Abstract
Background
Chorea may occur as a manifestation of an acute stroke. Patients with vascular‐related chorea typically present with an acute or subacute onset of hemichorea, contralateral to the lesion.
Methods and Findings
In this clinico‐pathological case, we report a 90‐year‐old female who presented, at age 81, with a transient episode of generalized chorea. Over the years, the patient continued to have intermittent episodes of generalized chorea or hemichorea, followed by a progressive dementia syndrome with gait and sphincter disturbance. There was no family history of chorea or dementia. Laboratory tests for paraneoplastic or autoimmune disorders and genetic testing for Huntington's disease were normal or negative. Magnetic resonance imaging showed subcortical and basal ganglia atrophy associated with ischemic leukoencephalopathy and lacunar infarcts. The post‐mortem examination identified multiple lacunar infarcts (cortex, white matter, thalamus, basal ganglia) and minor Alzheimer′s disease neuropathological changes.
Conclusions
Vascular disease, affecting the basal ganglia, is included in most lists of causes of generalized chorea. Proven cases are difficult to find. We present a rare case of vascular pathology causing late onset generalized and intermittent chorea. We highlight the intermittent nature of the chorea that could be explained by cumulative vascular lesions or functional disconnection in a previous deficient circuit.
Keywords: vascular chorea, generalized chorea, intermittent chorea, late‐onset chorea, cerebrovascular disease
Chorea is a hyperkinetic movement disorder characterized by involuntary brief, sudden, and irregular movements.1, 2 It can be hereditary or acquired, generalized or localized, and can have several aetiologies, such as acute stroke. The prevalence of hyperkinetic movement disorders after stroke is unknown. Some studies report hemichorea as the most frequent post‐stroke movement disorder.3, 4 Classically, it is caused by lesions of the subthalamic nuclei, however, it can also be associated with lesions in other basal ganglia nuclei.3 The precise mechanism underlying movement disorders associated with stroke in the basal ganglia and circuitry is not fully understood.5
Patients with vascular‐related chorea typically present with an acute or subacute onset of hemichorea contralateral to the lesion and only rarely as a progressive condition.1, 2, 3, 4, 5, 6 Some studies show that chorea could have spontaneous regression in about half of the patients, but others have persistent symptoms1, 2 and need to be treated.
Small vessel disease with small deep infarcts is the most common sub‐type of stroke leading to abnormal movements,1, 4, 5 and cerebrovascular disease is the most common cause of sporadic chorea.2 Generalized chorea in adults or elderly is usually attributed to degenerative causes.7, 8 Only rarely has acute‐onset generalized chorea been recognized as the result of cerebrovascular disease6, 8 mainly because it is difficult to prove. Herein, we describe a confirmed case of vascular pathology causing late onset, generalized, and intermittent chorea.
Case Report
A 90‐year‐old female was admitted to the emergency department due to a sudden and transient episode of generalized chorea at the age of 81. At that time, the family reported some memory and behavioral problems. Beside the generalized chorea, neurological examination revealed a frontal syndrome, with disinhibition and aggressiveness, and peri‐oral dyskinesia. She was treated with olanzapine and the chorea gradually improved over several days, until complete remission.
During the next three years, the cognitive deficits progressed and the patient was started on donepezil (up to 10 mg/day). She presented a multidomain cognitive impairment affecting memory, calculus, and spatial domains. The Mini‐Mental State Examination (MMSE) was 17/30. She had a shuffling gait with short steps and a slight wide‐base that progressed to an apraxic gait. At the same time, incontinence appeared and the patient became wheelchair‐bound and progressively more dependent.
Between the age of 84 and 89, intermittent and recurrent episodes of disabling chorea reappeared (either generalized or hemichorea), mostly without any identified precipitant factor, but requiring symptomatic treatment. During these periods, she was treated with different drugs, including olanzapine (up to 5 mg/day), quetiapine (up to 400 mg/day), haloperidol (up to 3 mg/day), and risperidone.
The patient had no other vascular risk‐factors besides age. She had no family history of neurological degenerative diseases, including dementia, movement disorders, or psychiatric disease.
An MRI scan at the age of 81 showed a dilated lateral ventricular system (Fig. 1A–D) due to subcortical and caudate atrophy (Fig. 1B). White‐matter changes predominated in periventricular and deep white matter (Fig. 1B–D), and there were lacunar infarcts in the right caudate (Fig. 1A) and cerebellum.
Figure 1.
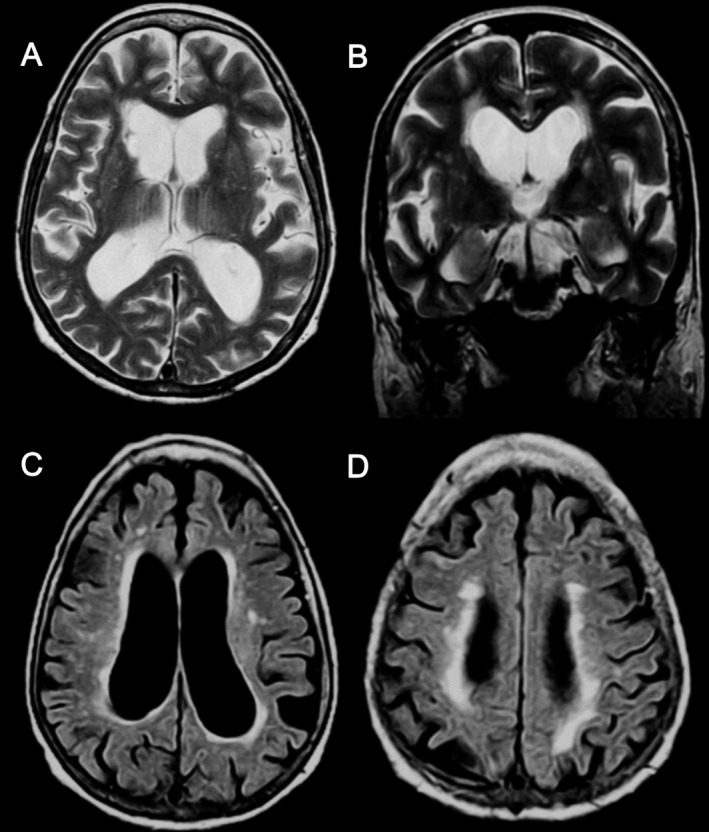
Patient brain MRI showing caudate lacunar infarction (A), ventricular enlargement (A–D), and caudate atrophy (B) and white matter changes with periventricular and deep white matter predominance (B–D).Axial T2‐weighted (A), coronal T2‐weighted (B), and axial T2‐weighted/fluid‐attenuated inversion recovery (C and D) sequences.
Blood tests revealed normal blood count and biochemistry (including renal, hepatic, and thyroid function). Iron, folic acid, and vitamin B12 levels were low, probably due to nutritional deficits. Immunologic tests, including antinuclear, antithyroid (antithyroglobulin, thyroid peroxidase antibodies and thyrotropin receptor antibodies), intrinsic factor, and antineuronal antibodies were normal. Tumour markers and genetic testing for Huntington's disease were also negative.
Iron and vitamin deficiency were corrected with supplementation, without clinical improvement.
With a ten‐year history of multiple intermittent episodes of chorea, and with a severe dementia, she died at the age of 90 from a respiratory infection.
Postmortem neuropathological examination revealed a 920 g brain, marked dilatation of the lateral ventricular system, with prominent subcortical white matter atrophy that particularly involved the frontal region. The head of the right caudate nucleus was flattened. Soft and discoloured lesions were noted in this location and in the thalamus bilaterally. There were no other basal ganglia abnormalities, including in the subthalamic nucleus (Fig. 2A). The hippocampus was mildly atrophic bilaterally. Horizontal sections of the brainstem showed normal pigmented substantia nigra and locus coeruleus. The circle of Willis vessels and vertebrobasilar arteries showed moderate atheroma plaques. Histological examination revealed severe atherosclerosis and small vessel disease (SVD) with old cortical microinfarcts and multiple lacunar infarcts in the white matter, basal ganglia (caudate and putamen bilaterally, nucleus accumbens on the right), and thalamus bilaterally (Fig. 2B–F). There were no vascular lesions in the subthalamic nucleus. There was subcortical white matter pallor, particularly around blood vessels. There were minor Alzheimer′s disease neuropathological changes (Braak stage II) and minor beta‐amyloid deposition in some leptomeningeal vessels, particularly in the occipital and cerebellar regions (Fig. 2J–L). Alpha‐synuclein and TDP43 immunohistochemical preparations were entirely negative.
Figure 2.
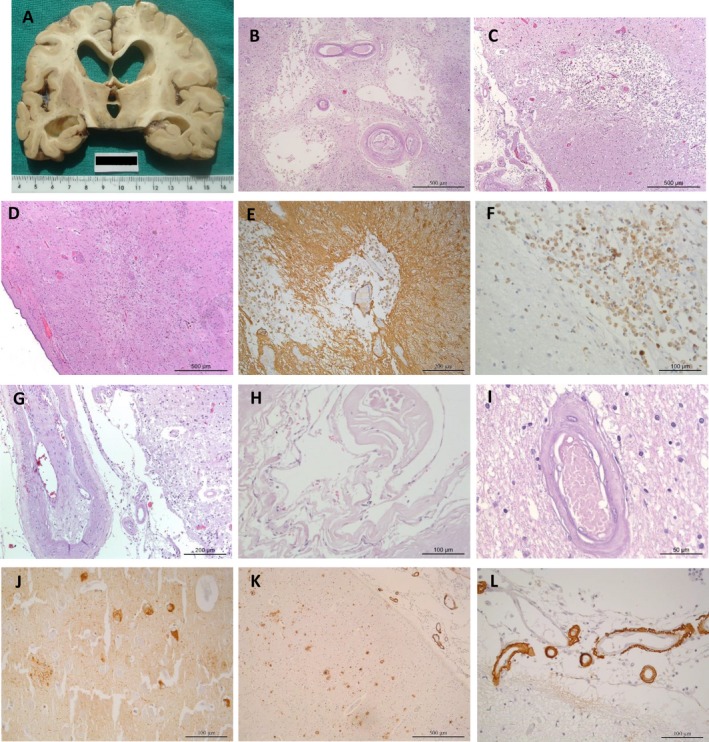
Brain macroscopic (A) and histologic findings (C–I) Coronal slice showing enlarged ventricles (particularly the frontal horns) cortical, and subcortical white matter atrophy. The right caudate is flattened and there are discoloured lesions in both thalamus. The subthalamic nucleus is normal. Histology showing infarct lesions in caudate, namely an old infarct with cystic change (B), and a more recent with glial scarring and macrophagic infiltration (D–F). In C, there is a small ischemic cortical lesion in temporal cortex. Atherosclerotic pathology in leptomeningeal vessels with endothelial proliferation, splitting of the internal elastic lamina and re‐canalized lumen (G). Vessel with cholesterol clefts and calcifications (H). Cortical occipital vessel with hyalinosis changes (I). Tau pathology (neurofibrillary tangles and neurites plaques) in CA1 region of hippocampus (J) and amyloid deposits (mature plaques and more frequent diffuse deposits) in temporal cortex (K). Higher magnification of leptomeningeal vessels showing beta‐amyloid deposition (L).Hematoxylin and eosin stain (B–D, G–I); Immunohistochemistry study with GFAP (E), CD68 (F), Tau (J) and Beta‐amyloid (K and L).Scale bars: 500 μm (B–D, K); 200 μm (E, G); 100 μm (F, H, J, L); 50 μm (I).
Discussion
We present a 90‐year‐old woman, without vascular risk‐factors, who developed a late onset generalized intermittent chorea, with progressive dementia. In this patient, although the acute onset of the chorea could suggest a vascular aetiology, the fact that it was generalized and the absence of known vascular risk‐factors lead us to exclude other entities, including degenerative and hereditary disorders.
The investigation ruled out other aetiologies, namely Huntington's disease, autoimmune, metabolic, toxic, nutritional, and infectious diseases. The imaging studies were suggestive of small vessel disease with white matter lesions, lacunar infarcts in the basal ganglia, and subcortical atrophy. The consequent ventriculomegaly could be interpreted as disproportionately enlarged subarachnoid‐space hydrocephalus (DESH),9, 10 feature of normal pressure hydrocephalus (NPH), or of a neurodegenerative disease. The presence of intermittent chorea, the distribution of white matter disease, and the absence of other typical imaging signs leave us to not consider NPH, at least as the only diagnosis.
Hemichorea has been frequently associated with vascular lesions of the basal ganglia, but generalized chorea has been rarely reported.2, 6, 7, 8 In these cases, lesions usually result from small vessel disease affecting the neostriatum with multiple bilateral areas of infarction,2 similar to the findings of the post‐mortem study in our case.
Although lacunar infarcts affecting basal ganglia and motor pathways circuits are common, clinically evident movement disorders attributed to them are difficult to prove. In fact, hyperkinetic movement disorders are relatively rare even when the basal ganglia are markedly damaged. This phenomenon could be explained by individual susceptibility and brain plasticity.5, 11 Additionally, it is thought that in some cases the decrease in cerebral blood flow to the basal ganglia can compromise the integrity of the blood‐brain barrier making the structures more vulnerable to the effects of circulating toxins or metabolic factors.12 Consequently, in some patients with previous lesions in the basal ganglia, episodic chorea became notable due to an infectious or metabolic complication. The patient had a urinary tract infection in one episode of generalized chorea. However, there were recurrent and self‐limited episodes of chorea in the absence of other precipitating factors. Each episode of chorea could be related to a new vascular lesion, an episodic hypoperfusion, or a functional disconnection3 in a brain with vascular disease. A brain MRI performed at the beginning of one of the intermittent episodes of chorea would have helped clarify whether the aetiology was related with a new ischemic lesion. The severity and topography of amyloid angiopathy in the post mortem study make the contribution of this pathology to the clinical symptoms less plausible.
Another hypothesis raised by recent evidence suggests that most movement disorders induced by basal ganglia lesions result from defects in functional connectivity rather than from a single lesion.5, 11 These defects might be a consequence of changes in synaptic activity, dendritic plasticity or brain metabolism in response to previous ischemic insults, factors that vary with age, or are most likely the cumulative effect of multiple of these variables.5, 11 Finally, co‐occurrence of neurodegenerative pathologies and nondegenerative pathologies is common, particularly at old age, and may explain some complex clinical presentations.13 This concept should be taken into account in our case and multiple pathologies found could contribute to the clinical picture.
Folstein et al.14 described a patient who developed progressive generalized chorea and gradual cognitive decline some years after the onset of chorea. The patient was clinically diagnosed with Huntington's disease, however, the postmortem study showed lesions consistent with vascular pathology instead of the histopathologic changes of Huntington's disease. Bhatia et al.6 also described one case of progressive generalized chorea with vascular aetiology, where the cognitive complains were the first symptoms. Although these two cases are similar to our case, none of them reported an intermittent pattern of chorea or an alternating hemichorea/generalized chorea as we saw in our patient. To our knowledge no other case with this intermittent pattern was previously reported in the literature.
In conclusion, we present a rare case of vascular pathology causing late onset generalized and intermittent chorea. We highlight the intermittent nature of the chorea that could be explained by cumulative vascular lesions or functional disconnection in a previous deficient circuit.
Author Roles:
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique
P.S.: 1B, 1C, 3A, 3B
R.T.: 1C, 3B
J.D.: 3B
D.D.: 1C, 3B
M.M.P: 1C, 3B
M.M: 1A, 1B, 1C, 3B
Disclosures
Funding Sources and Conflict of Interest: No specific funding was received for this work. The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the previous 12 months: The authors declare that there are no additional disclosures to report.
Ethical Compliance Statement: The authors confirm that the approval of an institutional review board was not required for this work. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Handley A, Medcalf P, Hellier K, Dutta D. Movement disorders after stroke. Age Ageing 2009;38(3):260–266. [DOI] [PubMed] [Google Scholar]
- 2. Zijlmans J. Vascular chorea in adults and children. Handb Clin Neurol 2011;100:261–270. [DOI] [PubMed] [Google Scholar]
- 3. Chung S, Im JH, Lee M, Kim J. Hemichorea after stroke: clinical‐radiological correlation. J Neurol 2004;251:725–729. [DOI] [PubMed] [Google Scholar]
- 4. Kwon DY. Management of post‐stroke movement disorders. Mov Disord 2016;9(2):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park J. Movement disorders following cerebrovascular lesion in the basal ganglia circuit. Mov Disord 2016;9(2):71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhatia K, Lera G, Luthert P, Marsden C. Vascular chorea: case report with pathology. Mov Disord 1994;4:447–450. [DOI] [PubMed] [Google Scholar]
- 7. Tabaton M, Mancardi G, Loeb C. Generalized chorea due to bilateral small deep cerebral infarcts. Neurology 1985;35:588–589. [DOI] [PubMed] [Google Scholar]
- 8. Sethi K, Nichols F, Yaghmai F. Generalized chorea due to basal ganglia lacunar infarcts. Mov Disord 1987;2(1):61–66. [DOI] [PubMed] [Google Scholar]
- 9. Ishii K, Kawaguchi T, Shimada K, et al. Voxel‐based analysis of gray matter and CSF space in idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Disord 2008;25:329–335. [DOI] [PubMed] [Google Scholar]
- 10. Kitagaki H, Mori E, Ishii K, Yamaji S, Hirono N, Imamura T. CSF spaces in idiopathic normal pressure hydrocephalus: morphology and volumetry. Am J Neuroradiol 1998;19:1277–1284. [PMC free article] [PubMed] [Google Scholar]
- 11. Mehanna R, Jankovic J. Movement disorders in cerebrovascular disease. Lancet Neurol 2013;12:597–608. [DOI] [PubMed] [Google Scholar]
- 12. Piccolo I, Defanti C, Soliveri P, Volontè M, Cislaghi G, Girotti F. Cause and course in a series of patients with sporadic chorea. J Neurol 2003;250:429–435. [DOI] [PubMed] [Google Scholar]
- 13. Rahimi J, Kovacs GG. Prevalence of mixed pathologies in the aging brain. Alzheimers Res Ther 2014;6:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Folstein S, Abbott M, Moser R, Parhad I, Clark A, Folstein M. A phenocopy of Huntington's disease: lacunar infarcts of the corpus striatum. Johns Hopkins Med J 1981;148:104–113. [PubMed] [Google Scholar]


