Abstract
Aims
Clinical heart failure (HF) guidelines recommend monitoring of creatinine and potassium throughout the initial weeks of mineralocorticoid receptor antagonists (MRAs) therapy. We here assessed the extent to which this occurs in our health care.
Methods and results
Observational study in 2007–2010 HF patients starting MRA therapy in Stockholm, Sweden. Outcomes included potassium and creatinine laboratory testing before MRA initiation and in the early (Days 1–10) and extended (Days 11–90) post-initiation periods. Exclusion criteria considered death/hospitalization within 90 days, and lack of a second MRA dispense. Of 4036 HF patients starting on MRA, 45% were initiated from a hospital, 24% from a primary care centre, and 30% from other private centres. Overall, 89% underwent pre-initiation testing, being more common among hospital (97%) than for primary care (74%) initiations. Only 24% were adequately monitored in all three recommended intervals, being again more frequent following hospital (33%) than private (21%) or primary care (17%) initiations. In multivariable analyses, adequate monitoring was more likely for hospital [odds ratio (OR) 2.85, 95% confidence interval (95% CI) 2.34–3.56] initiations, and for patients with chronic kidney disease (OR 1.79, 95% CI 1.30–2.43) and concomitant use of angiotensin-converting enzyme (OR 1.27, 95% CI 1.05–1.52), angiotensin receptor blockers (OR 1.19, 95% CI 1.01–1.40) or beta-blockers (OR 1.65, 95% CI 1.22–2.26). Age, sex, and prescribing centre explained a small portion of adequate monitoring (c-statistic 0.63). Addition of comorbidities and medications improved prediction marginally (c-statistic 0.65).
Conclusion
Although serum potassium and creatinine monitoring before MRA initiation for HF is frequent, rates of post-initiation monitoring remain suboptimal, especially among primary care centres.
Keywords: Hyperkalaemia, CKD, MRAs, SCREAM
Introduction
Mineralocorticoid receptor antagonists (MRAs; spironolactone and eplerenone), antagonize the effects of the aldosterone hormone and prevent sympathetic activation, parasympathetic inhibition, and myocardial remodelling. The use of MRA is recommended for persons with heart failure (HF),1,2 owing to their capacity to reduce the risk of cardiovascular complications and mortality as shown in large placebo-controlled randomized trials (RCTs).3,4
During the initial weeks of MRA therapy, serial monitoring of potassium and creatinine is recommended to identify therapy complications, adverse event risk and need for dose titration.2,5 Recent studies from real-world US health care systems have shown a low adherence to MRA monitoring guidelines.6–11 Equivalent studies in European settings are to date, lacking. In this study, we analyse the consistency between current guideline recommendations and the quality of creatinine and potassium monitoring upon initiation of MRAs in a region-representative cohort of HF patients from Sweden.
Methods
Data sources
This study utilizes the Stockholm CREAtinine Measurements (SCREAM) health care-utilization cohort,12 which includes all residents in the region of Stockholm, Sweden, undertaking at least one measurement of serum creatinine in ambulatory or hospital care during 2006–2010. Laboratory data were linked with regional and national administrative databases for complete information on health care utilization (including diagnoses and procedures undertaken), dispensed drugs and follow-up for death, with minimal loss to follow-up. The study was approved by Regional Institutional Review Boards and the Swedish National Board of Welfare and adheres to the Declaration of Helsinki. Since this was retrospective database and registry analysis, no individual patient consent was required.
Patient selection and study exposure
We included all adult (>18 years old) HF patients initiating MRA (spironolactone or eplerenone) therapy between 1 January 2007 and 31 December 2010. The study exposure was the initiation of MRA treatment which was considered the index date. New use of MRA was defined as a first-time MRA dispensation (with no previous dispensation of an MRA recorded) between 1 January 2007 and 31 December 2010. Information on MRA dispensations was obtained from the Dispensed Drug Registry, a nationwide register that records complete information on all prescribed drugs actually dispensed at Swedish pharmacies. The coverage of this register is considered complete, as all outpatient drug dispensations in Sweden are done via each citizen’s unique personal identification number. A total of 18 197 Stockholm residents initiated MRA therapy during 2007–2010. Of those, 7982 initiated therapy with comorbid HF (definition in Supplementary material online, Table S1). For the purpose of evaluating monitoring patterns, we excluded new users who died or were hospitalized within 4 months of drug initiation (n = 1296), as these events would have modified laboratory monitoring practice. We also excluded 2650 individuals who did not get a second MRA dispensation, as we wanted to ensure that MRA was prescribed for more than 3 months. After applying exclusion criteria, the cohort study considered in this analysis included 4036 persons (patient selection flow chart described in Supplementary material online, Figure S1).
Study covariates
Study covariates were assessed at index date and included age, sex, laboratory values, comorbidities, concomitant medications, and prescriber characteristics. Laboratory values considered in this analysis were measurements of potassium and creatinine in outpatient care, although patients lacking follow-up outpatient measurements were kept in the cohort. Potassium was measured in either plasma or serum by potentiometric titration. Serum creatinine was measured with either enzymatic or corrected Jaffe method (alkaline picrate reaction), both methods being traceable to isotope dilution mass spectroscopy standards. Potassium values >10 mmol/L and creatinine values <25 and >1500 μmol/L were considered implausible and were discarded. We excluded inpatient creatinine measurements, as they may not reflect baseline kidney function but rather acute kidney failure. When more than two measurements of an analyte were available in the same day, the median value was used if the concentrations differed by less than 30%. In case of >30% difference between measurements, the lowest value was used, in an attempt to discard incorrect measurements that may have required repeated testing. Inter- as well as intra-laboratory variation was considered minimal, with the three laboratories being frequently audited for quality and harmonization by the national organization EQUALIS (www.equalis.se).
Comorbid conditions included diabetes mellitus, history of myocardial infarction, peripheral vascular disease, cerebrovascular disease, and diagnosed chronic kidney disease (CKD), assessed using International Classification of Disease-Tenth Edition (ICD-10) diagnostic codes (definitions in Supplementary material online, Table S1). Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) 2009 creatinine equation.13 We defined CKD as eGFR ≤ 60 mL/min/1.73 m2, using the closest measurement recorded at index date. Medications included diuretics (both loop- and thiazides), angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARBs), beta-blockers, and other antihypertensive medications (definitions in Supplementary material online, Table S2). Medications were assumed to be concomitant if there was a pharmacy dispensation within 6 months before- and up to 15 days after MRA initiation. Finally, we retrieved information about the medical facility from which the first MRA prescription was issued. Information on prescribing centre is available in the Swedish national Drug register at each dispensation. Using thereafter an established classification system from our health care provider, prescribing centres were categorized as primary health care centres, hospital centres, and others. The latter included private subsidized health care services not fitting the other categories, such as geriatric clinics, psychiatric clinics, or nursing homes.
Study outcome
The study outcome was the adequacy of potassium and creatinine monitoring during the first 3 months of MRA therapy.2,5 For comparison with preceding literature,9,11 we considered three distinct laboratory monitoring periods: (i) pre-initiation testing: measurement of potassium and creatinine at least once within 120 days prior to MRA therapy initiation; (ii) early post-initiation testing: measurement of potassium and creatinine at least once within the first 10 days after MRA initiation; (iii) extended post-initiation testing: measurement of potassium and creatinine at least once within 11 and 90 days after MRA initiation. We explored two different patterns of adequate monitoring: first, we defined a combined indicator of appropriate monitoring across all three monitoring periods. Secondly, we created a combined indicator of appropriate post-initiation monitoring among those who underwent pre-initiation testing. We note that our definition of adequate monitoring is comparable to previous studies but requires less frequent post-initiation monitoring than what is recommended in the guidelines. The ESC guidelines recommend checking labs at 1 and 4 weeks, and again at 8 weeks and 12 weeks after MRA initiation.5 The american college of cardiology foundation/american heart association (ACCF/AHA) guidelines recommend checking labs within 2–3 days after drug initiation, again at 7 days, and at least monthly for the first 3 months.2
Statistical analysis
Multivariable logistic regression models were fitted to identify significant predictors for each indicator. The Kaplan–Meier curves were used for describing time to the first serum potassium and creatinine measurement after MRA dispensing during the 120 days observation period. The c-statistic and pseudo-R squared index improvement were reported to show which covariables improved goodness of fit the most.
Results
General descriptive results for the 4036 persons with HF initiating MRA therapy are shown in Table 1, together with characteristics of subgroups with differing pattern of laboratory monitoring. Spironolactone was the MRA initiated in 99% of cases and eplerenone in the remaining 1%. Median age was 77 years [interquartile range (IQR) 67–85] and 45% were women. Median eGFR was 67 (51–82) mL/min/1.73 m2 and median plasma potassium at initiation was 4.0 mmol/L. In all, 45% of patients received their MRA prescription from a hospital centre, 24% from a primary health care centre, and 30% from other private practices in the region. A large proportion of patients were also using ACEi and/or ARBs. A small proportion of patients had baseline eGFR or potassium at levels at which MRA are not recommended2,5: 3% of participants had an eGFR below 30 mL/min, and 20 persons had potassium >5 mmol/L.
Table 1.
Baseline characteristics of heart failure patients initiating mineralocorticoid receptor antagonist therapy, overall and by the pattern of laboratory monitoring
| Parameter | All patients | Lacking pre-initiation monitoring | Undergoing pre-initiation monitoring | Appropriate monitoring (all three pre-/ post-occasions)a |
|---|---|---|---|---|
| N | 4036 (100%) | 448 (11%) | 3588 (89%) | 934 (23%) |
| Age (years) | 78 (67–85) | 78 (69–85) | 78 (67–85) | 75 (65–83) |
| Age (categories) | ||||
| <45 years | 70 (2%) | 5 (1.1%) | 65 (1.8%) | 23 (2%) |
| 45–64 years | 727 (18%) | 73 (16%) | 654 (18%) | 212 (23%) |
| 65–75 years | 903 (22%) | 97 (22%) | 806 (23%) | 232 (25%) |
| >75 years | 2336 (58%) | 273 (61%) | 2063 (58%) | 467 (50%) |
| Women | 1824 (45%) | 225 (50%) | 1599 (45%) | 361 (39%) |
| eGFR (mL/min/1.73 m2) | ||||
| Median (IQR) | 67 (51–82) | 69 (55–82) | 67 (51–82) | 66 (51–81) |
| Missing | 383 (9%) | 383 (85%) | 0 (0%) | 0 (0%) |
| eGFR (categories) | ||||
| >60 mL/min/1.73 m2 | 2245 (56%) | — | 133 (3.7%) | 570 (61%) |
| 45–60 mL/min/1.73 m2 | 803 (20%) | — | 464 (13%) | 186 (20%) |
| 30–45 mL/min/1.73 m2 | 471 (12%) | — | 790 (22%) | 125 (13%) |
| <30 mL/min/1.73 m2 | 134 (3%) | — | 2201 (61%) | 53 (6%) |
| Potassium (mmol/L) | ||||
| Median (IQR) | 4.1 (3.6–4.2) | 3.8 (3.4–3.9) | 4.1 (3.7–4.2) | 4.0 (3.9–4.1) |
| Missing | 427 (11%) | 427 (95%) | 0 (0%) | 0 (0%) |
| Potassium (categories) | ||||
| <4 mmol/L | 1800 (45%) | — | 1783 (50%) | 523 (56%) |
| 4–5 mmol/L | 1789 (44%) | — | 1785 (50%) | 406 (43%) |
| >5 mmol/L | 20 (0%) | — | 20 (0.6%) | 5 (1%) |
| Site of first prescription | ||||
| Hospital centres | 1816 (45%) | 55 (12%) | 1761 (49%) | 580 (62%) |
| Primary health care centres | 976 (24%) | 251 (56%) | 725 (20%) | 126 (13%) |
| Other centres | 1194 (30%) | 109 (24%) | 1085 (30%) | 226 (24%) |
| Missing | 50 (1%) | 33 (7%) | 17 (0.5%) | 2 (<0%) |
| Diabetes mellitus | 1278 (32%) | 130 (29%) | 1148 (32%) | 298 (32%) |
| Myocardial infarction | 1197 (30%) | 111 (25%) | 1086 (30%) | 285 (31%) |
| Peripheral vascular disease | 551 (14%) | 58 (13%) | 493 (14%) | 132 (14%) |
| Cerebrovascular disease | 733 (18%) | 75 (17%) | 658 (18%) | 157 (17%) |
| Chronic kidney disease (diagnosis) | 215 (5%) | 14 (3%) | 201 (5.6%) | 71 (8%) |
| ACEi | 2820 (70%) | 282 (63%) | 2538 (71%) | 706 (76%) |
| ARB | 1543 (38%) | 178 (40%) | 1365 (38%) | 384 (41%) |
| Beta-blocker | 3609 (89%) | 378 (84%) | 3231 (90%) | 878 (94%) |
| Diuretics | 3882 (96%) | 417 (93%) | 3465 (97%) | 907 (97%) |
| Other antihypertensive drugs | 1553 (38%) | 165 (37%) | 1388 (39%) | 366 (39%) |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; MRA, mineralocorticoid receptor antagonist.
aThis group is a subset of those undergoing pre-initiation laboratory monitoring.
As many as 446 (11%) of patients initiating MRA did not undergo laboratory monitoring at time of or within the 120 days prior to MRA initiation (Figure 1). These patients were more often women and initiated on MRAs at a primary health care centre. Among these, potassium measurements were not performed in 95% of cases and creatinine not performed in 85% (Table 1).
Figure 1.
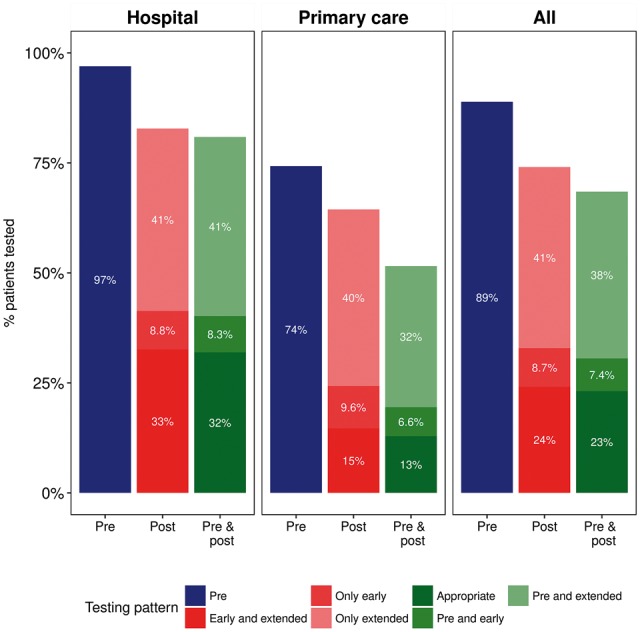
Patterns of laboratory testing in heart failure patients starting mineralocorticoid receptor antagonist therapy, overall and by the initial site of mineralocorticoid receptor antagonist prescription. Shown is the proportion of patients undergoing laboratory testing before (pre-) and after (post-)mineralocorticoid receptor antagonist initiation. Post-initiation testing is characterized as early (within 10 days) or extended (within 10–90 days). Appropriate monitoring denotes testing in all three studied intervals (pre, early, and extended).
The remaining 89% of patients underwent laboratory testing prior to MRA initiation (Figure 1), especially those whose first MRA prescription was issued at a hospital centre (97%) and less so among those first prescribed in primary health care centres (74%). Among patients undergoing pre-initiation laboratory testing, 23% had no detected follow-up monitoring at all. The time to first post-initiation measurement of potassium and creatinine is displayed in Supplementary material online, Figure S2. The median time to testing for creatinine was 26 days (IQR 12–82, mean 69 days) and 26 days (IQR 12–81) for potassium. Overall and throughout the different sites of prescription, ‘early’ monitoring after MRA initiation (within 10 days) was less common (33% overall, 42% in hospitals, and 25% in primary care) than monitoring in the ‘extended’ time period (within 10–90 days occurring in 50% overall, 50% in hospitals, and 50% in primary care). Once again, primary health care centres had a more suboptimal post-MRA initiation monitoring than hospital centres (Figure 1). In all, 69% of patients had one pre- and at least one post-initiation testing (either early or late follow-up testing or both). However, appropriate laboratory monitoring according to our definition (i.e. on all three occasions) was done only in 23% of patients. Appropriate monitoring was more common among persons whose first MRA was prescribed at a hospital centre (32%) compared to primary health care centres (13%) or other sites (19%, not shown in figure).
In order to identify factors associated with MRA-laboratory monitoring, we constructed two different multivariable logistic regression models. In the first model, we studied the likelihood to be appropriately monitored according to guideline recommendations (i.e. on all three occasions, Supplementary material online, Table S3). Briefly, patients whose first MRAs was prescribed at a hospital or private centre [odds ratio (OR) 2.85, 95% confidence interval (95% CI) 2.34–3.56, and OR 1.55, 95% CI 1.22–1.97, respectively, vs. primary health care centres], patients with clinically diagnosed CKD (OR 1.79, 95% CI 1.30–2.43), and using ACE (OR 1.27, 95% CI 1.05–1.52), ARB (OR 1.19, 95% CI 1.01–1.40), or beta-blockers (OR 1.65, 95% CI 1.22–2.26) were associated with higher odds of testing.
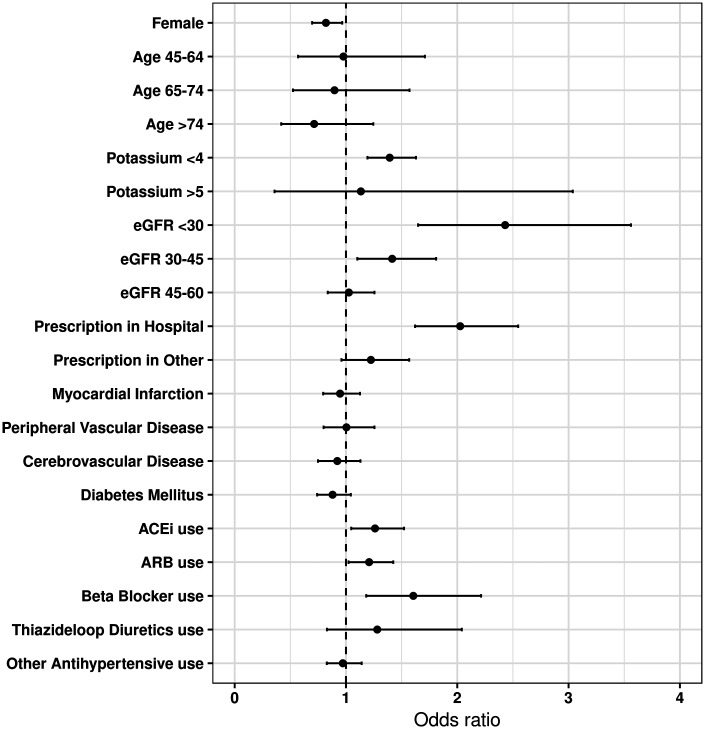
In the second model, we attempted to gain more precision by studying the likelihood to undertake post-MRA initiation laboratory monitoring (Figure 2 and Supplementary material online, Table S4). In this model, baseline potassium <4 mmol/L (OR 1.39, 95% CI 1.19–1.629), lower kidney function (OR 1.41, 95% CI 1.10–1.81 for eGFR < 45–30 mL/min/1.73 m2, and OR 2.43, 95% CI 1.64–3.56 for <30 mL/min/1.73 m2), MRAs initiated at hospital centres (OR 2.02, 95% CI 1.62–2.55), and concomitant use of ACEi (OR 1.26, 95% CI 1.05–1.52), ARB (OR 1.21, 95% CI 1.02–1.42), or beta-blockers (OR 1.60, 95% CI 1.18–2.21) associated with higher odds of post-initiation testing. Conversely, women (OR 0.82, 95% CI 0.69–0.96) were less likely to undergo post-initiation monitoring.
Figure 2.
Multivariable odd ratios and 95% confidence intervals of baseline factors associated with appropriate post-mineralocorticoid receptor antagonist initiation laboratory testing. Multivariable logistic regression was used for estimation and all the variables presented in the figure were included as covariables. eGFR, estimated glomerular filtration rate, in mL/min/1.73 m2; potassium, in mmol/L.
The model fit for these two regressions is displayed in Supplementary material online, Table S5. In both, the site of prescription was the covariate that improved model performance the most (from c-statistic 0.57 in and age- and sex-adjusted model to 0.61–0.63). Age, sex, and site of prescription explained; however, a moderate proportion of adequate monitoring (c-statistic 0.61–0.63). Addition of laboratory date, comorbidities, or concomitant medications improved prediction very marginally (c-statistic 0.64–0.65).
Discussion
Mineralocorticoid receptor antagonist is a mainstay treatment in patients with HF1,2 and frequent laboratory monitoring is advocated, especially during the initial weeks of therapy, to early detect and potentially avoid potential adverse events.2,5 In this study, we observe that monitoring of potassium and creatinine during MRA initiation in Swedish HF patients does not often meet current guideline recommendations. Although a large majority of participants underwent laboratory monitoring prior to MRA initiation, we observe insufficient post-initiation laboratory follow-up. Collectively, only about a third of patients initiated on MRAs in our region underwent the recommended laboratory monitoring frequency. In any case, it should be noted that the median time to first potassium measurement in clinical practice was 26 days, clearly exceeding the ESC guidelines recommendation of laboratory testing within the first week.5
Despite different patient selection and varying monitoring definitions used in previous studies, our results are consistent with US data. Allen et al.8 reported that among 490 hospitalized HF patients with reduced ejection fraction that started MRA therapy, 94.3% underwent laboratory monitoring before therapy initiation, but 46% were not followed within the first 7 days. Chauhan et al.10 studied serum potassium monitoring among 142 880 male patients with HF starting MRA treatment at the Veterans Affair health care system and found that only 41.6% had serum potassium monitoring within 14 days of MRA dispensing. Cooper et al.9,11 studied a sample of over 10 000 HF patients, observing that 25.2% of patients prescribed MRA during an inpatient visit had an appropriate frequency of laboratory testing (pre- and post-monitoring). Our study in Swedish patients shows higher rates of adequate monitoring as compared to US data. Nonetheless, rates are still largely suboptimal. Our analysis has the advantage of including the complete unselected HF population from a region with universal health care access, complete coverage of all MRA dispensations as well as of serum creatinine and potassium measurements in Stockholm health care. This may reduce the access to health care bias by reimbursement policies and monitoring ascertainment bias because of non-unified health care systems in the USA.
Cooper et al.9,11 reported a much lower proportion of appropriate monitoring in outpatient care (2.8%), which again agrees with our findings, although the authors used a somewhat different definition of monitoring than the present study. The apparent higher vigilance in hospital vs. primary care may be anticipated because of case-mix (i.e. more severe HF or comorbidities). However, hospital centres were associated with a higher probability of appropriate monitoring even when adjusted for comorbid conditions and concomitant medications. We acknowledge that our study assessed the actual performance of laboratory testing, not the intent of prescribing clinicians to obtain such testing in follow-up. Therefore, less adherent or less motivated patients may fail to attend follow-up check-ups in primary care. To contemplate this possibility, we base our exposure on MRA dispensations instead of MRA prescription claims, and we imposed the condition of purchasing a second MRA to ensure that this was a long-term therapy with sufficient pill supply throughout the monitoring period. In any case, the high rate of pre-initiation testing but subsequently low post-initiation testing suggests, collectively, that gaps in recommended testing may be related to system execution and patient adherence with such testing.
Identifying patient groups that are not adequately monitored is important to implement improvement strategies. Among the variety of patient characteristics (including comorbidities and laboratory values) and medications considered in our analysis, we found that CKD (either as defined by ICD-10 diagnosis or by measured eGFR), abnormal plasma potassium at therapy initiation, and concomitant use of potassium-altering medications (such as ACEi, ARB, beta-blockers) are associated with a higher likelihood or appropriate monitoring. All these are conditions known to increase hyperkalaemia risk. Interestingly, women had lower odds of appropriate testing, perhaps supporting the notion that men are perceived to be at higher adverse event risk. Because use of thiazide diuretics and baseline potassium <4 mmol/L were also associated with a higher odds of more stringent monitoring, we speculate that hypokalaemia concerns also influences monitoring practice. Although a substantial proportion of new MRA users (35%) in our study had reduced eGFR (<60 mL/min/1.73 m2), only 5% had a previous diagnosis of CKD, possibly indicating that pre-initiation creatinine tests were adequately interpreted by clinicians and translated into a higher post-MRA initiation rate in these patients particularly vulnerable to the adverse effects of MRA. Again, our findings agree with and expand US observations,8–11 with some differences likely attributed to inclusion/exclusion criteria and practice patterns. Nonetheless, the results collectively suggest that adequate laboratory monitoring during the initial weeks of MRA therapy is mainly performed in individuals with known or perceived higher risk of complications14,15 and complements other studies reporting underutilization of MRAs, cardiac resynchronization therapy and implantable cardioverter-defibrillators in HF patients.16,17
Although the importance of laboratory monitoring during MRA therapy is clear from RCTs,3,4 it is currently unknown whether patients who receive appropriate laboratory monitoring achieve greater benefits than those who do not. Such hypothesis cannot be adequately tested in observational designs such as this study, given that reverse causation can pose major biases. Observational reports have suggested that the marked increase in prescriptions of MRAs after the publication of the Randomized Aldactone Evaluation Study (RALES) was accompanied by parallel increases in detected hyperkalaemias18 and that such adverse event rate declined when the rise in MRA use was followed by a rise in laboratory monitoring.19 Although association does not prove causation, a higher adherence to guideline recommended laboratory monitoring can lead to better MRA dose titration as well as early identification (and avoidance) of undesired adverse events. Efforts on education/promotion about indications, use and monitoring strategies of these drugs,20,21 collaboration with clinical pharmacists, automated alert systems, and computerized monitoring of testing,22–25 are all initiatives that have been shown to improve rates of laboratory monitoring. Yet, interventions that improve laboratory monitoring may not necessarily translate into cost-effective measures to improve clinical outcomes.26,27
This study has limitations, and its observational nature should be brought upfront. We may risk selection bias because we excluded patients who died, were hospitalized within 4 months or did not purchase a second MRA dispensation. However, the appropriateness of laboratory monitoring could not be assessed in these patients. We acknowledge that our results are specific of Stockholm health care. Yet, the similarity between previous US evidence and ours makes us speculate that the situation is not different in similar clinical settings.
We conclude that regardless of the setting of MRA initiation, rates of appropriate laboratory monitoring before MRA initiation were high in an unselected population of Swedish adults with HF. Monitoring rates after MRA initiation were, however, poor though patients who initiated therapy in the hospital were more likely to receive appropriate laboratory monitoring. Patients with CKD and with concomitant use of other Renin-angiotensin-aldosterone inhibitors (RAASi) drugs were more likely to receive appropriate laboratory monitoring. We thus report a gap between guideline recommendations and real-world management of MRA therapy, which highlights a need for education and systems of care that enhance appropriate safety monitoring of MRA use.
Funding
An institutional grant from Vifor Fresenius Medical Care Renal Pharma to Karolinska Institutet; Grant support from the Swedish Heart and Lung Foundation, Swedish Research Council, AstraZeneca, the Stockholm County Council, Martin Rind’s and Westman’s Foundations, the National Institute of Diabetes and Digestive and Kidney Diseases (K08DK092287), and the US National Kidney Foundation, which receives support from Relyps; Erasmus Scholarship programme to PdD’s for the research stay at Karolinska Institutet.
Conflict of interest: L.H.L. has received consulting honoraria from Relypsa, Vifor Pharma, and AstraZeneca. J.J.C. has lectured at events sponsored by Vifor Pharma. Bengt Lindholm is employed by Baxter Healthcare. The other authors declare no conflicts of interest.
Supplementary Material
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola V-P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P.. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Rev Esp Cardiol (Engl Ed) 2016;69:1167.. [DOI] [PubMed] [Google Scholar]
- 2. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL.. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology foundation/American Heart Association task force on practice guidelines. Circulation 2013;128:1810–1327. [DOI] [PubMed] [Google Scholar]
- 3. Zannad F, McMurray JJV, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B.. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364:11–21. [DOI] [PubMed] [Google Scholar]
- 4. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J.. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 5. McMurray JJV, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GYH, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Ž, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Lamin BAH, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Lung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Ørn S, Parissis JT, Ponikowski P. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. The task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the heart failure association (HFA) of the ESC. Eur Heart J 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 6. Shah KB, Rao K, Sawyer R, Gottlieb SS.. The adequacy of laboratory monitoring in patients treated with spironolactone for congestive heart failure. J Am Coll Cardiol 2005;46:845–849. [DOI] [PubMed] [Google Scholar]
- 7. Raebel MA, McClure DL, Chan KA, Simon SR, Feldstein AC, Lafata JE, Andrade SE, Gunter MJ, Nelson WW, Roblin D, Platt R.. Laboratory evaluation of potassium and creatinine among ambulatory patients prescribed spironolactone: are we monitoring for hyperkalemia? Ann Pharmacother 2007;41:193–200. [DOI] [PubMed] [Google Scholar]
- 8. Allen LA, Shetterly SM, Peterson PN, Gurwitz JH, Smith DH, Brand DW, Fairclough DL, Rumsfeld JS, Masoudi FA, Magid DJ.. Guideline concordance of testing for hyperkalemia and kidney dysfunction during initiation of mineralocorticoid receptor antagonist therapy in patients with heart failure. Circulation: Heart Fail 2014;7:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cooper LB, Hammill BG, Peterson ED, Pitt B, Maciejewski ML, Curtis LH, Hernandez AF.. Consistency of laboratory monitoring during initiation of mineralocorticoid receptor antagonist therapy in patients with heart failure. JAMA 2015;314:1973–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chauhan V, Dev S, Pham M, Lin S, Heidenreich P.. Facility variation and predictors of serum potassium monitoring after initiation of a mineralocorticoid receptor antagonist in patients with heart failure. Am Heart J 2015;170:543–549. [DOI] [PubMed] [Google Scholar]
- 11. Cooper LB, Hammill BG, Peterson ED, Pitt B, Maciejewski ML, Curtis LH, Hernandez AF.. Characterization of mineralocorticoid receptor antagonist therapy initiation in high-risk patients with heart failure. Circ Cardiovasc Qual Outcomes 2017;10:e002946.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Runesson B, Gasparini A, Qureshi AR, Norin O, Evans M, Barany P, Wettermark B, Elinder CG, Carrero JJ.. The Stockholm CREAtinine Measurements (scream) project: protocol overview and regional representativeness. Clin Kidney J 2016;9:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J.. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chang AR, Sang Y, Leddy J, Yahya T, Kirchner HL, Inker LA, Matsushita K, Ballew SH, Coresh J, Grams ME.. Antihypertensive medications and the prevalence of hyperkalemia in a large health system. Hypertension 2016;67:1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nilsson E, Gasparini A, Ärnlöv J, Xu H, Henriksson KM, Coresh J, Grams ME, Carrero JJ.. Incidence and determinants of hyperkalemia and hypokalemia in a large healthcare system. Int J Cardiol 2017;245:277–284. [DOI] [PubMed] [Google Scholar]
- 16. Thorvaldsen T, Benson L, Dahlström U, Edner M, Lund LH.. Use of evidence-based therapy and survival in heart failure in Sweden 2003-2012. Eur J Heart Fail 2016;18:503–511. [DOI] [PubMed] [Google Scholar]
- 17. Lund LH, Braunschweig F, Benson L, Ståhlberg M, Dahlström U, Linde C.. Association between demographic, organizational, clinical, and socio-economic characteristics and underutilization of cardiac resynchronization therapy: results from the Swedish Heart Failure Registry. Eur J Heart Fail 2017;19:1270–1279. [DOI] [PubMed] [Google Scholar]
- 18. Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA.. Rates of hyperkalemia after publication of the randomized aldactone evaluation study. N Engl J Med 2004;351:543–551. [DOI] [PubMed] [Google Scholar]
- 19. Wei L, Struthers AD, Fahey T, Watson AD, Macdonald TM.. Spironolactone use and renal toxicity: population based longitudinal analysis. BMJ 2010;340:c1768.. [DOI] [PubMed] [Google Scholar]
- 20. Ghali JK, Massie BM, Mann DL, Rich MW.. Heart failure guidelines, performance measures, and the practice of medicine: mind the gap. J Am Coll Cardiol 2010;56:2077–2080. [DOI] [PubMed] [Google Scholar]
- 21. Zannad F, Gattis Stough W, Rossignol P, Bauersachs J, McMurray JJV, Swedberg K, Struthers AD, Voors AA, Ruilope LM, Bakris GL, O'Connor CM, Gheorghiade M, Mentz RJ, Cohen-Solal A, Maggioni AP, Beygui F, Filippatos GS, Massy ZA, Pathak A, Piña IL, Sabbah HN, Sica DA, Tavazzi L, Pitt B.. Mineralocorticoid receptor antagonists for heart failure with reduced ejection fraction: integrating evidence into clinical practice. Eur Heart J 2012;33:2782–2795. [DOI] [PubMed] [Google Scholar]
- 22. Johnson SG, Canty K, Billups S, Schimmer J.. Adherence to amiodarone monitoring recommendations before and after implementation of a centralized pharmacy service: a cohort study. J Pharm Pract 2010;23:536–539. [DOI] [PubMed] [Google Scholar]
- 23. Raebel MA, Lyons EE, Chester EA, Bodily MA, Kelleher JA, Long CL, Miller C, Magid DJ.. Improving laboratory monitoring at initiation of drug therapy in ambulatory care: a randomized trial. Arch Intern Med 2005;165:2395–2401. [DOI] [PubMed] [Google Scholar]
- 24. Schiff GD, Klass D, Peterson J, Shah G, Bates DW.. Linking laboratory and pharmacy: opportunities for reducing errors and improving care. Arch Intern Med 2003;163:893–900. [DOI] [PubMed] [Google Scholar]
- 25. Snider M, Kalbfleisch S, Carnes CA.. Initial experience with antiarrhythmic medication monitoring by clinical pharmacists in an outpatient setting: a retrospective review. Clin Ther 2009;31:1209–1218. [DOI] [PubMed] [Google Scholar]
- 26. Smith DH, Raebel MA, Chan KA, Johnson ES, Petrik AF, Weiss JR, Yang X, Feldstein A.. An economic evaluation of a laboratory monitoring program for renin-angiotensin system agents. Med Decis Making 2011;31:315–324. [DOI] [PubMed] [Google Scholar]
- 27. Smith DH, Feldstein AC, Perrin NA, Yang X, Rix MM, Raebel MA, Magid DJ, Simon SR, Soumerai SB. Improving laboratory monitoring of medications: an economic analysis alongside a clinical trial. Am J Manag Care 2009;15:281–289. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.