Abstract
Background
The diagnosis of Parkinson's disease (PD) can be challenging early in the disease course, when motor features are subtle. The objective of this study was to explore the diagnostic value of combining acute levodopa challenge and olfactory testing to predict PD.
Methods
Data from 210 patients with a recent onset of parkinsonism who had at least 2 years of follow‐up and underwent acute levodopa challenge for the clinical prediction of long‐term dopaminergic response and had olfactory testing with Sniffin’ Sticks Test were evaluated. Single and combined diagnostic measures were analyzed.
Results
After 2 years of follow‐up, a PD diagnosis was confirmed in 157 patients who fulfilled United Kingdom Parkinson's Disease Society Brain Bank criteria and was ruled out in 53. Sensitivity and specificity of acute levodopa challenge to predict PD diagnosis were 0.71 and 0.94, respectively. Sensitivity and specificity of olfactory tests were calculated according to the total olfactory score for hyposmia (0.61 and 0.77 respectively), the hyposmia identification subscore (0.63 and 0.74, respectively), and the anosmia score (0.40 and 0.85, respectively). The best combination identified was response to acute levodopa challenge together with hyposmia according to the total olfactory score (sensitivity, 0.90; specificity, 0.74; positive predictive value, 0.91; negative predictive value, 0.72; accuracy, 0.86).
Conclusion
The combination of response to acute levodopa challenge with hyposmia according to the total olfactory score improved sensitivity for the early diagnosis of PD.
Keywords: acute levodopa challenge, anosmia, hyposmia, olfaction, Parkinson's disease
The diagnosis of Parkinson's disease (PD) can be challenging early in the course of disease, when motor features are subtle. Unfortunately, clinical diagnostic accuracy has not improved in recent years for patients at this stage.1 However, to increase diagnostic reliability, the Movement Disorders Society has recently proposed clinical criteria for the diagnosis of PD that include olfactory dysfunction as well as a clear and sustained beneficial response to dopaminergic therapy as supportive criteria.2 Preserved or slightly impaired olfaction is also identified in patients with essential tremor and in those with vascular, drug‐induced, or atypical parkinsonisms, such as multiple system atrophy or corticobasal syndrome.3, 4 Acute levodopa (l‐dopa) challenge can be used to predict sustained, long‐term dopaminergic response and support a possible PD diagnosis.5 Although several screening batteries or 2‐step approaches combining olfactory testing with dopaminergic functional imaging, transcranial sonography, and myocardial scintigraphy, among others, have been proposed,6, 7, 8, 9, 10, 11, 12, 13 the need persists for inexpensive, reliable, bedside test combinations. The objective of this study was to compare the screening effectiveness of acute l‐dopa challenge combined with olfactory testing for the early diagnosis of PD versus the individual use of either test.
Patients and Methods
Study Sample
We conducted a retrospective review of medical records in our institutional electronic database to identify patients who had a recent onset of tremor or parkinsonism and underwent acute l‐dopa challenge from January 1, 2011, through May 31, 2015, for the clinical prediction of sustained, long‐term dopaminergic response and who also underwent olfactory testing. The population sample comprised various socioeconomic groups from the city of Buenos Aires and the rest of Argentina. Clinical assessment with acute l‐dopa challenge and olfactory testing within the first year is a typical practice in our Movement Disorders Clinic and is performed for all of our patients who have a recent onset of tremor or parkinsonism within 1 month of their first consultation. Patients who had at least 2 years of follow‐up were included. United Kingdom Parkinson's Disease Society Brain Bank criteria14 were applied for PD diagnosis; and the established diagnostic criteria for other parkinsonisms, like multiple system atrophy,15 corticobasal syndrome,16 progressive supranuclear palsy,17 dementia with Lewy bodies,18 drug‐induced parkinsonism,19 and dystonic or essential tremor,20 were applied by the corresponding treating movement disorders specialist (M.R., A.C., or M.M.). Dopamine transporter imaging or ultrasound were not routinely performed, because these modalities do not properly differentiate between various degenerative parkinsonisms, such as PD from multiple system atrophy.21 Patients who had a history of chronic sinusitis or current rhinorrhea and significant exposure to volatile substances were excluded, as were individuals who had suffered head trauma with loss of consciousness, had a history of drug abuse, had undergone nasal surgery, or were current smokers. The study protocol conformed to the principles of the Declaration of Helsinki and was approved by the local Institutional Review Board.
Evaluation
Olfactory function was evaluated in a quiet, well‐ventilated room using the extended version of the Sniffin’ Sticks Test (SST) (Burghart Messtechnik, Wedel, Germany), which consists of 3 subsets of felt‐tip whiteboard markers that assess different olfactory modalities (threshold, discrimination, and identification). Sixteen odors were used for the identification part. The SST establishes partial and total scores, generating an overall assessment of olfactory function. Subset scores range from zero to 16, with a maximum total score of 48. Hyposmia diagnosis was defined for results at or below the 10th percentile of the total age/sex‐stratified SST score, whereas anosmia was determined when SST scores were ≤16.5 (these are standardized results provided by the manufacturer). Three variables were computed: hyposmia according to total SST score (HT), hyposmia applying the identification subscore (HI), and anosmia according to the total SST score (AT). The SST took on average 30 to 45 minutes to be completed and was administered by trained physicians (C.T.C., A.B., and P.M.V.).
Acute l‐dopa challenge was conducted at least 72 hours after treatment with domperidone (60 mg daily), which was given to prevent adverse events related to l‐dopa–carbidopa administration. In the fasting state, patients were challenged with l‐dopa/carbidopa (single‐dose 250 mg/25 mg), at which time motor status was evaluated by applying the Movement Disorders Society‐Unified Parkinson's Disease Rating Scale‐part III (MDS‐UPDRS‐III). Clinical scores were evaluated at baseline, at 15‐minute intervals, and at any time during the assessment that the examiner, a movement disorders specialist, considered that significant modification in motor status had occurred. Monitoring continued either until the patient returned to baseline status or 4 hours had elapsed. For patients in whom an improvement of at least 25% in the MDS‐UPDRS‐III score was reached, acute l‐dopa challenge was rated as positive.22
Statistical Analysis
Descriptive data are presented as means ± standard deviations or proportions. Combination outcomes from acute l‐dopa challenge and SST olfactory testing were computed as negative when both tests yielded negative results. When both acute l‐dopa challenge and SST delivered positive results, the combination outcome was recorded as positive. Sensitivity, specificity, positive and negative predictive values (PPV and NPV, respectively), positive and negative likelihood ratios, diagnostic effectiveness (accuracy), and Youden's index were calculated for acute l‐dopa challenge in combination with all 3 olfaction variables (HT, HI, and AT). Accuracy expresses the proportion of correctly classified patients as a numeric value between 0.0 and 1.0. Youden's index is a magnitude commonly used to describe the performance of a diagnostic test and usually is computed to evaluate the overall discriminative power of a diagnostic procedure and for comparisons between tests.
Results
A flow diagram of the patient‐selection process for the study is provided in Figure 1. After clinical evaluation of 225 patient case histories, 210 individuals who had 2 years of follow‐up and underwent acute l‐dopa challenge as well as olfactory testing were identified (15 patients were excluded because of incomplete data). PD was diagnosed during follow‐up in 157 patients (75%), and 53 (25%) received a diagnosis other than PD. The latter included essential tremor (n = 16), multiple system atrophy (n = 9), drug‐induced parkinsonism or tremor (n = 9), dystonic tremor (n = 7), dementia with Lewy bodies (n = 4), vascular dementia‐parkinsonism, (n = 3), progressive supranuclear palsy (n = 3), and corticobasal syndrome (n = 2). Patient demographics, olfactory performance, and motor scores are provided in Table 1.
Figure 1.
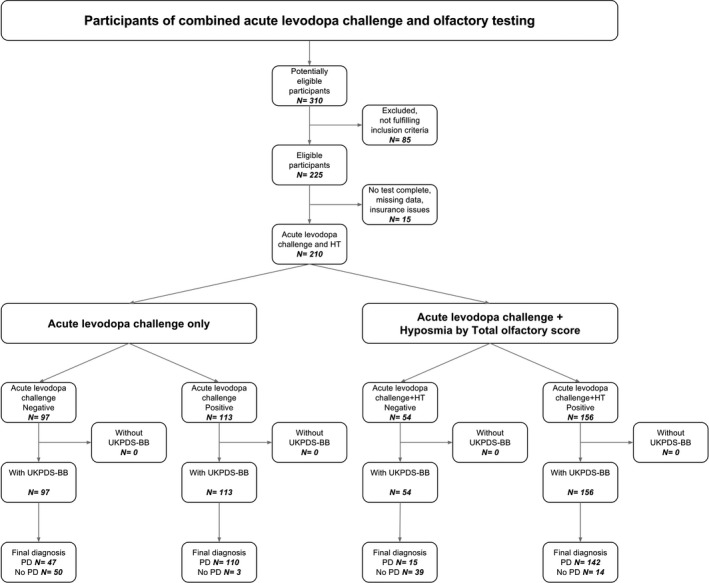
Flow chart of participants in the study. HT hyposmia according to total Sniffin’ Sticks Test score; UKPDS‐BB, United Kingdom Parkinson's Disease Society brain bank diagnostic criteria; PD, Parkinson's disease.
Table 1.
Patient demographic, olfactory performance, and motor scores
| Variable | Mean ± SD | P valuea | |
|---|---|---|---|
| PD patients, n = 157 | Non‐PD patients, n = 53 | ||
| Men, no. (%) | 94 (60) | 28 (53) | 0.1 |
| Age, y | 64.4 ± 9.5 | 67.8 ± 8.69 | 0.019 |
| SST | |||
| Threshold | 2.3 ± 2.4 | 3.7 ± 2.8 | <0.001 |
| Discrimination | 8.8 ± 3.1 | 9.6 ± 3.4 | 0.08 |
| Identification | 7.5 ± 3.4 | 10.2 ± 3.8 | <0.001 |
| Total | 18.7 ± 7.3 | 23.6 ± 8.3 | <0.001 |
| MDS‐UPDRS‐III | |||
| Off | 23.3 ± 11.1 | 19.9 ± 16.9 | 0.09 |
| On | 15.7 ± 8.8 | 18.0 ± 15.2 | 0.2 |
| Percentage improvement on acute levodopa challenge | 32.5 ± 15.1 | 7.9 ± 9.6 | <0.001 |
SD, standard deviation; PD, Parkinson's disease; SST, Sniffin’ Sticks Test; MDS‐UPDRS: Movement Disorders Society‐Unified Parkinson's Disease Rating Scale.
Significant differences are indicated in bold.
Single and combined diagnostic measures of acute l‐dopa challenge and olfactory testing indicated that acute l‐dopa challenge had 0.70 sensitivity and 0.94 specificity to predict a diagnosis of PD, whereas hyposmia based on the total SST score (HT) alone had 0.61 sensitivity and 0.77 specificity (Table 2). Combined acute l‐dopa challenge and olfactory testing had enhanced sensitivity, with the best results obtained by pooling together responses from acute l‐dopa challenge and hyposmia according to the total olfactory score (sensitivity, 0.90; specificity, 0.74; PPV, 0.91; NPV, 0.72; accuracy, 0.86) or the hyposmia identification subscore (sensitivity, 0.90; specificity, 0.70; PPV, 0.90; NPV, 0.71; accuracy 0.85). Diagnostic power evaluated according to accuracy produced superior results by combining results. The best diagnostic battery resulted from the combination of response to acute l‐dopa challenge and HT, followed by combination with HI and AT.
Table 2.
Single and combined diagnostic measures
| Diagnostic features | Se | Sp | PPV | NPV | LR+ | LR− | Accuracy | Youden's indexa |
|---|---|---|---|---|---|---|---|---|
| Single features | ||||||||
| Acute levodopa challenge | 0.70 | 0.94 | 0.97 | 0.52 | 12.3 | 0.32 | 0.76 | 0.64 |
| HI | 0.63 | 0.74 | 0.88 | 0.40 | 2.39 | 0.50 | 0.66 | 0.37 |
| HT | 0.61 | 0.77 | 0.89 | 0.40 | 2.68 | 0.51 | 0.65 | 0.38 |
| AT | 0.40 | 0.85 | 0.89 | 0.32 | 2.62 | 0.71 | 0.51 | 0.24 |
| Combined features | ||||||||
| Acute levodopa challenge + HI | 0.90 | 0.70 | 0.90 | 0.71 | 2.99 | 0.14 | 0.85 | 0.60 |
| Acute levodopa challenge + HT | 0.90 | 0.74 | 0.91 | 0.72 | 3.42 | 0.13 | 0.86 | 0.64 |
| Acute levodopa challenge + AT | 0.83 | 0.81 | 0.93 | 0.62 | 4.41 | 0.20 | 0.83 | 0.65 |
Se, sensitivity; Sp, specificity; PPV, positive predictive value; NPV, negative predictive value; LR, likelihood ratio; HI, hyposmia by identification olfactory subscore; HT, hyposmia by total olfactory score; AT, anosmia by total olfactory score.
Improved results are indicated in bold.
Discussion
Here, we observed that combining olfactory function assessment with response to acute l‐dopa challenge enhanced the diagnostic sensitivity for PD in patients who were experiencing early stages of disease. The composite results of acute l‐dopa challenge and hyposmia (assessed by total SST score) proved to be the combination with highest diagnostic prediction capacity. Combining the l‐dopa response with hyposmia according to the SST identification subscore also increased sensitivity, with similar values obtained for both HT and HI (sensitivity, 0.9). The NPV of all combinations studied also improved, but these improvements implied a detriment in both specificity and PPV. The method proposed aimed to improve the diagnostic yield of PD screening during early disease stages, as mentioned above, with the added benefit of reducing the false‐negative rate. This objective was achieved by combining test results, with the aforementioned enhancement of both sensitivity and NPV based on a true‐positive contribution of SST, which helped diagnose patients with early PD not detected by acute l‐dopa challenge. Conversely, false‐positive results also increased after combining tests, given that SST outcomes for hyposmic patients without PD contributed to misclassification, thus worsening specificity and PPV. Ultimately, however, accuracy for all combinations assessed increased, indicating a real improvement in the correct classification of both true‐positive and true‐negative results, which supports the trade‐off of adopting a combination of tests instead of continuing to use any single test alone. The reduction in specificity obtained by the combination approach is accepted in favor of the higher sensitivity gained when screening patients for PD.
Recently, Nalls and colleagues6 developed an algorithm based on olfactory function, family history, age, sex, and a composite genetic risk score, which was able to distinguish patients who had PD from healthy controls with 83% sensitivity and 90% specificity. Although that study compared patients who had PD with healthy controls, and ours differentiated patients who had PD from those who had other parkinsonisms, we observed similar results in terms of diagnostic accuracy for the diagnosis of PD using simpler and more applicable bedside tests.
Ross and colleagues7 noted that PD incidence was only modestly increased in men who had any single prodromal symptom at baseline, whereas patients who had 2 symptoms had a 10‐fold increase in the subsequent incidence of PD. Numerous other combinations of markers or screening batteries have been proposed8; for example, hyposmia has been combined with transcranial sonography,9 myocardial scintigraphy,10 and dopaminergic functional imaging,11 all of which increased diagnostic confirmation of PD. The potential problem of test or marker combinations is the increase in false‐negative results, especially when the instrument is used for screening purposes. In our study, the combination of response to acute l‐dopa challenge and hyposmia evaluated using SST scores increased sensitivity, thus reducing the problem. The selection of acute l‐dopa challenge and olfactory testing to predict PD does not preclude the value of other early biomarkers, such as genetic and family screening, rapid eye movement sleep behavior disorder, constipation, depression, and transcranial ultrasonography or dopaminergic functional neuroimaging. Finally, we should point out the limitations of this study: These include the lack of pathologic confirmation of the final diagnosis and the retrospective nature of the analysis, which is not exempt from selection or information retrieval biases. We minimized these limitations by using electronic chart reviews and by applying diagnostic criteria according to the treating movement disorders specialist. It should also be noted that SST scores were taken from the original test scores, and not from a local validation. This process is encouraged when using olfactory tests in order to achieve better diagnostic accuracy, but it does not invalidate current results.23
In conclusion, the combined response to acute l‐dopa challenge with the presence of hyposmia improved sensitivity for diagnosing PD during early stages of the disease and was superior to using the acute l‐dopa challenge test alone. However, with respect to specificity, acute l‐dopa challenge remains a better diagnostic tool for early differential diagnosis in patients with PD. The combination of l‐dopa response with hyposmia according to the SST identification subscore increased sensitivity similar to hyposmia according to the SST total olfactory score; thus, the use of the former is recommended in clinical practice because of its simplicity and time‐consuming properties, as also suggested by others.4, 24 Further studies that include the a longitudinal design or other nonmotor features are warranted to establish firmer conclusions.
Author Roles:
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
C.T.C.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
M.R.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
A.B.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
P.M.V.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
D.C.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
A.C.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
M.M.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: The authors report no sources of funding and no conflicts of interest.
Financial Disclosures for the previous 12 months: The authors declare that there are no disclosures to report.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Rizzo G, Coppeti M, Arcuti S, et al. Accuracy of clinical diagnosis of Parkinson disease—a systematic review and meta‐analysis. Neurology 2016;86:566–576. [DOI] [PubMed] [Google Scholar]
- 2. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2015;12:1591–1601. [DOI] [PubMed] [Google Scholar]
- 3. Hawkes C. Olfaction in neurodegenerative disorder. Mov Disord 2003;18:364–372. [DOI] [PubMed] [Google Scholar]
- 4. Krismer F, Pinter B, Mueller C, et al. Sniffing the diagnosis: olfactory testing in neurodegenerative parkinsonism. Parkinsonism Relat Disord 2017;35:36–41. [DOI] [PubMed] [Google Scholar]
- 5. Merello M, Nouzeilles MI, Arce GP, Leiguarda R. Accuracy of acute levodopa challenge for clinical prediction of sustained long‐term levodopa response as a major criterion for idiopathic Parkinson's disease diagnosis. Mov Disord 2002;17:795–798. [DOI] [PubMed] [Google Scholar]
- 6. Nalls MA, McLean CY, Rick J, et al. Diagnosis of Parkinson's disease on the basis of clinical and genetic classification: a population‐based modelling study. Lancet Neurol 2015;14:1002–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ross GW, Abbott RD, Petrovitch H, Tanner CM, White LR. Pre‐motor features of Parkinson's disease the Honolulu‐Asia Aging Study experience. Parkinsonism Relat Disord 2012;18(suppl 1):5199–5202. [DOI] [PubMed] [Google Scholar]
- 8. Tunc S, Graf J, Tadic V, et al. A population‐based study on combined markers for early Parkinson's disease. Mov Disord 2015;30:531–537. [DOI] [PubMed] [Google Scholar]
- 9. Busse K, Heilmann R, Kleinschimdt S, et al. Value of combined midbrain sonography, olfactory and motor function assessment in the differential diagnosis of early Parkinson's disease. J Neurol Neurosurg Psychiatry 2012;83:441–447. [DOI] [PubMed] [Google Scholar]
- 10. Izawa MO, Miwa H, Kajimoto Y, Kondo T. Combination of transcranial sonography, olfactory testing, and MIBG myocardial scintigraphy as a diagnostic indicator for Parkinson's disease. Eur J Neurol 2012;19:411–416. [DOI] [PubMed] [Google Scholar]
- 11. Borghammer P, Knudsen K, Ostergaard K, et al. Combined DaT imaging and olfactory testing for differentiating parkinsonian disorders. Int J Clin Pract 2014;68:1345–1351. [DOI] [PubMed] [Google Scholar]
- 12. Montgomery EB, Koller WC, LaMantia T. Early detection of probable idiopathic Parkinson's disease: I. Development of a diagnostic test battery. Mov Disord 2000;15:467–473. [PubMed] [Google Scholar]
- 13. Berendse HW. Diagnosing premotor Parkinson's disease using a two‐step approach combining olfactory testing and DAT SPECT imaging. Parkinsonism Relat Disord 2009;15(suppl 3):S26–S30. [DOI] [PubMed] [Google Scholar]
- 14. Hughes AJ, Ben‐Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology 1992;42:1142–1146. [DOI] [PubMed] [Google Scholar]
- 15. Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008;71:670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013;80:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele‐Richardson‐Olszewski syndrome): report of the NINDS‐SPSP international workshop. Neurology 1996;47:1–9. [DOI] [PubMed] [Google Scholar]
- 18. McKeith IG, Dickson DW, Lowe K, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 19. Wilson JA, Primrose WR, Smith RG. Prognosis of drug‐induced Parkinson's disease. Lancet 1987;1:443–444. [DOI] [PubMed] [Google Scholar]
- 20. Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord 1998;13(suppl 3):2–23. [DOI] [PubMed] [Google Scholar]
- 21. Saeed U, Compagnone J, Aviv RI, Strafella AP, Black SE, Lang AE, Masellis M. Imaging biomarkers in Parkinson's disease and parkinsonian syndromes: current and emerging concepts [serial online]. Transl Neurodegener 2017;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Merello M, Berschcovich ER, Ballesteros D, Cerquetti D. Correlation between the Movement Disorders Society Unified Parkinson's Disease rating scale (MDS‐UPDRS) and the Unified Parkinson's Disease rating scale (UPDRS) during L‐dopa acute challenge. Parkinsonism Relat Disord 2011;17:705–707. [DOI] [PubMed] [Google Scholar]
- 23. Millar Vernetti P, Rossi M, Cerquetti D, Perez Lloret S, Merello M. Comparison of olfactory identification patterns among Parkinson's disease patients from different countries. Chem Senses 2016;41:77–83. [DOI] [PubMed] [Google Scholar]
- 24. Mahlknecht P, Pechlaner R, Boesveldt S, et al. Optimizing odor identification testing as quick and accurate diagnostic tool for Parkinson's disease. Mov Disord 2016;31:1408–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]


