Abstract
Mutations in the gene encoding the main cardiac sodium channel (SCN5A) are the commonest genetic cause of Brugada syndrome (BrS). However, the effect of SCN5A mutations on the outcomes of ventricular fibrillation (VF) and syncope remains uncertain. To clarify this relationship, a meta‐analysis was performed. A comprehensive search was conducted to identify all eligible studies from PubMed, MEDLINE, EBSCO, ProQuest, Science Direct, Clinical Key, and Cochrane database for cohort studies of BrS populations that had been systematically tested for SCN5A mutations. We did meta‐analysis to see the relationship between SCN5A mutations and the occurrence of VF and/or syncope using RevMan 5.3. Five clinical studies met our criteria and included a total of 665 BrS patients. These studies included 45 patients with VF and 178 patients with syncope. We found that in BrS patients with SCN5A mutations the rate of VF event was 30.7% while in patients without mutations was 28.5% (Risk Ratio [RR] = 1.11, [95% CI: 0.61, 2.00], P = 0.73, I 2 = 0%). The occurrence of syncope events was 35.9% in patients with SCN5A mutations and 34.5% in patients without mutations (RR = 1.12, [95% CI: 0.87, 1.45], P = 0.37, I 2 = 39%). Furthermore, the occurrence of combined VF and syncope events were similar between the 2 groups (RR = 1.12, [95% CI: 0.89, 1.42], P = 0.34, I 2 = 11%). BrS patients with SCN5A mutations exhibit a similar risk of future occurence of VF and/or syncope as compared to those without SCN5A mutations.
Keywords: Brugada syndrome, SCN5A mutations, sodium channel, syncope, ventricular fibrillation
1. INTRODUCTION
Brugada syndrome (BrS) is an inherited cardiac channelopathy associated with genetic variants. Clinically, it presents with syncope or cardiac arrest due to ventricular fibrillation (VF), but the vast majority of the patients will remain asymptomatic. In the past two decades, several BrS‐associated genes and modifier genes were reported and most of these genes primarily encode sodium, potassium, and calcium channels or the proteins associated with these channels. Mutations in the gene encoding the main cardiac sodium channel (SCN5A) are the commonest genetic cause of BrS, identified in approximately 20%‐25% of BrS patients.1, 2
SCN5A is responsible for the fast upstroke in phase 0 of the cardiac action potential, and pathogenic variations will result in sodium channel dysfunction, which leads to conduction delay and predisposes the substrate for reentry.3, 4, 5 Functional studies of these mutations revealed that loss of function in the affected sodium channels was through different mechanisms including decreased expression of the sodium channel protein in the sarcolemma,6 expression of nonfunctional channels,7or altered gating properties (delayed activation, earlier inactivation, faster inactivation, enhanced slow inactivation, and delayed recovery from inactivation).8, 9
Although these studies already suggested that SCN5A mutations was associated with depolarization abnormality, the clinical importance of SCN5A mutations in BrS patients is still contradictory. Some studies suggested prognostic implications of SCN5A mutations with higher risk of arrhythmic events or syncopal episodes,10, 11 while others do not demonstrate such effects.12, 13, 14 Thus, this meta‐analysis sought to evaluate the prognostic significance of SCN5A mutations on the outcomes of VF and syncope in BrS patients.
2. METHODS
The protocol of this meta‐analysis was based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement.
2.1. Search strategy
We performed a comprehensive search to identify all published cohort studies that compared VF and syncope events in BrS populations that had been systematically tested for SCN5A mutations. We performed a literature search using the Pubmed, MEDLINE, EBSCO, ProQuest, Science Direct, Clinical Key, and Cochrane Databases. Searched terms included BrS; SCN5A mutation; VF; and syncope. A manual search was also performed to retrieve potential articles cited in previous meta‐analysis, systematic review, and those considered to be relevant by the reviewers.
2.2. Inclusion and exclusion criteria
Only cohort studies that compared documented VF and syncope events in BrS populations that had been systematically tested for SCN5A mutations were included in the meta‐analysis. BrS was diagnosed if a type 1 electrocardiogram (ECG) pattern was documented spontaneously or after provocation with a class I antiarrhytmic drug. Studies were excluded if they were cross‐sectional design, case reports or series, editorials, narrative reviews, non‐English articles, published before 2000, and if they didn't state clearly that structural heart disease were ruled out.
2.3. Statistical analysis
We performed the statistical calculations using RevMan version 5.3. Risk Ratio (RR) were used as a summary measure of efficacy for dichotomous data and its corresponding 95% CIs. Heterogeneity across studies was examined with the inconsistency index (I 2) test, which ranges from 0% to 100%. This index is defined as the percentage of the observed variability that is due to heterogeneity rather than chance alone. When I 2 was more than 50%, significant statistical heterogeneity was considered to be present. Pooled estimates of efficacy were calculated using the fixed‐effects model. If there was heterogeneity, the random‐effects model was used. In case of heterogeneity across the studies, we performed sensitivity analyses, serially excluding studies to determine the source of heterogeneity. All P values were two tailed, with statistical significance set at 0.05 or less.
3. RESULTS
The primary search for studies comparing VF and syncope events in BrS populations that had been systematically tested for SCN5A mutations yielded 103 potentially relevant articles published in English language. 55 studies were excluded after title and abstract screening. Therefore, 48 potential relevant studies were retrieved to read the entire manuscript. Following full‐text screening, 39 studies were excluded, leaving nine studies that qualified the inclusion and exclusion criteria. After careful examination, among nine studies, five studies were found to be published from the same institusion (Okayama University Hospital), therefore we only included one study with the largest sample number (Nishii et al10). Finally, five studies (n = 665 patients; mean age 45.98 ± 6.7 years; male: 77.5%) were included in this meta‐analysis. These studies included 45 patients with VF and 178 patients with syncope.
The prognostic values of SCN5A mutation on the outcomes of VF and/or syncope were analyzed from five studies (four prospective cohort studies and one retrospective cohort study). All studies included patients with either spontaneous type‐1 ECG of BrS at baseline or after provocation with a class I antiarrhthmic drug. The ranges of the proportion of SCN5A mutations were from 15.7% to 36.4%. The primary endpoints of these studies were documented VF and/or syncope (Table 1).
Table 1.
Baseline characteristics of published studies included in the meta‐analysis
| Study | Publication year | Country | Study design | Sample size (N) | Male / Female (N) | Spontaneous type 1 ECG, N (%) | Induced type 1 ECG, N (%) | Cascade testing, N (%) | Age (mean ± SD) | SCN5A mutation, N (%) | Documented VF, N (%) | Syncope, N (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eckardt, et al17 | 2005 | Germany, France, Netherlands | Prospective cohort | 183 | 140/43 | 125 (59) | 87 (41) | 47 (22) | 45 ± 6 | 57 (31.1) | Not reported | 65 (35.5) |
| Juang, et al15 | 2003 | Taiwan | Prospective cohort | 4 | 4/0 | Not reported | 4 (100) | Not reported | 45.2 ± 2.06 | 1 (25) | 3 (75) | 3 (75) |
| Nishii, et al10 | 2010 | Japan | Prospective cohort | 108 | 105/3 | 71 (65.7) | 37 (34.3) | Not reported | 46.8 ± 11.6 | 17 (15.7) | 23 (21.3) | 43 (39.8) |
| Amin, et al18 | 2011 | Netherlands | Retrospective cohort | 214 | 133/81 | 49 (22.9) | 165 (77.1) | Not reported | 46.9 ± 2.2 | 78 (36.4) | Not reported | 67 (31.3) |
| Yokokawa, et al16 | 2007 | Japan | Prospective cohort | 44 | 44/0 | 31 (70) | 13 (30) | Not reported | 46.0 ± 12.0 | 8 (18.2) | 19 (43.2) | 0 (0) |
ECG, electrocardiogram; VF, ventricular fibrillation.
3.1. Outcome: VF or syncope
The range of the occurrence of VF rate was between 21.3% to 75% (Table 1). Overall, in BrS patients with SCN5A mutations the rate was 30.7% while in patients without mutations it was 28.5%. This difference was not statistically significant (RR = 1.11, [95% CI: 0.61, 2.00], P = 0.73, I 2 = 0%; Figure 1).
Figure 1.
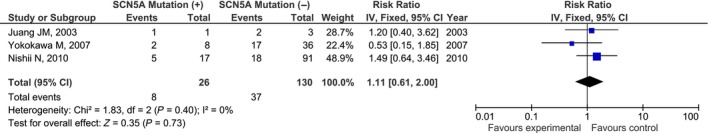
Forest plot of the association between SCN5A mutation and ventricular fibrillation events (RR = 1.11, [95% CI: 0.61, 2.00], P = 0.73, I 2 = 0%; References no. 10, 15, 16). RR, risk ratio
The range of syncope events was between 0% to 75% (Table 1). However, the occurrence of syncope events were also similar between BrS patients with SCN5A mutations (35.9%) and without SCN5A mutations (34.5%; RR = 1.12, [95% CI: 0.87, 1.45], P = 0.37, I 2 = 39%; Figure 2).
Figure 2.
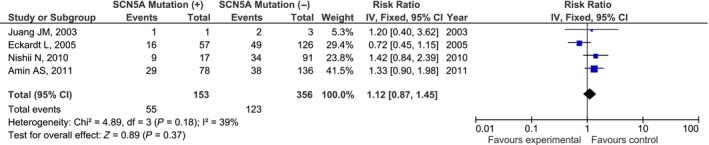
Forest plot of the association between SCN5A mutation and syncope events (RR = 1.12, [95% CI: 0.87, 1.45], P = 0.37, I 2 = 39%; References no. 10, 15, 17, 18). RR, risk ratio
3.2. Outcome: combined VF and syncope
Similar finding was observed when the events of VF and syncope were combined. Figure 3 showed the pool analysis of elligible studies suggesting that BrS patients with SCN5A mutations have similar risk of having combined VF and syncope as compared to BrS patients without mutations (RR = 1.12, [95% CI: 0.89, 1.42], P = 0.34). No significant heterogeneity among them was seen (I 2 index = 11%).
Figure 3.
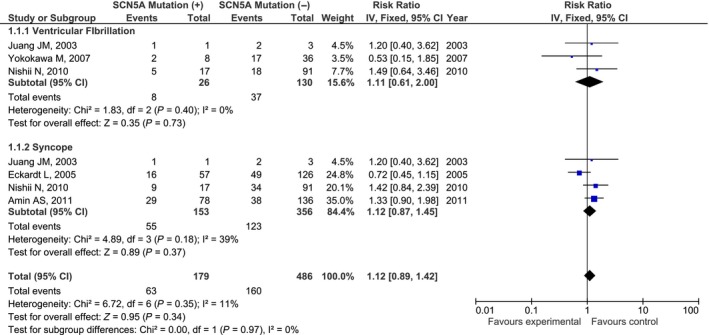
Forest plot of the association between SNC5A mutation and combined VF and syncope (RR=1.12, [95% CI: 0.89, 1.42], P = 0.34, I 2 = 11%). RR, risk ratio; VF, ventricular fibrillation
4. DISCUSSION
This meta‐analysis showed that BrS patients with SCN5A mutations exhibit a similar risk of future occurence of VF and/or syncope as compared with those without SCN5A mutations. Although our finding was negative (no association), we believe our finding have important clinical implications since mutations in the SCN5A gene are the commonest genetic defects found in BrS.
Our finding is dissimilar with previous cohort study conducted by Sommariva et al19 which found that SCN5A mutation carriers had an increased risk of major arrhythmic events (documented VT/VF, SCD or appropriate ICD shock) compared with noncarriers. Another study also observed that the presence of SCN5A mutations could be associated with recurrence of VF in BrS patients.10 However, our result is in agreement with the data from FINGER registry12 (France, Italy, Netherlands, and Germany) and previous meta‐analysis by Gehi et al14 suggesting that the status of SCN5A mutations did not seem to be an independent risk factors for future cardiac events. One possible explanation for the discrepancy between one studies with others probably due to the different location of SCN5A mutations.20, 21 Indeed, Yamagata et al11 reported that among all SCN5A (+) BrS probands, those with mutations located in the pore region may be at a higher risk for cardiac events compared to those with mutation in the nonpore region. In this meta‐analysis, we could not elaborate this issue since not all studies reported the precise location of the SCN5A mutations.
Several studies revealed that BrS patients with SCN5A mutations will have more severe clinical presentation, and are more likely to exhibit conduction abnormalities (longer PQ interval, longer QRS duration, frequent fragmentation) on ECG, and typically have a longer HV interval during electrophysiology study (EPS).3, 4, 21, 22, 23 Clinically, such conduction abnormalities may have important implication in the occurrence of syncope. Although our results showed a tendency toward a higher incidence of syncope (RR = 1.12; CI 0.87‐1.45), it was not statistically significant (P = 0.37). We speculate that if we could include more numbers of patients, the results might be different.
In conclusion, we have conducted a meta‐analysis to investigate whether the presence of an SCN5A mutation contributes to the risk of VF and/or syncope in BrS patients. We included 665 BrS patients from five cohort studies. Our results showed that the presence of an SCN5A mutation was not associated with the increased risk of developing VF and/or syncope in this patient population. Considering that mutations in the SCN5A gene are the commonest genetic defects found in BrS, we believe our finding have important implications in which the knowledge of a specific genetic mutation is unlikely to provide specific prognostic information at this time.
CONFLICT OF INTEREST
Authors declare no conflict of interests for this article.
Raharjo SB, Maulana R, Maghfirah I, et al. SCN5A gene mutations and the risk of ventricular fibrillation and syncope in Brugada syndrome patients: A meta‐analysis. J Arrhythmia. 2018;34:473–477. 10.1002/joa3.12097
REFERENCES
- 1. Juang JJ, Horie M. Genetics of Brugada syndrome. J Arrhythm. 2016;32:418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lieve KV, Wilde AA. Inherited ion channel diseases: a brief review. Europace. 2015;17(Suppl 2):ii1–6. [DOI] [PubMed] [Google Scholar]
- 3. Makiyama T, Akao M, Tsuji K, et al. High risk for bradyarrhythmic complications in patients with Brugada syndrome caused by SCN5A gene mutations. J Am Coll Cardiol. 2005;46:2100–6. [DOI] [PubMed] [Google Scholar]
- 4. Smits JP, Eckardt L, Probst V, et al. Genotype‐phenotype relationship in Brugada syndrome: electrocardiographic features differentiate SCN5A‐related patients from non‐SCN5A‐related patients. J Am Coll Cardiol. 2002;40:350–6. [DOI] [PubMed] [Google Scholar]
- 5. Tanaka M, Nakamura K, Kusano KF, et al. Elevated oxidative stress is associated with ventricular fibrillation episodes in patients with Brugada‐type electrocardiogram without SCN5A mutation. Cardiovasc Pathol. 2011;20:e37–42. [DOI] [PubMed] [Google Scholar]
- 6. Valdivia CR, Tester DJ, Rok BA, et al. A trafficking defective, Brugada syndrome‐causing SCN5A mutation rescued by drugs. Cardiovasc Res. 2004;62:53–62. [DOI] [PubMed] [Google Scholar]
- 7. Kyndt F, Probst V, Potet F, et al. Novel SCN5A mutation leading either to isolated cardiac conduction defect or Brugada syndrome in a large French family. Circulation. 2001;104:3081–6. [DOI] [PubMed] [Google Scholar]
- 8. Dumaine R, Towbin JA, Brugada P, et al. Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent. Circ Res. 1999;85:803–9. [DOI] [PubMed] [Google Scholar]
- 9. Lei M, Huang CL, Zhang Y. Genetic Na+ channelopathies and sinus node dysfunction. Prog Biophys Mol Biol. 2008;98:171–8. [DOI] [PubMed] [Google Scholar]
- 10. Nishii N, Ogawa M, Morita H, et al. SCN5A mutation is associated with early and frequent recurrence of ventricular fibrillation in patients with Brugada syndrome. Circ J. 2010;74:2572–8. [DOI] [PubMed] [Google Scholar]
- 11. Yamagata K, Horie M, Aiba T, et al. Genotype‐phenotype correlation of SCN5A mutation for the clinical and electrocardiographic characteristics of probands with Brugada syndrome: a Japanese multicenter registry. Circulation. 2017;135:2255–70. [DOI] [PubMed] [Google Scholar]
- 12. Probst V, Veltmann C, Eckardt L, et al. Long‐term prognosis of patients diagnosed with Brugada syndrome: results from the FINGER Brugada syndrome registry. Circulation. 2010;121:635–43. [DOI] [PubMed] [Google Scholar]
- 13. Priori SG, Napolitano C, Gasparini M, et al. Natural history of Brugada syndrome: insights for risk stratification and management. Circulation. 2002;105:1342–7. [DOI] [PubMed] [Google Scholar]
- 14. Gehi AK, Duong TD, Metz LD, Gomes JA, Mehta D. Risk stratification of individuals with the Brugada electrocardiogram: a meta‐analysis. J Cardiovasc Electrophysiol. 2006;17:577–83. [DOI] [PubMed] [Google Scholar]
- 15. Juang JM, Huang SKS, Tsai CT, et al. Characteristics of Chinese patients with symptomatic Brugada syndrome in Taiwan. Cardiology. 2003;99:182–9. [DOI] [PubMed] [Google Scholar]
- 16. Yokokawa M, Noda T, Okamura H, et al. Comparison of long‐term follow‐up of electrocardiographic features in Brugada syndrome between the SCN5A‐positive probands and the SCN5A‐negative probands. Am J Cardiol. 2007;100:649–55. [DOI] [PubMed] [Google Scholar]
- 17. Eckardt L, Probst V, Smits JPP, et al. Long‐term prognosis of individuals with right precordial ST‐segment–elevation Brugada syndrome. Circulation. 2005;111:257–63. [DOI] [PubMed] [Google Scholar]
- 18. Amin AS, Boink GJJ, Atrafi F, et al. Facilitatory and inhibitory effects of SCN5A mutations on atrial fibrillation in Brugada syndrome. Europace. 2011;13:968–75. [DOI] [PubMed] [Google Scholar]
- 19. Sommariva E, Pappone C, Martinelli Boneschi F, et al. Genetics can contribute to the prognosis of Brugada syndrome: a pilot model for risk stratification. Eur J Hum Genet. 2013;21:911–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Itoh H, Shimizu M, Mabuchi H, Imoto K. Clinical and electrophysiological characteristics of Brugada syndrome caused by a missense mutation in the S5‐pore site of SCN5A. J Cardiovasc Electrophysiol. 2005;16:378–83. [DOI] [PubMed] [Google Scholar]
- 21. Pfahnl AE, Viswanathan PC, Weiss R, et al. A sodium channel pore mutation causing Brugada syndrome. Heart Rhythm. 2007;4:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Probst V, Wilde AA, Barc J, et al. SCN5A mutations and the role of genetic background in the pathophysiology of Brugada syndrome. Circ Cardiovasc Genet. 2009;2:552–7. [DOI] [PubMed] [Google Scholar]
- 23. Morita H, Kusano KF, Miura D, et al. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation. 2008;118:1697–704. [DOI] [PubMed] [Google Scholar]


