Abstract
Background
Recent developments in magnetic resonance imaging (MRI) techniques have offered new research opportunities to visualize in vivo substantia nigra pathology in Parkinson's disease (PD). This paper summarizes the main findings of nigrosome imaging and neuromelanin sensitive MRI studies in patients with PD and other parkinsonisms.
Methods
The PubMed database was searched from 2005 to 2017 using the following keywords: Parkinson's disease and parkinsonism, in combination with MRI, nigrosome, neuromelanin, and iron. Only publications in English were included.
Results
Nigrosome or dorsal nigral hyperintensity abnormalities are studied using T2* and susceptibility weighted imaging MRI sequences in most studies, whereas Neuromelanin imaging is usually performed using T1‐weighted fast spin echo sequence. Nigrosome abnormalities have been consistently demonstrated in PD patients, and nigrosome imaging has high sensitivity and specificity in distinguishing PD from healthy controls, though it is unable to reliably separate PD from atypical parkinsonisms. Reduced neuromelanin‐related signals and/or volume loss in neuromelanin containing structures have been found in PD patients, and neuromelanin sensitive MRI imaging can also discriminate PD patients from healthy controls with high accuracy, though there is a degree of heterogeneity in the imaging findings. Preliminary findings suggested that longitudinal change of neuromelanin signal could be detected in PD, raising the possibility of using it as a marker of disease progression.
Conclusion
Nigrosome imaging and neuromelanin sensitive MRI are promising tools to study nigral pathology and to improve the diagnosis of PD. However, further studies are required to standardize analysis approaches, confirm longitudinal changes, and assess their generalizability.
Keywords: MRI, neuromelanin, nigrosome, Parkinson's disease
Introduction
Traditionally, structural imaging of the brain using Magnetic Resonance Imaging (MRI) plays little role in the diagnosis of idiopathic Parkinson's disease (PD), as there are few changes that positively support its diagnosis. MRI brain is sometimes performed to exclude other causes of parkinsonism (e.g., atypical or vascular parkinsonism).1, 2 However, characteristic abnormalities detected with conventional MRI techniques in other neurodegenerative parkinsonisms are usually seen only in the later stages of the disease, and therefore show low initial diagnostic accuracy for these conditions. Additionally, they do not appear to provide objective markers of disease progression. Functional assessments of nigrostriatal dysfunction with PET (positron emission tomography) and SPECT (single photon emission tomography) modalities (e.g., dopamine transporter [DAT] SPECT, have shown high sensitivity and specificity to differentiate PD from healthy controls, ranging from 80–100%,3, 4 but they are unable to differentiate PD from other neurodegenerative parkinsonisms).5 New sensitive and non‐invasive imaging modalities are, therefore, required to detect the early brain abnormalities that occur in PD and other neurodegenerative parkinsonisms and possibly identify in vivo biomarkers to improve the early diagnosis of these conditions in clinical practice and their progression over time.
Recent advancements in MRI technology and the introduction of high‐field 3 Tesla (3T) and ultra‐high‐field 7 Tesla (7T) MRI have led to improved spatial resolution and contrast. These advancements, in conjunction with the development of newer MRI sequences, result in greater ability to image underlying substantia nigra pathology in PD. In this review, we will focus on recent developments in MRI techniques which allow us to visualize nigrosome abnormalities and to quantify neuromelanin content in brainstem structures in patients with parkinsonisms. We will refer to the former as nigrosome imaging and the latter as neuromelanin sensitive MRI, as are commonly used in published literature.
Methods
We searched he PubMed database from 2005 to 2017 to identify articles relevant to this topic. Keywords for the search included Parkinson's disease and parkinsonism in combination with MRI, nigrosome, neuromelanin, and iron. Only publications in English were included, without bias or exclusion. The studies discussed in this review are listed in tables 1, 2, 3, which also describe the MRI sequences used.
Table 1.
Summary of Studies Using Nigrosome MRI to Study Parkinsonisms
| Study | MRI field (Tesla) | MRI sequence | Study population | Main findings |
|---|---|---|---|---|
| Blazejewska et al. (2013)13 | 7T | T2* | 10 PD, 8 HC | Abnormal SN in 10 PD and 1 HC |
| Cosottini et al. (2014)15 | 7T | SWI | 17 PD, 13 HC | Sn‡ 100%, Sp‡ 96.2% in distinguishing PD from HC |
| Kim et al. (2016)14 | 7T | T2* | 30 PD, 26 HC, 10 APD | Abnormal SN in all PD and APD except one MSA‐C |
| Schwarz et al. (2014)12 | 3T | T2*/SWI | 19 PD, 90 non‐PD | Sn 100%, Sp 95% in distinguishing PD from non‐PD |
| Reiter et al. (2015)19 | 3T | SWI | 104 PD, 42 HC, 44 APD | Correctly classified 95.2% of neurodegenerative parkinsonisms vs HC |
| Noh et al. (2015)16 | 3T | MEDIC | 24 PD, 13 HC | Sn‡ 100%, Sp‡ 84.6% |
| Sung et al. (2016)17 | 3T | MEDIC | 29 PD, 20 HC, 20 DIP | Sn‡ 88.8%, Sp‡ 83.6% |
| Bae et al. (2016)18 | 3T | SWI | 126 PD, 62 HC, 22 APD | Sn 88.8%, Sp 83.6% in distinguishing PD/APD from HC. 86.2% concordance between SWI and DAT findings |
| Oh et al. (2016)20 | 3T | 3D FLAIR | 19 PD, 13 non‐PD | Abnormal SN has Sn of 85.7% and Sn of 85.4% in predicting ipsilateral DAT scan abnormality |
| Sung et al. (2017)27 | 3T | SMWI | 89 early PD, 39 advanced PD | 61.5% of advanced PD showed bilateral loss of signal hyperintensity in N1 and N4 compared with 19.1% of early PD patients. The loss of N1 signal only was seen in 65.2% of early PD compared with 25.6% of advanced PD. |
Abbreviations: APD, atypical parkinsonisms; DAT, dopamine transporter; DIP, drug‐induced parkinsonism; FLAIR, fluid‐attenuation inversion recovery; HC, healthy controls; MEDIC, multiecho data image combination; MSA‐C, multiple system atrophy‐cerebellar; N1, nigrosome‐1; N4, nigrosome‐4; PD, Parkinson's disease; SMWI, susceptibility map‐weighted imaging; Sn, sensitivity; SN, substantia nigra; Sp, specificity; SWI, susceptibility weighted imaging; T2*, T2 star.
‡Sensitivity and specificity of abnormal nigrosome imaging in distinguishing PD from HC (unless otherwise stated).
Table 2.
Summary of Studies Using Neuromelanin‐Sensitive MRI in Parkinson's Disease
| MR sequence | Primary outcome | Automated or manual approach |
Results PD versus controls |
Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| Sasaki et al. (2006)29 | T1w FSE | Contrast ratios SNc and LC relative to pontine tegmentum | Manual | Reduced SNc and LC | NA | NA |
| Kashihara et al. (2011)38 | T1w FSE | Volume of the SNc neuromelanin‐sensitive region | Manual | Reduced SNc in HY stages 4 but not HY stages I and II | NA | NA |
| Schwarz et al. (2011)36 | T1w FSE, MT |
Size and signal intensity of SNc and LC normalized to mean values of surrounding background regions a |
Manual | Reduced SNc but not LC | NA | NA |
| Ogisu et al. (2013)47 | T1w 3D FSE | Volume of the SNc neuromelanin‐sensitive region | Semi‐automated | Reduced SNc | 83% | 85% |
| Otshuka et al. (2013)30 | T1w 3D FSE | Contrast ratios of lateral, central, and medial SNc and LC b | Manual | Reduced lateral part of the SNc and LC |
SNc: 73% LC: 82% |
87% 90% |
| Otshuka et al. (2014)45 | T1w 3D FSE | Contrast ratios of lateral, central, and medial SNc and LC b | Manual | Reduced lateral part of the SNc and LC | NA | NA |
| Matsuura et al. (2014)39 | T1wFSE | Contrast ratios lateral, central, and medial SNc and LC c | Manual | Reduced SNc and LC. Stage‐dependent decrease in SNc subregions | NA | NA |
| Reimao et al. (2015)37 | T1wFSE |
Area and length of SN high signal neuromelanin/midbrain ratio |
Semi‐automated |
Reduced area and length of SN signal and neuromelanin/midbrain ratio |
Area: 70% Length:83% |
65% 70% |
| Reimao et al. (2015)44 | T1wFSE | Area and width of the SN region with high signal | Semi‐automated | Reduced area and width of SN region | Area: 71% | 80% |
| Castellanos et al. (2015)43 | T1wFSE | SNc and LC volumes | Fully automated | Reduced SNc and LC | 91%–92% d | 89% e |
| Isaias et al (2016)34 | T1wFSE, MT |
SN volume and contrast‐to‐noise‐ratio (cerebral crus) LC contrast relative to surrounding tissue |
Manual |
Reduced SN volume and contrast‐to‐noise‐ratio correlated with corresponding striatal DAT density. Reduced LC contrast ratio |
SN volume
f
: 89% |
83% |
| Schwarz et al. (2017)31 | Three differing protocols f | Normalized neuromelanin volumes of anterior and posterior SNc, LC, and ventral tegmental area | Manual | Reduced posterior and the anterior SNc and LC |
Whole SNc: 82% Posterior SNc: 89.7% |
83% 83.3% |
Abbreviations: DAT = Dopamine transporter; LC = Locus coeruleus; MT = magnetization transfer, PD = Parkinson's disease; SN = Substantia nigra; SNc = Substantia nigra pars compacta; T1w FSE = T1‐weighted fast spin‐echo.
aCerebral peduncles and tegmentum for SN and pontine tegmentum for LC.
bRelative to superior cerebellar peduncle for SNc and pontine tegmentum for LC.
cRelative to dorsal side of the midbrain for SNc and pontine tegmentum for LC.
dContralateral atrophy in the SNc.
eSide opposite to the clinically most impaired hemibody.
fProtocol 1: T1‐weighted imaging with spectral presaturation inversion‐recovery pulse. Protocol 2: T1‐weighted sequence, replacing the spectral presaturation inversion‐recovery pulse with a standard Philips “off‐resonance” MT pulse. Protocol 3: T1‐weighted spin‐echo sequence with additional “off‐resonance” MT pulse.
Table 3.
Summary of Studies Using Neuromelanin‐Sensitive MRI in Parkinsonisms and Essential Tremor
| Cases | Primary outcome |
Results PD versus controls |
Sensitivity | Specificity | |
|---|---|---|---|---|---|
| Kashihara et al. (2011)46 |
80 PD 28 MSA 11 PSP 10 CBD 9 SCA |
Volume of the SNc neuromelanin‐sensitive region | No differences between PD and parkinsonisms | NA | NA |
| Otshuka et al. (2014)45 |
30 PD 10 MSA 13 PSP |
Contrast ratios of lateral, central, and medial SNc and LC a |
Reduced lateral/central SNc in PD and MSA. LC reduced in PD but not in parkinsonisms. No differences between MSA and PD |
PD from MSA LC: 60% PD from PSP LC: 63% Lateral SNc: 77% MSA‐P from PSPS Lateral SNc: 80% |
90% 88% 92% 85% |
| Matsuura et al. (2013)39 |
32 PD 9 MSA |
Contrast ratios lateral, central, and medial SNc and LC b |
SNc reduced in PD but not in MSA. LC reduced in MSA more than PD. |
NA | NA |
| Castellanos et al. (2015)43 |
23 PD 8 LRRK2 5 Parkin |
SNc and LC volumes |
Reduced SNc and LC but no differences between PD groups |
NA | NA |
| Reimao et al. (2015)44 |
12 PD 15 ET |
Area and width of the SN region with high signal | Reduced area and width of SN region in PD but not ET | SN Area: 66.7% | 93.3% |
Abbreviations: CBD = Cortical basal degeneration; ET = Essential Tremor; LC = Locus coeruleus; MSA = Multiple system atrophy; PD = Parkinson's disease; PSP = Progressive supranuclear palsy; SCA = Spinocerebellar ataxia; SN = Substantia nigra; SNc = Substantia nigra pars compacta.
arelative to superior cerebellar peduncle for SNc and pontine tegmentum for LC.
brelative to dorsal side of the midbrain for SNc and pontine tegmentum for LC.
Nigrosome Imaging
Substantia Nigra Anatomy
The substantia nigra (SN) is divided functionally and structurally into two different parts: pars compacta (SNc) and pars reticulata (SNr). SNr is located in the ventral part of SN and contains predominantly GABAergic neurons that project to thalamus; whereas SNc is located dorsally and contains dopaminergic neurons.6 SNc is preferentially affected in PD, particularly the caudal and ventrolateral tiers, impacting on the nigrostriatal dopaminergic pathway that accounts for many of the cardinal symptoms of PD.7
In humans, the boundaries between SNc and SNr can be difficult to distinguish anatomically; immunoreactivity with calbindin D28k can also be used to subdivide the SN into different sub‐structures.8 60% of SN dopaminergic neurons are found in calbindin‐positive/rich areas, with the rest found in five calbindin‐poor zones also known as nigrosomes. The largest nigrosome (nigrosome‐1) is found in the dorsal region of SN.6 The loss of dopaminergic neurons in the SN of PD is greatest in nigrosome‐1.9
Within the substantia nigra, iron is mostly found in SNr, though it is also found in the dopaminergic neurons of SNc as it is a cofactor of tyrosine hydroxylase, an enzyme essential for dopamine synthesis. Iron content in SNc increased by about 30% in PD compared to controls in histological studies.10 This increased iron accumulation is found in neuromelanin granules within the dopaminergic neurons, and also in the surrounding microglia and astrocytes.11
Nigrosome Imaging Studies
On 1.5T MRI, the SN appears diffusely hypointense on conventional T2‐weighted sequence due to the presence of iron, but the relatively low spatial resolution does not allow further identification of structures within the SN. The advent of ultra‐high‐field MRI, with its greater spatial resolution and signal‐to‐noise ratio, has enabled us to visualize sub‐regions of SN.
Using 7T MRI and iron sensitive sequences, it is possible to detect a hyperintense ovoid area in the dorsolateral area of SN in healthy individuals, which resembles the appearance of a “swallow tail,” a description coined by one group of authors (Fig. 1).6, 12 Histopathological study has confirmed that this dorsolateral nigral hyperintensity corresponds to nigrosome‐1,13 and its hyperintense appearance is likely due to the low iron content in this area. In PD patients, the increased iron content in nigrosome‐1 leads to the loss of this dorsolateral hyperintensity, as shown by three recent 7T MRI studies (Fig. 1).13, 14, 15 Subsequent studies using 3T MRI have also been able to replicate this finding in most PD patients (refer to Table 1 for list of studies).12, 16, 17, 18, 19, 20 This replication has generated considerable interest to use nigrosome imaging as a way of studying and diagnosing nigral pathology in PD. However, it should be noted that nigrosome is traditionally a histopathological concept as described above, and dorsolateral nigral hyperintensity might be a more appropriate term to describe the area visualized on MRI.21
Figure 1.
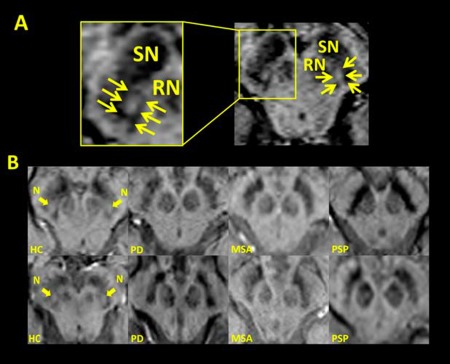
(A) Susceptibility weighted (SWI) MRI axial images of the substantia nigra (SN) of a healthy control (HC), with the yellow arrows outlining the boundary of the dorsolateral nigral hyperintensity. RN = red nucleus. (B) SWI MRI showing that the dorsolateral nigral hyperintensity seen in HC (arrows, N) is absent in patients with neurodegenerative Parkinsonisms including Parkinson's disease (PD), multiple system atrophy (MSA) and progressive supranuclear palsy (PSP). With permission, from Reiter et al.19
PD Versus Healthy Controls
Most studies have used T2* and/or susceptibility weighted imaging (SWI) as iron sensitive sequences to image dorsolateral nigral hyperintensity, with one study using 3D FLAIR (Table 1). A recent meta‐analysis showed that using 7T MRI to detect the presence or absence of dorsolateral nigral hyperintensity; there was an overall sensitivity of 97.7% (95% CI, 85.5%–99.7%) and specificity of 94.6% (95% CI, 89.8–97.2%) in distinguishing PD from healthy controls. Using 3T MRI, the sensitivity and specificity were 94.6% (95% CI, 85.8%–98.0%) and 94.4% (95% CI, 87.7%–97.5%), respectively.21 The intrarater reliability for detecting changes in the dorsolateral nigral hyperintensity was high, with the intrarater kappa ranging from 0.82 to 1.0, while the interrater kappa ranged from 0.63 to 1.0. The one study that used 3D FLAIR for nigrosome imaging had a lower interrater kappa of 0.63.20
All 7T MRI studies have demonstrated bilateral loss of dorsolateral nigral hyperintensity in PD subjects that were correctly identified, whereas studies using 3T MRI have more consistently shown only unilateral changes in PD patients, likely reflecting the differing spatial resolution/contrast between the high‐field and ultra‐high‐field imaging.21
The nigrosome abnormality also correlates with nigrostriatal dopaminergic dysfunction as detected by DAT SPECT. The absence of dorsolateral nigral hyperintensity in PD subjects predicted ipsilateral striatal DAT signal abnormality with a sensitivity of 87.5% (95% CI, 83.5%–90.7%) and specificity of 83.6% (95% CI, 77.6%–88.2%).18, 20, 21
Nigrosome imaging appears to be sensitive to early changes in PD, as patients with milder disease or short duration of motor symptoms, and prodromal subjects, also demonstrated loss of dorsolateral nigral hyperintensity.14, 15, 16, 17, 18, 21 Longitudinal follow‐up studies in patients with idiopathic rapid eye movement sleep behavior (iRBD) have shown that over a period of about ten years, the majority of these patients will develop PD or another synucleinopathy associated with dopamine deficiency such as dementia with Lewy bodies or multiple system atrophy (MSA). One study showed that out of 15 patients with iRBD, two‐thirds have loss of dorsolateral nigral hyperintensity.22 This implies that using neuroimaging techniques in iRBD patients could help identify the earliest pathological changes of these conditions.
PD Versus Atypical/Non‐Neurodegenerative Parkinsonisms
Only a few studies have looked at nigrosome changes in atypical parkinsonisms and non‐neurodegenerative parkinsonisms. The loss of dorsolateral nigral hyperintensity is seen in 89.4% of patients with atypical parkinsonisms (MSA, corticobasal degeneration [CBD], and progressive supranuclear palsy [PSP]),14, 18, 19 in similar proportion to PD population (Fig. 1). On the other hand, 21.7% of subjects with non‐neurodegenerative parkinsonism exhibited the loss of dorsolateral nigral hyperintensity.17, 18, 20, 21 However, it should be noted that the number of patients with non‐neurodegenerative parkinsonism included in these studies was small and the diagnoses heterogeneous, and they included patients with essential tremor (ET), vascular, psychogenic and drug‐induced parkinsonism (among others). In summary, nigrosome imaging may help distinguish neurodegenerative from non‐neurodegenerative parkinsonisms but is less helpful in distinguishing PD from atypical parkinsonisms. In this context, its role is broadly similar to DAT SPECT.
Virtually all studies reported the absence or presence of the dorsolateral hyperintensity qualitatively. One study measured quantitatively an increase in the volume of nigral hypointensity.23 This merely reflected the corresponding loss of the dorsolateral hyperintensity. So far, there has been limited information on longitudinal nigrosome changes. Currently, nigrosome imaging is useful in visualizing nigral pathology and aiding clinical diagnosis, but is unsuitable as a biomarker of disease progression. The development of a new MRI modality, quantitative susceptibility mapping (QSM), has led to the ability to quantify iron content in the SN accurately. Increased QSM values in the SN, indicating increased iron content, have been found in PD patients compared to controls, and the values correlated with motor disability in PD.24, 25 However, it should be noted that increased iron content in the SN does not necessarily equate nigrosome or SN dopaminergic neurodegeneration, as there have been some reported discordances between susceptibility MRI and DAT SPECT findings.26 Nevertheless, it may reflect a related pathological process and QSM provides an avenue to quantify it.
Using susceptibility map‐weighted imaging (SMWI), a novel method which combines magnitude image with QSM‐based weighting factor, a recently published study has shown that loss of signal hyperintensity in nigrosome‐4 is more commonly seen in advanced PD compared with early PD.27 61.5% of advanced PD showed bilateral loss of signal hyperintensity in putative nigrosome‐1 and nigrosome‐4, compared with only 19.1% of early PD patients. The loss of nigrosome‐1 hyperintensity only, without nigrosome‐4 involvement, was seen in 65.2% of early PD compared with 25.6% of advanced PD. Though further studies are required to replicate the findings of this new report, these suggest that this imaging technique may be able to demonstrate the progression of pathology in nigral subregions from early‐to‐advanced PD.
Limitations
While ultra‐high‐field imaging offers greater spatial resolution and contrast compared to high‐field MRI, it is far less readily available. Also, the images are more susceptible to motion artifacts, and there are more patient contraindications to scanning. Across studies, even in experienced centers, there are approximately 8% non‐diagnostic scans using 7T MRI scanners mainly due to motion artifacts.21
In experienced hands, 3T MRI appears to be able to achieve a similar level of diagnostic sensitivity and specificity in separating PD from healthy controls, and there remains a high degree of inter‐rater reliability. 3T scanners are more widely available and are less prone to motion artifacts. Further studies are required to see if the results obtained using 3T scanners in these research studies can be replicated more widely across different centers for routine clinical diagnostic use. There also needs to be comparative studies to investigate which MRI sequences (e.g., T2*, SWI) are more sensitive in detecting nigrosome changes and have the greater intra‐ and inter‐rater reliability.
Most studies thus far have investigated the sensitivity and specificity of nigrosome imaging in distinguishing PD patients from healthy controls. In clinical practice, however, this is not necessarily the most relevant for clinicians. Rather, it would be more helpful if such investigative modalities can accurately distinguish neurodegenerative from non‐neurodegenerative parkinsonisms or other movement disorders such as Essential Tremor; or PD from atypical parkinsonisms. At present, DAT SPECT is the investigative modality often used to fulfill the former role, but it is not usually helpful for the latter. However, DAT SPECT is costly and involves exposure of patients to ionizing radiation. Further studies (e.g., http://ClinicalTrials.gov Identifier NCT03022357) are required to demonstrate that nigrosome imaging can at least match, if not better, what DAT SPECT can offer to clinicians.
Neuromelanin Sensitive MRI
Neuromelanin is a brown‐to‐black colored granular pigment found naturally in specific populations of catecholaminergic neurons in the human brain. The dopaminergic neurons of the SNc and the noradrenaline‐containing neurons of the locus coeruleus (LC) contain high levels of this pigment that is responsible for the dark color of these structures in postmortem tissue samples. In patients with PD, the amount of neuromelanin in the SN is known to decrease as the disease progresses.28 Therefore, neuromelanin imaging could potentially be an important in vivo biomarker not only for the diagnosis of PD but also for the longitudinal assessment of disease progression.
When combined with metals such as copper and iron, neuromelanin shows paramagnetic properties resulting in T1‐shortening effects.29 Therefore by using MRI with a T1‐weighted fast spin‐echo sequence at 3 Tesla, where the signal of the brain tissue is suppressed due to the prolongation of its T1 relaxation time, neuromelanin‐containing nuclei can be visualized as distinct high‐intensity areas compared with surrounding brain tissue (Fig. 2).
Figure 2.
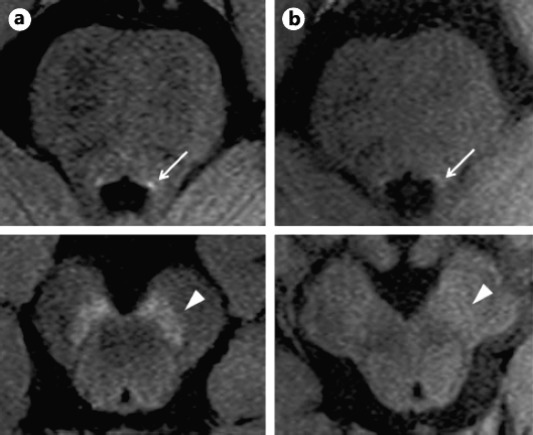
Neuromelanin sensitive MRI of the locus coeruleus and substantia nigra in a healthy control (A) and in a patient with Parkinson's disease (B). With permission, from Matsuura et al.39
PD Versus Healthy Controls
Over the last ten years, neuromelanin sensitive MRI techniques have been increasingly employed to assess patients with PD. Despite significant differences in MRI protocols, analysis approaches, and nomenclature used to describe the assessed structures, MRI studies with neuromelanin sensitive sequences have consistently demonstrated the presence of a significant reduction of neuromelanin‐related signal in the brainstem structures of PD brains, which appears to discriminate PD patients from healthy controls with high accuracy (Fig. 2, Table 2).
The earliest studies that used this technique to assess patients with PD focused on reduction (area, volume, or contrast ratio [CR] to reference brain tissue) of the neuromelanin‐positive region of the SNc, and, occasionally, the LC. More recently, with the development of the methodology and in light of the well‐known progression of the neurodegenerative process within the SNc,7 sub‐regional analyses of this structure have been reported.
In early PD patients, the lateral part of the SNc showed the most significant signal attenuation.30 The central part of the SNc was also reduced (to a lesser extent) while the medial part of the SNc was mostly preserved. In the same cohort of patients, a significant signal attenuation compared with controls was also observed in the LC. Interestingly, the change in the LC was comparable to that in the lateral part of the SNc and significantly greater than the ones observed in the central and medial parts of the SNc, suggesting early neuronal depletion in this structure in PD. Additionally, measures of signal attenuation in LC had higher sensitivity and specificity than those of the lateral part of the SNc for discriminating early PD patients from healthy controls (LC: 82% sensitivity and 90% specificity; SNc: 73% sensitivity and 87% specificity). Taken together these findings would suggest that Lewy body deposition and neuronal degeneration of the LC could precede those of the SNc in PD patients as supported by recent pathological reports.
Similarly, in another study, where the SNc was geometrically divided into anterior and posterior halves by using manually placed regions of interest, reduction of normalized neuromelanin volume was most pronounced in the posterior SNc than in the anterior SNc of PD patients with mild to moderate disease severity.31 However, at variance with the previous study, the normalized neuromelanin volume of the LC was significantly reduced in these patients but less than the anterior and posterior SNc (‐37%, ‐49%, and ‐87%, respectively). Additionally, the normalized neuromelanin volume loss of the posterior and whole SNc provided the best differentiation of PD patients and healthy controls.
The pathological correlates of neuromelanin‐sensitive MRI findings have been investigated by Kitao and colleagues in two autopsy‐proven cases of parkinsonism (one PD and one DLB) and a normal case.32 They found a significant positive correlation between signal intensity on post‐mortem imaging and the density of neuromelanin‐containing neurons within the SNc. Interestingly, the signal intensity in the SNc was apparently not influenced by iron deposition. The latter observation has recently been confirmed in an in vivo study where a neuromelanin‐sensitive MR sequence was combined with T2* relaxometry for iron quantification analysis in PD patients and age‐matched controls. No significant correlation was found between the two parameters within the SN region, suggesting that iron accumulation and neuromelanin reduction may be independent contributors to the neurodegenerative process in PD.33
Isaias and colleagues have investigated the relationship between neuromelanin‐sensitive MRI findings and striatal dopamine terminal loss as measured by 123I‐FP‐CIT SPECT.34 Compared to age‐matched controls, PD patients showed reduced volume and contrast‐to‐noise‐ratio of the SNc, and both measurements, though SNc volume more than contrast ratio, showed a positive correlation with the corresponding striatal 123I‐FP‐CIT binding. A similar significant correlation between SNc volume and striatal specific binding ratio of 123I‐FP‐CIT has been reported in a cohort of 23 patients with parkinsonism (17 PD, 3 MSA, 1 PSP, 1 spinocerebellar degeneration, and 1 ET).35 Taken together findings from these two studied suggest that loss of nigral neuromelanin is directly associated with loss/dysfunction of striatal dopamine presynaptic terminals and can be potentially used as a biomarker of nigrostriatal dysfunction.
The clinical correlates of MRI findings have also been investigated. Unfortunately, in the earlier studies, the relationship between MRI parameters and clinical measures such as the UPDRS scores was not assessed. Using regression analysis, Schwarz and colleagues observed a significant association between reduction in size of SNc hyperintensity and UPDRS scores in a small group of ten patients with PD (six patients classified as early‐stage PD and four patients as late stage).36 More recently, the same team has reported that normalized neuromelanin volume of the anterior, posterior, and whole SNc was inversely correlated with UPDRS score in a larger group of 39 PD patients with mild to moderate disease severity.31 In a cohort of 18 PD patients with Hoehn and Yahr stage 2, Isaias and colleagues found that the SN volume on the side opposite to the clinically most impaired hemibody was negatively correlated with total UPDRS‐III motor scores and sub‐scores related to rigidity and bradykinesia.34
While these studies seem to suggest a strong relationship between neuromelanin measurements and severity of motor symptoms, it should be noted that the sample size in these studies is relatively small. Therefore, larger cohorts, including patients at different disease stages are required to confirm this finding.
A stage‐dependent reduction of neuromelanin‐related MRI signal has been found in some but not all the studies where this relationship was investigated.37 Schwarz and colleagues reported that signal loss within the SNc was more pronounced in PD patients with more advanced disease (four patients with Hoehn and Yahr stage 2 and 3, UPDRS motor scores 26–60) compared to patients at an early disease stage (six patients with Hoehn and Yahr stage 1 or 1.5, UPDRS motor scores 8–25).36 Other studies, performed in larger cohorts of PD patients, have reported significant inverse correlations between Hoehn and Yahr stage and either neuromelanin‐sensitive SNc volume38 or the CR of the SNc39. However, in other studies, no significant differences were observed in the CRs of the SNc or LC between groups of PD patients with early and advanced stages30 and normalized neuromelanin volume of the whole SNc did not correlate with Hoehn and Yahr stage in patients with mild to moderate disease severity.31
A recent study has investigated longitudinal changes in the area and CR of the SNc in a small cohort of 14 patients with PD.40 After a follow‐up of 2.3 ± 1.1 years (range 1.0–4.9 years), both the total area and the CR of the SNc were significantly smaller compared to baseline imaging (from 33.5 ± 18.9 pixels and 6.35 ± 2.86% to 21.5 ± 16.7 pixels and 4.19 ± 2.11%). The total area and CR of the SNc negatively correlated with disease duration. Analysis of individual cases showed that the area of the SNc decreased consistently in all patients. Additionally, both intra‐ and inter‐rater reliability analyses showed very high intraclass correlation coefficients, suggesting that this technique could be a suitable tool to assess longitudinal changes in SNc pathology in PD patients.
Ehrminger and colleagues have recently investigated the integrity of the locus coeruleus/subcoeruleus complex in a cohort of iRBD using neuromelanin‐sensitive MRI approach.41 They found reduced signal intensity in the locus coeruleus/subcoeruleus complex of these patients compared to age‐matched healthy volunteers. The magnitude of reduction in iRBD patients was similar to the one previously observed in affected PD patients with iRBD.42 The authors claimed that decreased signal intensity in the coeruleus/subcoeruleus complexes can be detected on an individual basis and could be an early marker of non‐dopaminergic alpha‐synucleinopathy. Longitudinal studies in larger cohort of iRBD patients, however, are required to elucidate if the presence of signal loss in the coeruleus/subcoeruleus complexes can be used as imaging biomarker to predict if, and when, individual iRBD patients will manifest parkinsonism.
PD Versus Atypical/Non‐Neurodegenerative Parkinsonisms
Neuromelanin‐sensitive MRI has also shown a potential utility to differentiate idiopathic PD from ET and other types of parkinsonism, whereas no differences have been found between patients with idiopathic PD and genetic PD (Table 3).39, 43, 44, 45 Reimao and colleagues used Neuromelanin‐sensitive MRI to assess 15 patients with ET and 12 recently diagnosed, untreated patient with PD.44 They found that, compared with age‐matched healthy controls, PD patients had marked decreases in the area and width of the T1 high signal in the SN region, particularly in its ventrolateral segment but this was not the case of patients with ET. Using a cutoff value of 24.08 mm,2 the SN high signal area discriminated ET patients from the PD patients with 66.7% sensitivity and 93.3% specificity.
In a recent paper, Ohtsuka and colleagues have reported a cohort of 53 patients with early parkinsonism who after an observational period of at least 1.5 years (range 1.5–6.0 years; median 3.5 years) received a diagnosis of idiopathic PD (n = 30), MSA with predominant parkinsonism (MSA‐P; n = 10), or PSP syndrome (PSPS; n = 13).45 They found that the CR of LC was lower in the PD group compared to the control group and patients with other parkinsonism. Whereas, compared to control the CR of the lateral SNc was lower in the patients with PD and MSA‐P groups but not in patients with PSPS. CRs showed good sensitivity and specificity for discriminating PD from MSA‐P (LC: 60% sensitivity and 90% specificity), PD from PSPS (LC: 63% sensitivity and 88% specificity; Lateral SNc: 77% sensitivity and 92% specificity), and MSA‐P from PSPS (Lateral SNc: 80% sensitivity and 85% specificity). Interestingly, patients in this study also had MIBG scintigraphy and sensitivity/specificity of CR values that were comparable to the sensitivity/specificity of MIBG scintigraphy for discriminating PD from MSA‐P and PD from PSPS.
These findings are interesting and promising, but they need to replication in larger cohorts of patients. In fact, they are at odds with previously published papers. Matsuura and colleagues reported that the CRs of SNc were decreased in PD but not MSA patients.39 Conversely, the CRs of the LC were more prominently reduced in MSA patients than in PD patients and correlated with the severity of the typical neuroradiological abnormalities seen in MSA patients (‘hot cross bun’ sign). Additionally, Kashihara and colleagues have reported that the volumes of the neuromelanin‐positive SNc region in patients with MSA, PSP, and CBD were reduced to the same extent as PD patients compared to age‐matched healthy controls.46 While these discrepancies could be related to the small samples of patients and the different analysis techniques, more work is required in this field.
Limitations
Neuromelanin imaging appears to be a promising tool to improve the diagnosis of PD and to assess longitudinal changes in SNc pathology in PD patients. However, before this technique is routinely used in clinical practice, there are some issues that need to be addressed. In fact, this review of the literature shows that the results of the published studies are heterogeneous, often with significant discrepancies between studies, particularly regarding the relationship between changes in neuromelanin‐related MRI signal and disease progression. The discrepancies among reports could in part be explained by the relatively small sample size in these studies, but also by significant differences in MRI protocols, analysis approaches, and terminology used to describe the assessed structures. Therefore, at this stage, a community‐based consensus on technical standards, imaging protocols, nomenclature, and methods of analysis is an essential requirement to achieve a standardized methodological framework for neuromelanin imaging. It should also be noted that despite MRI systems being widely accessible and neuromelanin sensitive MRI sequences have been available for more than ten years, only a limited number of papers, often by the same research teams, have been published so far. Therefore, large multi‐center studies are warranted to confirm the potential of neuromelanin imaging in parkinsonisms.
Conclusions
Nigrosome and neuromelanin imaging offer an exciting opportunity to visualize nigral pathology/changes in PD and can aid diagnostic process. Their ability to identify changes in prodromal PD subjects also make them a useful research tool.
There is a degree of heterogeneity in the outcome of neuromelanin imaging as outlined above, and further studies will be helpful to improve the consistency. By comparison, nigrosome changes are more consistently demonstrated in studies performed so far, but it is a relatively new technique and further experience gained in its use, by studying larger number of subjects in different conditions and across a wider number of centers, will hopefully confirm the findings reported thus far. Additionally, at present, nigrosome imaging enables only a visual qualitative assessment of the dorsolateral nigral hyperintensity whereas neuromelanin imaging offers the opportunity of quantitative and possible longitudinal, assessment of MRI signal. With the use of more advance iron‐sensitive techniques such as QSM, multi‐susceptibility map‐weighted imaging, or adiabatic T1rho,26 quantitative nigrosome imaging of the SN may also be possible in the future.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
N.P.: 1A, 1B, 1C, 3A, 3B
Y.F.T.: 1A, 1B, 1C, 3A, 3B
Disclosures
Funding Sources and Conflict of Interest: YFT received funding from Imperial College Healthcare Trust Biomedical Research Center. The authors report no conflicts of interest.
Financial Disclosures for the previous 12 months: The authors report no financial disclosures.
Ethical Compliance Statement: The authors confirm that the approval of an institutional review board was not required for this work. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Heim B, Krismer F, De Marzi R, Seppi K. Magnetic resonance imaging for the diagnosis of Parkinson's disease. J Neural Transm (Vienna) 2017;124(8):915–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Primers 2017;3:17013. [DOI] [PubMed] [Google Scholar]
- 3. Suwijn SR, van Boheemen CJ, de Haan RJ, Tissingh G, Booij J, de Bie RM. The diagnostic accuracy of dopamine transporter SPECT imaging to detect nigrostriatal cell loss in patients with Parkinson's disease or clinically uncertain parkinsonism: a systematic review. EJNMMI Res 2015;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bajaj N, Hauser RA, Grachev ID. Clinical utility of dopamine transporter single photon emission CT (DaT‐SPECT) with (123I) ioflupane in diagnosis of parkinsonian syndromes. J Neurol Neurosurg Psychiatry 2013;84(11):1288–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brooks DJ, Pavese N. Imaging biomarkers in Parkinson's disease. Prog Neurobiol 2011;95(4):614–628. [DOI] [PubMed] [Google Scholar]
- 6. Lehericy S, Bardinet E, Poupon C, Vidailhet M, Francois C. 7 Tesla magnetic resonance imaging: a closer look at substantia nigra anatomy in Parkinson's disease. Mov Disord 2014;29(13):1574–1581. [DOI] [PubMed] [Google Scholar]
- 7. Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain 1991;114(Pt 5):2283–2301. [DOI] [PubMed] [Google Scholar]
- 8. Gibb WR. Melanin, tyrosine hydroxylase, calbindin and substance P in the human midbrain and substantia nigra in relation to nigrostriatal projections and differential neuronal susceptibility in Parkinson's disease. Brain Res 1992;581(2):283–291. [DOI] [PubMed] [Google Scholar]
- 9. Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine‐containing neurons in Parkinson's disease. Brain 1999;122 (Pt 8):1437–1448. [DOI] [PubMed] [Google Scholar]
- 10. Dexter DT, Wells FR, Lees AJ, et al. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson's disease. J Neurochem 1989;52(6):1830–1836. [DOI] [PubMed] [Google Scholar]
- 11. Hirsch EC, Brandel JP, Galle P, Javoy‐Agid F, Agid Y. Iron and aluminum increase in the substantia nigra of patients with Parkinson's disease: an X‐ray microanalysis. J Neurochem 1991;56(2):446–451. [DOI] [PubMed] [Google Scholar]
- 12. Schwarz ST, Afzal M, Morgan PS, Bajaj N, Gowland PA, Auer DP. The ‘swallow tail’ appearance of the healthy nigrosome ‐ a new accurate test of Parkinson's disease: a case‐control and retrospective cross‐sectional MRI study at 3T. PLoS One 2014;9(4):e93814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blazejewska AI, Schwarz ST, Pitiot A, et al. Visualization of nigrosome 1 and its loss in PD: pathoanatomical correlation and in vivo 7 T MRI. Neurology 2013;81(6):534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim JM, Jeong HJ, Bae YJ, et al. Loss of substantia nigra hyperintensity on 7 Tesla MRI of Parkinson's disease, multiple system atrophy, and progressive supranuclear palsy. Parkinsonism Relat Disord 2016;26:47–54. [DOI] [PubMed] [Google Scholar]
- 15. Cosottini M, Frosini D, Pesaresi I, et al. MR imaging of the substantia nigra at 7 T enables diagnosis of Parkinson disease. Radiology 2014;271(3):831–838. [DOI] [PubMed] [Google Scholar]
- 16. Noh Y, Sung YH, Lee J, Kim EY. Nigrosome 1 detection at 3T MRI for the diagnosis of early‐stage idiopathic Parkinson disease: assessment of diagnostic accuracy and agreement on imaging asymmetry and clinical laterality. AJNR Am J Neuroradiol 2015;36(11):2010–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sung YH, Noh Y, Lee J, Kim EY. Drug‐induced Parkinsonism versus Idiopathic Parkinson Disease: Utility of Nigrosome 1 with 3‐T Imaging. Radiology 2016;279(3):849–858. [DOI] [PubMed] [Google Scholar]
- 18. Bae YJ, Kim JM, Kim E, et al. Loss of Nigral Hyperintensity on 3 Tesla MRI of Parkinsonism: Comparison With (123) I‐FP‐CIT SPECT. Mov Disord 2016;31(5):684–692. [DOI] [PubMed] [Google Scholar]
- 19. Reiter E, Mueller C, Pinter B, et al. Dorsolateral nigral hyperintensity on 3.0T susceptibility‐weighted imaging in neurodegenerative Parkinsonism. Mov Disord 2015;30(8):1068–1076. [DOI] [PubMed] [Google Scholar]
- 20. Oh SW, Shin NY, Lee JJ, et al. Correlation of 3D FLAIR and Dopamine Transporter Imaging in Patients With Parkinsonism. AJR Am J Roentgenol 2016;207(5):1089–1094. [DOI] [PubMed] [Google Scholar]
- 21. Mahlknecht P, Krismer F, Poewe W, Seppi K. Meta‐analysis of dorsolateral nigral hyperintensity on magnetic resonance imaging as a marker for Parkinson's disease. Mov Disord 2017;32(4):619–623. [DOI] [PubMed] [Google Scholar]
- 22. De Marzi R, Seppi K, Hogl B, et al. Loss of dorsolateral nigral hyperintensity on 3.0 tesla susceptibility‐weighted imaging in idiopathic rapid eye movement sleep behavior disorder. Ann Neurol 2016;79(6):1026–1030. [DOI] [PubMed] [Google Scholar]
- 23. Kwon DH, Kim JM, Oh SH, et al. Seven‐Tesla magnetic resonance images of the substantia nigra in Parkinson disease. Ann Neurol 2012;71(2):267–277. [DOI] [PubMed] [Google Scholar]
- 24. Barbosa JH, Santos AC, Tumas V, et al. Quantifying brain iron deposition in patients with Parkinson's disease using quantitative susceptibility mapping, R2 and R2. Magn Reson Imaging 2015;33(5):559–565. [DOI] [PubMed] [Google Scholar]
- 25. Du G, Liu T, Lewis MM, et al. Quantitative susceptibility mapping of the midbrain in Parkinson's disease. Mov Disord 2016;31(3):317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frosini D, Cosottini M, Volterrani D, Ceravolo R. Neuroimaging in Parkinson's disease: focus on substantia nigra and nigro‐striatal projection. Curr Opin Neurol 2017. [DOI] [PubMed] [Google Scholar]
- 27. Sung YH, Lee J, Nam Y, et al. Differential involvement of nigral subregions in idiopathic parkinson's disease. Hum Brain Mapp 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zecca L, Tampellini D, Gerlach M, Riederer P, Fariello RG, Sulzer D. Substantia nigra neuromelanin: structure, synthesis, and molecular behavior. Mol Pathol 2001;54(6):414–418. [PMC free article] [PubMed] [Google Scholar]
- 29. Sasaki M, Shibata E, Tohyama K, et al. Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson's disease. Neuroreport 2006;17(11):1215–1218. [DOI] [PubMed] [Google Scholar]
- 30. Ohtsuka C, Sasaki M, Konno K, et al. Changes in substantia nigra and locus coeruleus in patients with early‐stage Parkinson's disease using neuromelanin‐sensitive MR imaging. Neurosci Lett 2013;541:93–98. [DOI] [PubMed] [Google Scholar]
- 31. Schwarz ST, Xing Y, Tomar P, Bajaj N, Auer DP. In Vivo Assessment of Brainstem Depigmentation in Parkinson Disease: Potential as a Severity Marker for Multicenter Studies. Radiology 2017;283(3):789–798. [DOI] [PubMed] [Google Scholar]
- 32. Kitao S, Matsusue E, Fujii S, et al. Correlation between pathology and neuromelanin MR imaging in Parkinson's disease and dementia with Lewy bodies. Neuroradiology 2013;55(8):947–953. [DOI] [PubMed] [Google Scholar]
- 33. Reimao S, Ferreira S, Nunes RG, et al. Magnetic resonance correlation of iron content with neuromelanin in the substantia nigra of early‐stage Parkinson's disease. Eur J Neurol 2016;23(2):368–374. [DOI] [PubMed] [Google Scholar]
- 34. Isaias IU, Trujillo P, Summers P, et al. Neuromelanin Imaging and Dopaminergic Loss in Parkinson's Disease. Front Aging Neurosci 2016;8:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kuya K, Shinohara Y, Miyoshi F, Fujii S, Tanabe Y, Ogawa T. Correlation between neuromelanin‐sensitive MR imaging and (123)I‐FP‐CIT SPECT in patients with parkinsonism. Neuroradiology 2016;58(4):351–356. [DOI] [PubMed] [Google Scholar]
- 36. Schwarz ST, Rittman T, Gontu V, Morgan PS, Bajaj N, Auer DP. T1‐weighted MRI shows stage‐dependent substantia nigra signal loss in Parkinson's disease. Mov Disord 2011;26(9):1633–1638. [DOI] [PubMed] [Google Scholar]
- 37. Reimao S, Pita Lobo P, Neutel D, et al. Substantia nigra neuromelanin magnetic resonance imaging in de novo Parkinson's disease patients. Eur J Neurol 2015;22(3):540–546. [DOI] [PubMed] [Google Scholar]
- 38. Kashihara K, Shinya T, Higaki F. Neuromelanin magnetic resonance imaging of nigral volume loss in patients with Parkinson's disease. J Clin Neurosci 2011;18(8):1093–1096. [DOI] [PubMed] [Google Scholar]
- 39. Matsuura K, Maeda M, Yata K, et al. Neuromelanin magnetic resonance imaging in Parkinson's disease and multiple system atrophy. Eur Neurol 2013;70(1–2):70–77. [DOI] [PubMed] [Google Scholar]
- 40. Matsuura K, Maeda M, Tabei KI, et al. A longitudinal study of neuromelanin‐sensitive magnetic resonance imaging in Parkinson's disease. Neurosci Lett 2016;633:112–117. [DOI] [PubMed] [Google Scholar]
- 41. Ehrminger M, Latimier A, Pyatigorskaya N, et al. The coeruleus/subcoeruleus complex in idiopathic rapid eye movement sleep behavior disorder. Brain 2016;139(Pt 4):1180–1188. [DOI] [PubMed] [Google Scholar]
- 42. Garcia‐Lorenzo D, Longo‐Dos Santos C, Ewenczyk C, et al. The coeruleus/subcoeruleus complex in rapid eye movement sleep behavior disorders in Parkinson's disease. Brain 2013;136(Pt 7):2120–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Castellanos G, Fernandez‐Seara MA, Lorenzo‐Betancor O, et al. Automated neuromelanin imaging as a diagnostic biomarker for Parkinson's disease. Mov Disord 2015;30(7):945–952. [DOI] [PubMed] [Google Scholar]
- 44. Reimao S, Pita Lobo P, Neutel D, et al. Substantia nigra neuromelanin‐MR imaging differentiates essential tremor from Parkinson's disease. Mov Disord 2015;30(7):953–959. [DOI] [PubMed] [Google Scholar]
- 45. Ohtsuka C, Sasaki M, Konno K, et al. Differentiation of early‐stage parkinsonisms using neuromelanin‐sensitive magnetic resonance imaging. Parkinsonism Relat Disord 2014;20(7):755–760. [DOI] [PubMed] [Google Scholar]
- 46. Kashihara K, Shinya T, Higaki F. Reduction of neuromelanin‐positive nigral volume in patients with MSA, PSP and CBD. Intern Med 2011;50(16):1683–1687. [DOI] [PubMed] [Google Scholar]
- 47. Ogisu K, Kudo K, Sasaki M, et al. 3D neuromelanin‐sensitive magnetic resonance imaging with semi‐automated volume measurement of the substantia nigra pars compacta for diagnosis of Parkinson's disease. Neuroradiology 2013;55(6):719–724. [DOI] [PubMed] [Google Scholar]


