmotor symptoms in Parkinson's disease (the ONSET PD study). Mov Disord 2015;2:229–237. [DOI] [PubMed] [Google Scholar]
- 22. Chaudhuri KR, Sauerbier A, Rojo JM, et al. The burden of non‐motor symptoms in Parkinson's disease using a self‐completed non‐motor questionnaire: a simple grading system. Parkinsonism Relat Disord 2015;21:287–291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Mean disease durations for MDS‐UPDRS severity level group.
Figure S1. Mean MDS‐UPDRS scores for each year of disease duration.


