Abstract
Objectives
Subthalamic nucleus deep brain stimulation (STN‐DBS) is an effective treatment for improving the motor symptoms of Parkinson's disease (PD). Overall, cognitive function remains stable after STN‐DBS in most patients. However, cognitive decline, specifically in the verbal fluency domain, is seen in a subset of STN‐DBS patients. Currently, predictors of cognitive decline in PD patients treated with STN‐DBS are not well known. Thus, identification of presurgical predictors might provide an important clinical tool for better risk‐to‐benefit assessment. This study explores whether whole brain white matter lesion (WML) volume, or hippocampal and forebrain volumes, measured quantitatively on MRI, are associated with cognitive changes following STN‐DBS in PD patients.
Methods
We conducted a retrospective study using presurgical, and ≥ 6‐month postsurgical neuropsychological (NP) evaluation scores from 43 PD patients with STN‐DBS. Mean pre/post NP test scores for measures of executive function, attention, verbal fluency, memory, and visuospatial function were analyzed and correlated with WML volume, and brain volumetric data.
Results
Although cognitive measures of verbal fluency, executive function, attention, memory, and visuospatial function showed declines following STN‐DBS, we observed limited evidence that white matter lesion burden or cortical atrophy contributed to cognitive change following STN‐DBS.
Conclusions
These results suggest that post‐STN‐DBS cognitive changes may be unrelated to presurgical WML burden and presence of cortical atrophy.
Keywords: STN‐DBS, Parkinson's disease, cognitive outcomes
Introduction
Deep brain stimulation (DBS) is an established treatment in advanced Parkinson's disease (PD) patients experiencing motor fluctuations and dyskinesia despite optimal dopamine drug therapies.1, 2, 3, 4, 5, 6 Numerous studies demonstrate that the subthalamic nucleus (STN) is a target that results in favorable improvement in motor disability, reductions in anti‐Parkinsonian medications, and improvements in quality of life.4, 5, 7, 8 Though STN‐DBS is a proven and effective treatment of the motor symptoms of PD, challenges remain in differentiating non‐motor impairments as a function of disease progression or a result of DBS.
Cognitive changes are reported in a subset of patients following STN‐DBS, including declines in verbal fluency, executive function, attention, and memory.3, 7, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 Pathological mechanisms have been hypothesized to contribute to cognitive changes in PD including brain volume changes and degeneration of dopaminergic and other non‐dopaminergic neurotransmitter systems (e.g., cholinergic projection pathways).22 Previous studies have also attributed cognitive changes in the domains of attention, learning and memory, visuospatial function, and executive function to white matter lesions (WML) observed on brain MRI,23, 24, 25, 26, 27 as well as volumetric changes to neuroanatomical structures.28, 29, 30, 31, 32
Evidence suggests that cognitive decline in PD patients correlates with white matter volume 23, 24, 25, 26, 27 and brain volumetric changes.22, 28, 29, 30, 31, 32 Therefore, it is critical to determine whether STN‐DBS correlates with WML or volumetric changes in PD patients who demonstrate postsurgical cognitive changes. To date, the relationship between quantitative measures of presurgical brain MRI and cognitive declines post‐STN‐DBS surgery remains unclarified and the best predictor of postsurgical decline is presurgical cognitive status.20, 21, 33
This study evaluated whether (1) there was a decrease in cognitive performance in patients following STN‐DBS and (2) whether there was a relationship between quantitative measures of presurgical brain MRI (WML volume, forebrain, and hippocampal volumes) and change in cognitive performance between pre‐ and post‐STN DBS surgery. We hypothesized that greater WML volume and lower forebrain and hippocampal volumes would be associated with greater decline in performance on neuropsychological (NP) tests post‐DBS surgery. Importantly, this relationship has not been explored in post‐DBS patients. Therefore, the results of this study could impact the clinical management decisions of DBS teams in assessing possible risks and benefits of DBS surgery.
Methods
Population Identification
The Colorado Multiple Institutional Review Board (COMIRB) approved this retrospective study. The University of Colorado Hospital (UCH) neuropsychology database was retrospectively screened for idiopathic PD (iPD) patients who had undergone STN DBS between January 2011 and June 2016 and for which presurgical NP testing and ≥ 6‐month postsurgical NP testing were available. Data were collected in three categories. The UCH cloud‐based neuropsychology database was used to obtain the presurgical and postsurgical neuropsychology evaluation records. The health record management system (EPIC) and medical imaging management system (PACS) were reviewed to extract relevant clinical (PD history, patient demographics, and comorbidities) surgical and imaging data (T2‐fluid attenuated inversion recovery (FLAIR) and T1‐3D). Of the 162 patients total with STN‐DBS surgery between January 2011 and June 2016, 43 iPD patients met the inclusion criteria for our study: (1) STN‐DBS, (2) presurgical NP and ≥ 6‐month postsurgical NP testing, and (3) presurgical T2‐FLAIR and 3D T1‐weighted MRI. 119 patients were excluded due to lack of postsurgical cognitive testing (N = 94); reimplantation of the DBS stimulator (N = 11); < 6 months between surgery and June 2016 (N = 8); postsurgical cognitive testing occurred < 6 months after surgery; and lack of pre/postsurgical cognitive testing (N = 3).
Patients
Table 1 summarizes demographic, clinical, and surgical details for 43 patients. The mean age at the time of surgery was 63.1 ± 7.3 years, and the mean duration of PD was 9.4 ± 4.1 years. The mean duration of NP follow‐up after surgery was 14.4 ± 6.4 months (median, 13 months, range 6 to 43 months).
Table 1.
Demographic, clinical, and surgical characteristics of patients.
| Subjects | N = 43 |
| Age @ baseline mean (± SD) | 62.3 (± 7.3) |
| Age @ PD onset mean (± SD) | 52.8 (± 9.2) |
| Disease duration, years | |
| Mean (± SD) | 9.4 (± 4.1) |
| Median (min/max) | 9 (2/22) |
| Sex | |
| Male/Female | 30/13 |
| Education, years | |
| Mean (± SD) | 15.3 (± 2.7) |
| Median (min/max) | 16 (10/20) |
| Duration of surgery after Pre‐NP eval, months | |
| Mean (± SD) | 5.7 (± 4.0) |
| Median (min/max) | 5 (1/23) |
| Duration of Post‐NP eval after surgery, months | |
| Mean (± SD) | 14.4 (± 6.4) |
| Median (min/max) | 13 (6/43) |
| Percent change in UPDRS* | |
| Mean (± SD) | −36.8 (± 27.4) |
| Median (min/max) | −37 (‐88/26) |
| Post‐surgical Levodopa equivalent dose, mg | |
| Mean (± SD) | 534.3 (± 397.1) |
| Median (min/max) | 525 (0/2000) |
| Surgical characteristics | |
| Age @ implantations mean (± SD) | 63.1 (± 7.3) |
| Unilateral STN‐DBS | 14 |
| left | 7 |
| right | 7 |
| Bilateral STN‐DBS | 29 |
| Vascular risk factors | |
| Diabetes % | 2 |
| Controlled Hypertension % | 18.6 |
| Hypercholesterimia/Hyperlipidemia % | 18.6 |
| History of smoking % | 37.2 |
Pre‐DBS Evaluation and Selection, and Neurosurgical Procedure
As part of the DBS evaluation process, the UPDRS score was obtained ON and OFF L‐DOPA to confirm medication response, brain MRI were obtained and assessed for surgical risks, and NP testing was administered and evaluated for cognitive functioning. Candidates were deemed appropriate for DBS from a neurological and neurosurgical perspective when there was no evidence of cognitive impairment indicative of dementia (e.g., PD‐Dementia, comorbid Alzheimer's disease) and no concerning psychological risk factors (e.g., significant depression with suicidal ideation/intent, significant anxiety, current substance abuse) Surgical implantation of the DBS electrode and generator was carried out in accordance with the established UCH staged DBS‐surgery protocol (i.e., DBS lead and implantable pulse generator [IPG] are implanted during separate surgeries roughly two weeks apart). Briefly, awake microelectrode recordings, intraoperative kinesthetic and macro‐stimulation testing were used to implant the DBS lead (Medtronic model 3389 quadripolar electrode: contacts were 1.5 mm in length and spaced 0.5 mm apart). Postoperative MRI and CT were used to verify accurate placement. Three weeks following implantation of the lead, the IPG would be implanted under general anesthesia.
Relevant Neuropsychological (NP) Tests
To determine cognitive status pre‐ and postsurgically, all patients completed a standardized UCH movement disorder NP battery. On average, testing was performed 5.7 months (± 4.0; range: 1 to 23 months) before surgery. Sixteen test scores from the full battery were selected and group changes within various domains were assessed. The selection of tests relevant for this study was based on suggestions from the literature.10, 11, 12, 13, 16, 20, 21, 34 The Stroop color‐word and interference tasks (Golden version), and the Wisconsin card sorting test‐64 (WCST‐64) Perseverative errors score were used for executive function. The block‐design subtest (WAIS‐IV) and judgment of line orientation (JOLO) were used for visuospatial function. The brief test of attention (BTA), coding subtest (WAIS‐IV), and the digit span subtest (WAIS‐IV) were used for attention. The California verbal learning test‐II (CVLT‐II) long‐delay recall, recognition hits and total scores, and the brief visuospatial memory test‐revised (BVMT‐R) delayed recall; discrimination index and total scores were used for memory. Animal naming and letter fluency tests were used for semantic and phonemic verbal fluency, respectively.
MRI Processing Pipeline and Segmentation
All MRI files for each study subject were stripped of identifying information. Axial T2‐FLAIR MRIs with a slice thickness of 3 mm were available for all 43 patients. For calculation of T2 hyperintense white matter lesion volumes (mm3), each subject's T2‐FLAIR images were processed with the Lesion Prediction Algorithm (LPA) as implemented in the Lesion Segmentation Tool (LST) toolbox, version 2.0.13 (http://www.applied-statistics.de/lst.html) for SPM12. LPA results from the 43 T2‐FLAIR scans were reviewed in FSL View from the FMRIB Software Library (FSL: http://www.fmrib.ox.ac.uk/fsl) to assess accuracy of lesion segmentation. Manual editing to correct erroneous lesion segmentation of non‐brain tissue in nine scans was completed. An expert neuroradiologist reviewed and gave final approval of corrections. All 43‐lesion probability masks were binarized at a threshold value of 0.5 recommended by LST developers. Lesion volumes were then calculated for each patient's binarized thresholded probability mask.
Axial 3D T1‐weighted brain MRIs with a slice thickness of 1.2 mm were available for 30 of 43 patients. For calculation of brain structure volume (cm3), each patient's 3D T1‐weighted brain MRI was processed with Neuroquant (CorTechs Labs). Volumes for the left and right forebrain parenchyma and left and right hippocampi were extracted from the Neuroquant segmentation.
Statistical Analysis
All analyses were completed with SAS software, Version 9.4. Data was graphically checked for outliers, skew, and deviation from normality. Covariates were set to their mean values in the usable sample when testing the pre–post difference. Additionally, responses were grouped into several domains, and mixed model regressions were used to perform overall F tests for a pre–post difference among any of the responses. The strength of relationships between pre–post differences in scales and volumetrics were examined with the non‐linear Spearman correlation. Due to the range in magnitude of lesion volume, the natural log transformation (base e) of lesion volumes was used for all data analyses. Partial correlations controlled for covariates. Spearman correlation analyses between log (lesion volume) and the change in performance on each of the 16 cognitive tests was employed to investigate if presurgical WML volume was a significant predictor of postsurgical changes in performance on cognitive tests with a significance level of p < 0.05. To explore if brain region volume was associated with postsurgical changes in performance on cognitive tests, Spearman correlation analyses between left forebrain parenchyma, right forebrain parenchyma, left hippocampus or right hippocampus volume and change in performance on each of the 16 cognitive tests was used with a significance level of p < 0.05. Given the importance of WML and vascular risk factors (VRFs),35, 36 we controlled for the latter in our analysis. Covariates for statistical analysis were identified from extracted clinical data and included: age at baseline, education at baseline, number of VRFs, disease duration, percent change in UPDRS, postsurgical levodopa equivalent dose, and time from surgery to postsurgical NP testing. VRFs were defined as a history of diabetes, controlled hypertension (managed by medication), hypercholesterolemia and/or hyperlipidemia, and smoking. Patient's history across VRFs were summed to calculate number of VRFs. Percent change in UPDRS was calculated from presurgical UPDRS score OFF medication and postsurgical UPDRS score OFF mediation/ON stimulation. Due to the small sample size and the number of responses, outcomes were mostly analyzed individually, on available case data, and without adjustment for multiple comparisons to avoid loss power. Similarly, full regression models were not fit with all the volumetric measurements because of the number of variables compared with the number of usable observations.
Results
Neuropsychological (NP) Testing
There were significant declines (p < 0.05) in pre‐ to post‐ performance for cognitive tests in the domains of executive function, verbal fluency, attention, memory, and visuospatial function (Table 2; Fig. 1). There were also non‐significant pre‐ to post‐changes in performance for cognitive measures in the domains of memory, executive function, and visuospatial function. (Table 2; Fig. 1).
Table 2.
Group neuropsychological evaluation scores for presurgical, postsurgical, and pre/post changes, organized by cognitive domain.
| Total N | Pre‐surgical score | Post‐surgical score | Pre/post change in score | p‐value | |
|---|---|---|---|---|---|
| Attention | |||||
| BTA | 35 | 15.4 ± 3.4) | 13.5 ± 4.9) | −1.8 (± 3.3) | 0.003 |
| Digit span | 36 | 27.1 (± 4.2) | 25.3 (± 4.2) | −1.9 (± 3.5) | 0.003 |
| Coding | |||||
| Executive Function | 37 | 55.5 (± 13.5) | 47.9 (± 13.7) | −7.6 (± 10.6) | <0.0001 |
| Stroop color‐word | 30 | 35.2 (± 7.6) | 29.7 (± 7.7) | −5.4 (± 5.4) | <0.0001 |
| Stroop interference | 27 | 2.0 (± 12.3) | 6.9 (± 19.2) | 4.9 (± 21.4) | 0.25 |
| WSCT‐64 perseverative errors | 33 | 20.0 (± 12.2) | 19.2 (± 12.8) | −0.8 (± 14.7) | 0.77 |
| Memory | |||||
| BVMT‐R delayed recall | 39 | 7.8 (± 2.5) | 7.4 (± 3.2) | −0.4 (± 2.4) | 0.29 |
| BVMT‐R discrimination index | 39 | 5.6 (± 0.8) | 5.2 (± 1.2) | −0.4 (± 1.1) | 0.05 |
| BVMT‐R total | 39 | 19.1 (± 6.7) | 17.7 (± 8.3) | −1.4 (± 6.0) | 0.14 |
| CVLT‐II long‐delay recall | 38 | 9.7 (± 3.4) | 10.1 (± 3.1) | 0.4 (± 2.8) | 0.42 |
| CVLT‐II recognition hits | 38 | 14.8 (± 1.3) | 14.6 (± 1.6) | −0.2 (± 1.7) | 0.5 |
| CVLT‐II total | 38 | 46.3 (± 13.0) | 45.2 (± 12.3) | −1.1 (± 10.2) | 0.51 |
| Verbal fluency | |||||
| Animal naming | 36 | 21.9 (± 5.4) | 18.7 (± 5.0) | −3.1 (± 4. 7) | 0.0003 |
| Letter fluency | 38 | 41.8 (± 10.4) | 35.1 (± 12.1) | −6.7 (± 9.1) | <0.0001 |
| Visuospatial function | |||||
| Block design | 36 | 34.8 (± 11.1) | 32.4 (± 11.4) | −2.4 (± 6.4) | 0.03 |
| JOLO | 37 | 23.6 (± 4.7) | 23.4 (± 4.5) | −0.3 (± 3.8) | 0.67 |
All values are Mean (± SD).
Abbreviations: BTA, Brief Test of Attention; BVMT‐R, Brief Visual Memory Test‐Revised; CVLT‐II, California Verbal Learning Test‐II; JOLO, Judgement of Line Orientation; WCST‐64, Wisconsin Card Sorting Test‐64.
Figure 1.
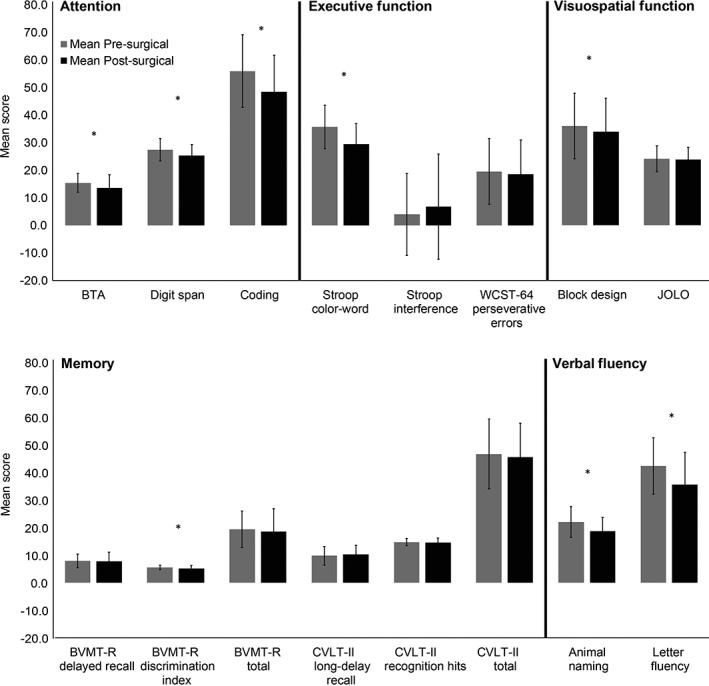
Mean pre‐ and postsurgical group neuropsychological evaluation scores organized by cognitive domain.
*Significant change in performance on cognitive test.
Abbreviations: BTA, Brief Test of Attention; BVMT‐R, Brief Visual Memory Test‐Revised; CVLT‐II, California Verbal Learning Test‐II; JOLO, Judgement of Line Orientation; WCST‐64, Wisconsin Card Sorting Test‐64.
Correlation Between Change in Performance and WML Volume
Presurgical T2 lesion volume was not correlated with change in performance on cognitive measures in the domains of memory, verbal fluency, executive function, or attention, even after adjusting for covariates (age at baseline, education at baseline, number of VRFs, disease duration, percent change in UPDRS, postsurgical levodopa equivalent dose, and time from surgery to postsurgical NP testing; Table 3). There was a significant negative correlation observed between lesion volume and decline in performance on a visuospatial task, which persisted when adjusting for covariates (r = ‐0.44, p = 0.02; Table 3). Age (r = 0.46, p < 0.002), number of vascular comorbidities (r = 0.35, p < 0.02) and percent change in UPDRS (r = 0.35, p = 0.03) were positively correlated with lesion volume (Table 4).
Table 3.
Correlation between change in performance on neuropsychological tests and log (lesion volume), forebrain and hippocampal volumes, covarying for age, education, number of vascular risk factors, duration of PD, percent change in UPDRS, postsurgical levodopa equivalence score, and time from surgery to postsurgical NP testing.
| White matter lesion | Left forebrain parenchyma | Right forebrain parenchyma | Left hippocampus | Right hippocampus | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total N | r | p‐value | r | p‐value | r | p‐value | r | p‐value | r | p‐value | |
| Attention | |||||||||||
| BTA | 35 *; 23 | −0.02 | 0.93 | 0.38 | 0.14 | 0.31 | 0.24 | 0.04 | 0.88 | 0.30 | 0.25 |
| Digit span | 36 *; 23 | 0.02 | 0.91 | −0.01 | 0.97 | −0.11 | 0.68 | −0.18 | 0.49 | 0.38 | 0.15 |
| Coding | 37 *; 25 | −0.28 | 0.13 | −0.27 | 0.28 | −0.27 | 0.27 | −0.10 | 0.70 | −0.02 | 0.92 |
| Executive Function | |||||||||||
| Stroop color‐word | 30 *; 19 | 0.01 | 0.96 | 0.38 | 0.23 | 0.40 | .020 | −0.08 | 0.80 | 0.03 | 0.93 |
| Stroop interference | 27 *; 17 | −0.02 | 0.94 | 0.59 | 0.07 | 0.55 | 0.10 | 0.32 | 0.36 | 0.27 | 0.45 |
| WSCT‐64 perseverative errors | 33 *; 20 | −0.01 | 0.97 | 0.03 | 0.93 | 0.16 | 0.60 | −0.40 | 0.18 | −0.37 | 0.21 |
| Memory | |||||||||||
| BVMT‐R delayed recall | 39 *; 26 | 0.03 | 0.88 | 0.00 | 0.99 | 0.03 | 0.90 | −0.33 | 0.17 | −0.13 | 0.60 |
| BVMT‐R discrimination index | 39 *; 26 | 0.08 | 0.64 | −0.12 | 0.61 | −0.09 | 0.73 | 0.01 | 0.96 | −0.20 | 0.40 |
| BVMT‐R total | 39 *; 26 | 0.05 | 0.77 | 0.12 | 0.63 | 0.19 | 0.44 | −0.33 | 0.17 | −0.09 | 0.71 |
| CVLT‐II long‐delay recall | 38 *; 25 | −0.04 | 0.83 | 0.22 | 0.38 | 0.14 | 0.58 | 0.18 | 0.48 | −0.11 | 0.66 |
| CVLT‐II recognition hits | 38 *; 25 | 0.05 | 0.81 | −0.07 | 0.79 | −0.08 | 0.75 | 0.26 | 0.30 | 0.48 | 0.04 |
| CVLT‐II total | 38 *; 25 | 0.03 | 0.87 | 0.39 | 0.11 | 0.35 | 0.16 | 0.19 | 0.45 | 0.04 | 0.88 |
| Verbal fluency | |||||||||||
| Animal naming | 36 *; 23 | −0.33 | 0.08 | 0.08 | 0.76 | 0.11 | 0.69 | 0.10 | 0.72 | 0.09 | 0.73 |
| Letter fluency | 38 *; 25 | −0.29 | 0.11 | 0.07 | 0.77 | 0.05 | 0.85 | 0.08 | 0.75 | 0.27 | 0.28 |
| Visuospatial function | |||||||||||
| Block design | 36 *; 24 | −0.44 | 0.02 | −0.13 | 0.63 | −0.14 | 0.59 | −0.18 | 0.49 | 0.20 | 0.45 |
| JOLO | 37 *; 24 | 0.13 | 0.48 | −0.14 | 0.6 | −0.16 | 0.55 | −0.08 | 0.75 | 0.02 | 0.93 |
*Total N for lesion volume analysis
Abbreviations: BTA, Brief Test of Attention; BVMT‐R, Brief Visual Memory Test‐Revised; CVLT‐II, California Verbal Learning Test‐II; JOLO, Judgement of Line Orientation; WCST‐64, Wisconsin Card Sorting Test‐64.
Table 4.
Correlation between each quantitative measures of brain MRI and covariates. All values are Spearman r; p value.
| Log(Lesion Volume) 0.Left FB Parenchyma 0.Right FB Parenchyma 0.Left Hippocampus 0.Right Hippocampus |
Education | Age | Number of Vascular Risk Factors | Disease duration | Percent change in UPDRS | Post−surgical levodopa equivalent dose | Time from surgey to post−surgical NP testing | |
|---|---|---|---|---|---|---|---|---|
| Log(Lesion Volume) 0.Left FB Parenchyma 0.Right FB Parenchyma 0.Left Hippocampus 0.Right Hippocampus |
||||||||
| Education | 0.03; 0.86 0.15; 0.44 0.14; 0.45 0.25; 0.17 0.16; 0.39 |
|||||||
| Age | 0.46; 0.00
*
−0.30; 0.11 −0.32; 0.09 −0.50; 0.00 * −0.55; 0.00 * |
0.04; 0.79 −0.15; 0.44 −0.15; 0.44 −0.15; 0.44 −0.15; 0.44 |
||||||
| Number of Vascular Risk Factors | 0.35; 0.02
*
−0.03; 0.88 −0.12; 0.53 0.06; 0.74 −0.16; 0.41 |
0.01; 0.96 0.07; 0.72 0.07; 0.72 0.07; 0.72 0.07; 0.72 |
0.28; 0.07 0.26; 0.16 0.26; 0.16 0.26; 0.16 0.26; 0.16 |
|||||
| Disease duration | −0.04; 0.82 −0.15; 0.42 −0.21; 0.28 −0.07; 0.71 0.00; 0.99 |
0.12; −0.30 0.14; 0.46 0.14; 0.46 0.14; 0.46 0.14; 0.46 |
−0.30; 0.05
*
−0.19; 0.31 −0.19; 0.31 −0.19; 0.31 −0.19; 0.31 |
−0.16; 0.30 −0.01; 0.96 −0.01; 0.96 −0.01; 0.96 −0.01; 0.96 |
||||
| Percent change in UPDRS | 0.35; 0.03
*
0.02; 0.93 0.09; 0.68 0.16; 0.43 0.27; 0.18 |
−0.04; 0.80 −0.13; 0.53 −0.13; 0.53 −0.13; 0.53 −0.13; 0.53 |
0.18; 0.27 0.21; 0.30 0.21; 0.30 0.21; 0.30 0.21; 0.30 |
0.05; 0.75 −0.09; 0.67 −0.09; 0.67 −0.09; 0.67 −0.09; 0.67 |
−0.33; 0.04
*
−0.25; 0.22 −0.25; 0.22 −0.25; 0.22 −0.25; 0.22 |
|||
| Post−surgical levodopa equivalent dose | 0.12; 0.45 −0.03; 0.88 −0.06; 0.78 −0.10; 0.62 −0.30; 0.13 |
−0.22; 0.16 −0.12; 0.54 −0.12; 0.54 −0.12; 0.54 −0.12; 0.54 |
0.13; 0.43 0.06; 0.77 0.06; 0.77 0.06; 0.77 0.06; 0.77 |
0.24; 0.13 0.05; 0.78 0.05; 0.78 0.05; 0.78 0.05; 0.78 |
0.05; 0.74 0.22; 0.26 0.22; 0.26 0.22; 0.26 0.22; 0.26 |
−0.11; 0.52 −0.12; 0.57 −0.12; 0.57 −0.12; 0.57 −0.12; 0.57 |
||
| Time from surgey to post‐surgical NP testing | 0.01; 0.93 0.28; 0.14 0.31; 0.10 0.27; 0.16 0.27; 0.15 |
0.15; 0.35 0.00; 1.00 0.00; 1.00 0.00; 1.00 0.00; 1.00 |
0.13; 0.39 −0.02; 0.91 −0.02; 0.91 −0.02; 0.91 −0.02; 0.91 |
0.06; 0.72 −0.31; 0.50 −0.31; 0.50 −0.31; 0.50 −0.31; 0.50 |
−0.36; 0.02
*
−0.31; 0.09 −0.31; 0.09 −0.31; 0.09 −0.31; 0.09 |
0.31; 0.05
*
0.54; 0.005 * 0.54; 0.005 * 0.54; 0.005 * 0.54; 0.005 * |
0.22; 0.17 0.09; 0.64 0.09; 0.64 0.09; 0.64 0.09; 0.64 |
Reported scores: Spearman r and p−values
*Indicate significant correlations (p < 0.05).
Correlation Between Change in Performance and Brain Region Volumes
Presurgical forebrain parenchyma and hippocampal volumes were not correlated with declines in performance on cognitive measures, even after adjusting for age at baseline, education at baseline, number of VRFs, disease duration, percent change in UPDRS, postsurgical levodopa equivalent dose, and time from surgery to postsurgical NP testing (Table 3). There was a significant positive correlation observed between right hippocampus volume and change in performance on the CVLT‐II recognition hits score (r = 0.48, p = 0.04), which persisted after adjusting for covariates, as shown in Table 3. Age was negatively correlated with left (r = ‐0.50, p < 0.004) and right (r = ‐0.55, p < 0.002) hippocampus volumes, but not left or right forebrain parenchyma volumes (Table 4).
Discussion
This study is the first to test the association of quantitative measures of whole brain WML, forebrain parenchymal, and hippocampal volumes in presurgical brain MRI with cognitive change following STN‐DBS. We observed significant group declines in performance on cognitive test scores in the five cognitive domains evaluated. We also observed that a significant decline in cognitive performance on a visuospatial task post‐STN‐DBS was significantly correlated with a higher burden of WML. Additionally, greater presurgical right hippocampus volume was associated with less change in performance on a memory task from pre‐ to post‐surgery. However, this study also showed that in this cohort larger WML volume before surgery was not strongly associated with the cognitive changes in the domains of attention, executive function, or verbal fluency. Likewise, lower presurgical forebrain parenchyma and hippocampal volumes were not associated with most postsurgical cognitive declines experienced by this cohort.
Neuropsychological Evaluation Performance
We observed significant group declines in performance on cognitive tests scores in the domains of executive function (Stroop‐color word task), verbal fluency (animal naming and letter fluency tasks), attention (brief test of attention, coding, and digit span), memory (BVMT‐R discrimination index), and visuospatial function (block design). Our results are generally consistent with previous cognitive outcome studies; however, many studies also inconsistently report impairment in domains of executive function, memory, attention, and visuospatial function.3, 7, 9, 11, 12, 13, 15, 16, 18, 19, 20, 21 Our observations could be attributed to the demographic and clinical characteristics of our cohort, as well as the UCH DBS for PD evaluation and selection protocol for determining suitable surgical candidates.
We did not observe significant declines in performance on two measures of executive function (Stroop interference task and WSCT‐64 perseverative errors score), or most measures of memory (BVMT‐R delayed recall and total scores, and CVLT‐II long‐delay recall, recognition hits and total scores), which is consistent with some prior reports,13 but inconsistent with others.11, 12, 19, 20, 21 Some of these studies11, 13, 20 included a control group of PD patients managed with standard clinical care, a lack of which is a limitation of our current study. In one control matched study, STN‐DBS for PD patients demonstrated a selective decline in verbal memory compared to a control group.11 In addition, significant declines on tasks of executive function (measured by the stroop color‐word task), and attention (measured by the digit span task), were exhibited by both groups. These results were suggestive that the observed declines were a result of the progression of the disease, rather than the DBS alone.
Although significant group declines in performance were observed in all five cognitive domains, it is important to note that our cohort was a limited sampling of STN‐DBS patients, and represents patients with advanced PD and no significant cognitive impairments for a mean disease duration of 9.4 years before surgery. Moreover, it is standard protocol to evaluate each DBS candidate and carefully assess the risk‐to‐benefit ratio, to approve candidates without clinical, surgical, or psychiatric contra‐indicators for the surgery. Nonetheless, it is somewhat unusual that in the present study we observed declines in performance within all cognitive domains assessed. The reason for these results is unclear, but it possible that selection bias may be one factor in this clinical sample. While repeat evaluations following STN‐DBS were recommended to all patients, not all patients were responsive to scheduling requests. Therefore, it is possible those patients who presented for repeat evaluation differed from those who did not (e.g., those with worries regarding cognitive functioning may have been more likely to schedule repeat evaluation than those with no worries), thereby influencing our findings. Overall, our neuropsychological results taken with previously reported findings emphasize the importance of counseling DBS candidates on the potential risk of cognitive declines following the surgery to appropriately manage expectations.
Correlation of Lesion Volume to Change in Performance on Cognitive Tests
Our results did demonstrate a statistically significant correlation between decline in visuospatial performance (as measured by block design subtest) and a greater burden of WML, even after correcting for age at baseline, education, number of VRFs, disease duration, percent change in UPDRS, postsurgical levodopa equivalent dose, and time from surgery to postsurgical NP testing. These findings contrast previous observations that PD‐MCI patients treated with medication demonstrated impaired visuospatial function that was not correlated with increased WML volume,24 or DTI metrics.23 These studies employed different methodologies and instruments for assessing visuospatial function, which can explain the difference in our observations. A different DTI study27 did demonstrate a relationship between visuospatial ability and white matter changes in the anterior corona radiata. These results suggest that the prefrontal control processes important for intact visuospatial function were disrupted. However, caution must be taken when comparing these results to ours since these studies employed DTI metrics in patients who had not undergone STN‐DBS surgery. Overall, the contribution of increased WML volume to decline in performance on a visuospatial task is unclear, however, it is possible the observed finding reflects a decline in speeded performance associated with a greater burden of WML rather than a “true” visuospatial change. Nonetheless, future studies should explore whether this decline is correlated with WML burden in specific cortical or sub‐cortical brain region areas.
Our results did reveal a significant correlation between greater burden of WML, increased age at baseline and increased number of vascular comorbidities (diabetes mellitus, hypertension, hyperlipidemia/hypercholesterolemia, and smoking). These results are consistent with a recent critical review36 of studies exploring the contribution of WML to the cognitive impairments experienced by PD patients treated with medication. This analysis implicated vascular risk factors as a main contributing factor of greater WML volume, and, therefore, cognitive dysfunction.
Correlation of Bilateral Brain Region Volumes to Change in Performance on Cognitive Tests
We observed a significant positive relationship between right hippocampus volume and less change in performance on CVLT‐II recognition hits score. There are no reports of correlates between intact memory functions and increased brain volume, and few reports of correlates28, 32, 37, 38 of impaired memory and volumetric changes to brain regions, in either PD or STN‐DBS patients. Our observations are consistent with broader reports11, 28, 29, 32, 37, 38 that associate impaired memory with volume changes to the medial temporal lobe, and frontostriatal networks. Indeed, medial temporal lobe structures, including the hippocampus, are established substrates for memory functions, and implicated in memory networks.32 Based on these reports, it is reasonable that unimpaired performance on the CVLT‐II recognition hits is associated with less atrophy in the right hippocampus, as our results suggested.
Overall, there is a paucity of literature exploring the relationship of quantitative measures of brain MRI and the cognitive outcomes of STN‐DBS. This is the first study to explore the correlation between quantitative measures of presurgical WML volumes and bilateral forebrain parenchyma and hippocampus brain volumes in STN‐DBS patients, and the cognitive changes following STN‐DBS. This study, therefore highlights the need for further exploration of this topic.
Limitations and Future Directions
This study is not without some limitations. As a retrospective study, the collection of the neuropsychological data and MRI data was “non‐systematic,” and thus, there were some missing data. A limitation of the brain volume correlation was the exclusion of the 13 patients with missing 3D T1‐weighted MRI. In addition, our cohort's MRI data were collected on two different scanners, and future studies should aim to have an established MRI acquisition protocol. Nonetheless, the LPA tool within the Lesion Segmentation Tool (LST) toolbox, version 2.0.13 for SPM12, and Neuroquant segmentation tool for our neuroanatomical quantifications helped us overcome this limitation in our study.
Another limitation of our study was our relatively small sample size, restricted by the completion of ≥ 6‐month postsurgical neuropsychological evaluation. Although we performed well over 43 STN‐DBS surgeries between January 2011 and June 2016, many of the STN‐DBS patients were excluded in the preliminary screening of this study's population because they lacked ≥ 6‐month postsurgical cognitive testing. It is not clear why some patients did not have postsurgical cognitive testing; however, any number of variables could have contributed to these patients not returning. First, postsurgical cognitive testing is not required for standard clinical care; therefore, many patients could have been prohibited from completing this testing because it was not covered by their insurance policy. It is also possible that the patients who did not complete postsurgical testing represent patients who were doing either very well or very poorly, and therefore, were not inclined to return for the extensive neuropsychological battery. Likewise, some patients could have decided that they did not wish to participate in an additional cognitive battery, regardless of an ability to do so, as it is a demanding and exhaustive evaluation. Additionally, the University of Colorado Hospital, being a regional center, serves patients from several surrounding states, and many patients treated at this facility commute from long distances. It is possible patients completed postsurgical cognitive testing at a different site, and these records were not accessible for this study. Regardless of the reasons for patients' not having postsurgical cognitive testing, future studies should implement a standardized presurgical and postsurgical cognitive testing protocol to allow for a more systematic collection of patient data.
It is possible that our decision to broadly group our cohort's neuropsychological data kept relationships hidden. Therefore, future studies should stratify patients in some manner, such as individual cognitive changes, vascular comorbidities, or even unilateral vs. bilateral STN‐DBS. Last, future studies evaluating whether quantitative measures of brain MRI are associated with the cognitive outcomes of STN‐DBS should also include a control group of age and disease duration, matched PD patients treated with medication. Future exploration of this relationship could also evaluate increased lesion volumes in specific cortical brain regions as a predictor of cognitive changes in domains associated with those regions, such as changes in frontal white matter regions and worsened verbal fluency and executive function performance.
Conclusions
These results are encouraging since they suggest that presurgical lesion and brain region volumes, beyond any clinical predictors, do not put STN‐DBS candidates at an increased risk for postsurgical cognitive impairments.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
O.K.: 1A, 3B
B.H.: 1A, 1B, 1C, 3B
L.W.: 1B, 1C, 2A, 2B, 2C, 3A, 3B
J.H.: 1B, 1C, 3B
J.T.: 1B, 3B
J.A.T: 1B, 1C, 2C, 3A, 3B
S.S.: 1C, 2A, 2B, 2C
Disclosures
Ethical Compliance Statement: The design of this study satisfied the ethical standards of the Colorado Multiple Institutional Review Board (COMIRB) and received IRB approval. Written consent for use of neuropsychological outcomes and clinical data was obtained for all subjects during their pre‐DBS evaluation. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. We confirm that we have read the journal's position on issues involved in ethical publicationand affirm that this work is consistent with those guidelines.
Financial Disclosures and Conflicts of Interest: None
Financial Disclosures for previous 12 months: None
Acknowledgments
We thank Drs. Aviva Abosch and Steven Ojemann for critical review of the manuscript. In addition, we thank Joy Abraham for assistance with data collection.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Kalia LV, Lang AE. Parkinson's disease. Lancet. 2015;386:896–912. [DOI] [PubMed] [Google Scholar]
- 2. Lang AE, Widner H. Deep brain stimulation for Parkinson's disease: patient selection and evaluation. Mov Disord. 2002;17 Suppl 3:S94–101. [DOI] [PubMed] [Google Scholar]
- 3. Parsons TD, Rogers SA, Braaten AJ, Woods SP, Troster AI. Cognitive sequelae of subthalamic nucleus deep brain stimulation in Parkinson's disease: a meta‐analysis. Lancet Neurol. 2006;5:578–588. [DOI] [PubMed] [Google Scholar]
- 4. Rodriguez‐Oroz MC, Obeso JA, Lang AE, et al. Bilateral deep brain stimulation in Parkinson's disease: a multicentre study with 4 years follow‐up. Brain. 2005;128:2240–2249. [DOI] [PubMed] [Google Scholar]
- 5. Kleiner‐Fisman G, Fisman DN, Sime E, Saint‐Cyr JA, Lozano AM, Lang AE. Long‐term follow up of bilateral deep brain stimulation of the subthalamic nucleus in patients with advanced Parkinson disease. J Neurosurg. 2003;99:489–495. [DOI] [PubMed] [Google Scholar]
- 6. Munhoz RP, Picillo M, Fox SH, et al. Eligibility Criteria for Deep Brain Stimulation in Parkinson's Disease, Tremor, and Dystonia. Can J Neurol Sci. 2016;43:462–471. [DOI] [PubMed] [Google Scholar]
- 7. Massano J, Garrett C. Deep brain stimulation and cognitive decline in Parkinson's disease: a clinical review. Front Neurol. 2012;3:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krack P, Batir A, Van Blercom N, et al. Five‐year follow‐up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 2003;349:1925–1934. [DOI] [PubMed] [Google Scholar]
- 9. Combs HL, Folley BS, Berry DT, et al. Cognition and Depression Following Deep Brain Stimulation of the Subthalamic Nucleus and Globus Pallidus Pars Internus in Parkinson's Disease: A Meta‐Analysis. Neuropsychol Rev. 2015;25:439–454. [DOI] [PubMed] [Google Scholar]
- 10. Kim HJ, Jeon BS, Paek SH, et al. Long‐term cognitive outcome of bilateral subthalamic deep brain stimulation in Parkinson's disease. J Neurol. 2014;261:1090–1096. [DOI] [PubMed] [Google Scholar]
- 11. York MK, Dulay M, Macias A, et al. Cognitive declines following bilateral subthalamic nucleus deep brain stimulation for the treatment of Parkinson's disease. J Neurol Neurosurg Psychiatry. 2008;79:789–795. [DOI] [PubMed] [Google Scholar]
- 12. Heo JH, Lee KM, Paek SH, et al. The effects of bilateral subthalamic nucleus deep brain stimulation (STN DBS) on cognition in Parkinson disease. J Neurol Sci. 2008;273:19–24. [DOI] [PubMed] [Google Scholar]
- 13. Castelli L, Rizzi L, Zibetti M, Angrisano S, Lanotte M, Lopiano L. Neuropsychological changes 1‐year after subthalamic DBS in PD patients: A prospective controlled study. Parkinsonism Relat Disord. 2010;16:115–118. [DOI] [PubMed] [Google Scholar]
- 14. Kim HJ, Jeon BS, Yun JY, Kim YE, Yang HJ, Paek SH. Initial cognitive dip after subthalamic deep brain stimulation in Parkinson disease. J Neurol. 2013;260:2130–2133. [DOI] [PubMed] [Google Scholar]
- 15. Witt K, Pulkowski U, Herzog J, et al. Deep brain stimulation of the subthalamic nucleus improves cognitive flexibility but impairs response inhibition in Parkinson disease. Arch Neurol. 2004;61:697–700. [DOI] [PubMed] [Google Scholar]
- 16. Odekerken VJ, Boel JA, Geurtsen GJ, et al. Neuropsychological outcome after deep brain stimulation for Parkinson disease. Neurology. 2015;84:1355–1361. [DOI] [PubMed] [Google Scholar]
- 17. Wyman‐Chick KA. Verbal Fluency in Parkinson's Patients with and without Bilateral Deep Brain Stimulation of the Subthalamic Nucleus: A Meta‐analysis. J Int Neuropsychol Soc. 2016;22:478–485. [DOI] [PubMed] [Google Scholar]
- 18. Witt K, Daniels C, Reiff J, et al. Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson's disease: a randomised, multicentre study. Lancet Neurol. 2008;7:605–614. [DOI] [PubMed] [Google Scholar]
- 19. Smeding HM, Speelman JD, Koning‐Haanstra M, et al. Neuropsychological effects of bilateral STN stimulation in Parkinson disease: a controlled study. Neurology. 2006;66:1830–1836. [DOI] [PubMed] [Google Scholar]
- 20. Smeding HM, Speelman JD, Huizenga HM, Schuurman PR, Schmand B. Predictors of cognitive and psychosocial outcome after STN DBS in Parkinson's Disease. J Neurol Neurosurg Psychiatry. 2011;82:754–760. [DOI] [PubMed] [Google Scholar]
- 21. Yaguez L, Costello A, Moriarty J, et al. Cognitive predictors of cognitive change following bilateral subthalamic nucleus deep brain stimulation in Parkinson's disease. J Clin Neurosci. 2014;21:445–450. [DOI] [PubMed] [Google Scholar]
- 22. Braak H, Rub U, Del Tredici K. Cognitive decline correlates with neuropathological stage in Parkinson's disease. J Neurol Sci. 2006;248:255–258. [DOI] [PubMed] [Google Scholar]
- 23. Melzer TR, Watts R, MacAskill MR, et al. White matter microstructure deteriorates across cognitive stages in Parkinson disease. Neurology. 2013;80:1841–1849. [DOI] [PubMed] [Google Scholar]
- 24. Kandiah N, Mak E, Ng A, et al. Cerebral white matter hyperintensity in Parkinson's disease: a major risk factor for mild cognitive impairment. Parkinsonism Relat Disord. 2013;19:680–683. [DOI] [PubMed] [Google Scholar]
- 25. Bohnen NI, Albin RL. White matter lesions in Parkinson disease. Nat Rev Neurol. 2011;7:229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Agosta F, Canu E, Stefanova E, et al. Mild cognitive impairment in Parkinson's disease is associated with a distributed pattern of brain white matter damage. Hum Brain Mapp. 2014;35:1921–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Theilmann RJ, Reed JD, Song DD, et al. White‐matter changes correlate with cognitive functioning in Parkinson's disease. Front Neurol. 2013;4:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Filoteo JV, Reed JD, Litvan I, Harrington DL. Volumetric correlates of cognitive functioning in nondemented patients with Parkinson's disease. Mov Disord. 2014;29:360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ibarretxe‐Bilbao N, Junque C, Marti MJ, Tolosa E. Brain structural MRI correlates of cognitive dysfunctions in Parkinson's disease. J Neurol Sci. 2011;310:70–74. [DOI] [PubMed] [Google Scholar]
- 30. Alzahrani H, Venneri A. Cognitive and neuroanatomical correlates of neuropsychiatric symptoms in Parkinson's disease: A systematic review. J Neurol Sci. 2015;356:32–44. [DOI] [PubMed] [Google Scholar]
- 31. Pereira JB, Junque C, Marti MJ, Ramirez‐Ruiz B, Bartres‐Faz D, Tolosa E. Structural brain correlates of verbal fluency in Parkinson's disease. Neuroreport. 2009;20:741–744. [DOI] [PubMed] [Google Scholar]
- 32. Geevarghese R, Lumsden DE, Costello A, et al. Verbal Memory Decline following DBS for Parkinson's Disease: Structural Volumetric MRI Relationships. PLoS One. 2016;11:e0160583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abboud H, Floden D, Thompson NR, et al. Impact of mild cognitive impairment on outcome following deep brain stimulation surgery for Parkinson's disease. Parkinsonism Relat Disord. 2015;21:249–253. [DOI] [PubMed] [Google Scholar]
- 34. Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27:349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Malek N, Lawton MA, Swallow DM, et al. Vascular disease and vascular risk factors in relation to motor features and cognition in early Parkinson's disease. Mov Disord. 2016;31:1518–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vesely B, Rektor I. The contribution of white matter lesions (WML) to Parkinson's disease cognitive impairment symptoms: A critical review of the literature. Parkinsonism Relat Disord. 2016;22 Suppl 1:S166–170. [DOI] [PubMed] [Google Scholar]
- 37. Song SK, Lee JE, Park HJ, Sohn YH, Lee JD, Lee PH. The pattern of cortical atrophy in patients with Parkinson's disease according to cognitive status. Mov Disord. 2011;26:289–296. [DOI] [PubMed] [Google Scholar]
- 38. Mak E, Zhou J, Tan LC, Au WL, Sitoh YY, Kandiah N. Cognitive deficits in mild Parkinson's disease are associated with distinct areas of grey matter atrophy. J Neurol Neurosurg Psychiatry. 2014;85:576–580. [DOI] [PubMed] [Google Scholar]


