Abstract
Background
Implantable cardioverter defibrillator (ICD) and cardiac resynchronization with a defibrillator (CRT‐D) are established therapies for secondary prevention of sudden cardiac death (SCD) in patients with structural heart disease (SHD), but the rates of subsequent ICD/CRT‐D therapy widely differ among patients with SHD. The aim of this study was to determine clinical factors associated with appropriate therapy for preventing SCD in patients with SHD.
Methods
We enrolled 147 patients with SHD (mean age, 59 ± 15 years; mean ejection fraction [EF], 45 ± 15%) who underwent ICD/CRT‐D implantation for secondary prevention of SCD (ischemic heart disease, n = 50; nonischemic heart disease, n = 97). ICD/CRT‐D was implanted for aborted cardiopulmonary arrest (CPA, n = 65) or sustained ventricular tachycardia (VT, n = 82).
Results
During a follow‐up period of 3.2 ± 3.6 years, 79 of the 147 patients had appropriate ICD/CRT‐D therapies. A Kaplan‐Meier survival curve showed that the rate of appropriate therapy was 54% at 5‐year follow‐up. Prior sustained VT, lower EF, and use of a class I antiarrhythmic drug were significantly more frequent in patients with appropriate therapy. In multivariate analysis, prior sustained VT (hazard ratio, 2.8; 95% CI, 1.60‐4.46; P = .001) was the only independent predictor for appropriate ICD/CRT‐D therapy. Kaplan‐Meier survival curves showed that rates of appropriate therapy during a 5‐year follow‐up period were 70% and 34% in patients with sustained VT and those with CPA, respectively (P = .001).
Conclusions
In SHD patients implanted with an ICD/CRT‐D, prior sustained VT as an indication of ICD/CRT‐D implantation, but not EF or an antiarrhythmic drug, predicts a high rate of appropriate therapy.
Keywords: implantable cardioverter defibrillator, out‐of‐hospital cardiac arrest, secondary prevention, structural heart disease, sustained ventricular tachycardia
1. INTRODUCTION
Implantable cardioverter defibrillator (ICD) is an established therapy for secondary prevention of sudden cardiac death (SCD) in patients with structural heart disease (SHD).1, 2, 3 The rate of appropriate therapy by ICD insertion has been reported to be higher in patients in whom the device was implanted for secondary prevention than in patients in whom the device was implanted for primary prevention.4, 5, 6, 7, 8, 9, 10, 11 Clinical trials on ICD therapy have been mainly conducted in European countries and the United States, where most cases of underlying heart disease are ischemic heart disease,1, 2, 3, 5, 7, 8, 9, 10, 11 and thus, the findings may not be necessarily applicable to countries with different prevalences of heart diseases such as Japan, where SHD in 51%‐69% of candidates for ICD therapy is cardiomyopathy.12, 13, 14, 15 In the Japanese guidelines of ICD implantation for secondary prevention,16 both documented ventricular fibrillation (VF) and hemodynamically unstable ventricular tachycardia (VT) in SHD are class I indication. However, it is unclear whether the rate of appropriate therapy differs depending on the type of prior ventricular arrhythmia or other clinical parameters. The aim of this study was to determine which clinical factors are associated with appropriate therapy in Japanese SHD patients in whom an ICD has been implanted.
2. METHODS
2.1. Study subjects
We consecutively enrolled patients with SHD who underwent ICD or cardiac resynchronization therapy with defibrillator (CRT‐D) implantation between 1999 and 2015 at our institute for secondary prevention of SCD due to lethal ventricular arrhythmia. Lethal ventricular arrhythmia in this study was defined as resuscitated cardiopulmonary arrest (CPA), in most cases of which VF was documented, and/or sustained VT in patients with SHD. CPA was defined as ventricular arrhythmia resulting in loss of consciousness and requiring out‐of‐hospital cardiac resuscitation. Sustained VT was defined as VT requiring medical or external cardioversion for termination but being hemodynamically tolerable long enough for the patient to seek medical assistance. Patients in whom ventricular arrhythmias were associated with severe electrolyte imbalance, acute myocardial ischemia or proarrhythmic drug effects were excluded from this study.
2.2. Diagnosis of cardiovascular disease
Diagnosis of cardiovascular disease was made by standard examinations including laboratory examinations of blood and urine, chest X‐ray, computed tomography, cardiac magnetic resonance imaging, echocardiography, and cardiac catheterization (including coronary angiography and myocardial biopsy in cases of suspected cardiomyopathy). Ischemic heart disease (IHD) was diagnosed by severe (>75%) coronary artery stenosis, the presence of a Q wave on an electrocardiogram, or a previous history of percutaneous coronary angioplasty or coronary artery bypass graft. Left ventricular ejection fraction (LVEF) and left ventricular diastolic diameter were evaluated by two‐dimensional echocardiography. Plasma brain natriuretic peptide (BNP) was measured as a marker of severity of heart failure in a stable condition, mostly at the time of discharge after ICD/CRT‐D implantation.
2.3. Device implantation, programming, pharmacological therapy, and follow‐up
ICD/CRT‐D implantation was performed by the subclavian venous approach in all cases. CRT‐D was selected for patients who met the following criteria: (i) reduced LVEF < 35%, (ii) symptomatic heart failure (NYHA II or III), and (iii) QRS width >120 ms with left bundle branch block morphology. Device interrogation was scheduled every three months, and an intracardiac electrocardiogram stored in the ICD or CRT‐D was analyzed to determine whether the therapies were delivered appropriately or not by two specialized electrophysiologists. Discrimination between ventricular arrhythmia and supraventricular arrhythmia was made by standard criteria such as abrupt or gradual onset, regularity of tachycardia, morphology of ventricular electrogram, and atrioventricular dissociation. Appropriate ICD/CRT‐D therapy was defined as ICD shock or antitachycardia pacing (ATP) for VT or VF. Therapies were classified as inappropriate when they are triggered by sinus or supraventricular tachycardia, T wave oversensing, or electrode dysfunction. When appropriate therapy was initially ATP and followed by shock because of failure of ATP to terminate the VT by ATP, the therapy was classified as ATP. Although detailed device programming was left to the discretion of treating electrophysiologists, we basically tried to detect as many ventricular arrhythmias as possible and we programmed the VT zone even in relatively lower heart rate, expecting effective therapy by ATP. Our basic ICD/CRT‐D programming was as follows. In patients with cardiac arrest in whom VF was the only ventricular arrhythmia documented until the event of cardiac arrest, VF zone with a threshold of 320 ms was programmed. In patients with documented sustained VT, programming of the VT zone was set to a value of 40 ms below the clinically documented VT, and the VF zone was set to a value less than 320 ms. Even in patients with CPA, if frequent nonsustained VT was observed during follow‐up period, VT zone was programmed. The primary endpoint of this study was the occurrence of first appropriate device therapy during the follow‐up period. The secondary endpoint was first appropriate therapy or all‐cause death. Details of the pharmacological therapy were registered at the time of discharge after ICD/CRT‐D implantation.
2.4. Statistical analysis
Statistical values are shown as means ± 1 SD. Patients were divided into two groups according to the presence or absence of at least one appropriate ICD/CR‐TD therapy during the follow‐up period. The Mann‐Whitney U test was used for comparison of mean values in the two groups, and the chi‐square test was used for comparison of prevalences. Event‐free rates were calculated by the Kaplan‐Meier method, and the rates in the two groups were compared by the log‐rank test. A P value <.05 was considered significant. Cox proportional hazard analysis was used to identify independent predictors of appropriate ICD/CRT‐D therapy. Variables with a P value <.05 in univariate analysis were selected for use in multivariate models. The analyses were performed using JMP software (version 8.0.2, SAS Institute, Cary, North Carolina, USA).
3. RESULTS
The baseline characteristics of the study patients and comparison of clinical variables between the groups with and without episodes of appropriate ICD/CR‐TD therapy during follow‐up are shown in Table 1. The study population consisted of 147 patients who underwent ICD (n = 136) or CRT‐D (n = 11) implantation for secondary prevention of SCD. The mean age of the patients was 59 ± 15 years, and 73% of the patients were male. IHD accounted for 34% of the cases of SHD in the total study subjects. Reasons for device implantation were a prior history of sustained VT in 82 patients (56%) and history of CPA in 65 patients (44%). Mean LVEF was 45 ± 15%, and mean serum BNP level was 308 ± 399 pg/mL. Seventy‐nine patients (54%) had at least one appropriate ICD/CRT‐D therapy during an average follow‐up period of 3.2 ± 3.6 years. When clinical variables were compared between the two groups with and without appropriate ICD/CRT‐D therapy, the rate of sustained VT was higher (71% vs 38%, P < .001), the rate of CPA was lower (29% vs 62%, P < .001), LVEF was lower (42% vs 49%, P = .014), and left ventricular diameter was larger (57 mm vs 53 mm, P = .031) in the group with appropriate therapy than in the group without an episode of appropriate therapy. There was no significant difference in the rate of ischemic heart disease (49% vs 55%) or serum BNP level (291 pg/mL vs 328 pg/mL) between the two groups. Cardiac death occurred in 18 patients (heart failure in 13, sudden cardiac death in 4, and device lead infection in 1), and it was not associated with appropriate therapy. Data for the type of appropriate device therapy, that is, shock therapy or ATP, were available in 95% of patients. In patients with prior sustained VT who underwent appropriate therapy, 45 of 53 therapies (85%) were ATP. On the other hand, ATP was performed only in 10 of 22 (46%) in patients with CPA (P = .001). In patients with CPA who did not undergo appropriate therapy, three cases had an episode of nonsustained VT which lasted more than 20 beats. Although these episodes were of therapy zone and not treated, they could have been treated if VT zone was programmed.
Table 1.
Patient characteristics and comparison of clinical parameters
| All patients (n = 147) | Appropriate therapy(−) (n = 68) | Appropriate therapy(+) (n = 79) | P value | |
|---|---|---|---|---|
| Age, y | 59.2 ± 14.6 | 60.1 ± 15.2 | 58.5 ± 14.3 | .512 |
| Male, n (%) | 107 (72.8) | 47 (69.1) | 60 (75.9) | .353 |
| Underlying heart disease | ||||
| Ischemic/nonischemic | 50/97 | 24/44 | 26/53 | .897 |
| 34%/66% | 35%/65% | 33%/67% | ||
| Cause of implantation | ||||
| History of sustained VT, n (%) | 82 (55.8) | 26 (38.2) | 56 (70.9) | <.001 |
| History of CPA, n (%) | 65 (44.2) | 42 (61.8) | 23 (29.1) | <.001 |
| LVEF, % | 45.3 ± 15.2 | 48.7 ± 16.8 | 42.4 ± 13.1 | .014 |
| LVDd, mm | 55.2 ± 9.6 | 53.4 ± 10.0 | 56.8 ± 8.9 | .031 |
| BNP, pg/mL | 308.2 ± 399.2 | 327.9 ± 455.6 | 291.1 ± 345.3 | .592 |
| Follow‐up period, years | 3.2 ± 3.6 | 5.0 ± 4.0 | 1.6 ± 2.3 | |
| ICD/CRT‐D | 136/11 | 64/4 | 72/7 | .711 |
| 93%/7% | 94%/6% | 91%/9% | ||
| Cardiac death (SCD/pump failure) | 18 (4/13) | 6 (1/5) | 12 (3/9) | .315 |
BNP, brain natriuretic peptide; CPA, cardiopulmonary arrest; CRT‐D, cardiac resynchronization therapy with defibrillator; ICD, implantable cardioverter‐defibrillator; LVDd, left ventricular diastolic diameter; LVEF, left ventricular ejection fraction; SCD, sudden cardiac death; VT, ventricular tachycardia.
Table 2 shows underlying cardiac diseases: old myocardial infarction (n = 50), dilated cardiomyopathy (n = 15), hypertrophic cardiomyopathy (n = 33), arrhythmogenic right ventricular cardiomyopathy (n = 10), cardiac sarcoidosis (n = 16), valvular heart disease (n = 11), other secondary cardiomyopathy (n = 9), tetralogy of fallot (n = 1), atrial septal defect post patch closure (n = 1), and left ventricular noncompaction (n = 1). There was no significant difference in etiology of SHD between the two groups with and without appropriate ICD/CRT‐D therapy. At discharge, amiodarone, a beta‐blocker, and class I antiarrhythmic drugs (AAD) were prescribed in 61%, 68%, and 29% (class Ib AAD accounted for 78% of class I AAD) of the patients, respectively (Table 3). The percentage of patients in whom amiodarone was prescribed was lower (53% vs 71%, P = .031), and the percentage of patients in whom class I AAD was prescribed was higher (38% vs 19%, P = .012) in patients with appropriate therapy than in those without appropriate therapy.
Table 2.
Underlying heart disease
| All patients (n = 147) | Appropriate therapy(−)(n = 68) | Appropriate therapy(+)(n = 79) | P value | |
|---|---|---|---|---|
| IHD, n (%) | 50 (34.0) | 24 (35.3) | 26 (33.3) | .761 |
| DCM, n (%) | 15 (10.2) | 6 (8.8) | 9 (11.4) | .608 |
| HCM, n (%) | 33 (22.4) | 17 (25.0) | 16 (20.2) | .492 |
| ARVC, n (%) | 10 (6.8) | 4 (5.9) | 6 (7.6) | .681 |
| CS, n (%) | 16 (10.9) | 8 (11.8) | 8 (11.4) | .751 |
| VHD, n (%) | 11 (7.5) | 3 (8.8) | 8 (10.1) | .198 |
| Secondary CM, n (%) | 9 (6.1) | 4 (5.9) | 5 (6.3) | .910 |
| Others, n (%) | 3 (2.0) | 2 (2.9) | 1 (1.3) | .474 |
ARVC, arrhythmogenic right ventricular cardiomyopathy; CM, cardiomyopathy; CS, cardiac sarcoidosis; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; IHD, ischemic heart disease; VHD, valvular heart disease.
Table 3.
Oral drug treatment
| All patients(n = 147) | Appropriate therapy(−)(n = 68) | Appropriate therapy(+)(n = 79) | P value | |
|---|---|---|---|---|
| Amiodarone, n (%) | 90 (61.2) | 48 (70.6) | 42 (53.1) | .031 |
| Sotalol, n (%) | 14 (9.5) | 3 (4.4) | 11 (13.9) | .050 |
| Bepridil, n (%) | 3 (2.0) | 0 (0.0) | 3 (3.8) | .105 |
| Class I AAD, n (%) | 43 (29.3) | 13 (19.1) | 30 (38.0) | .012 |
| Ia | 7 (4.8) | 4 (5.9) | 3 (3.8) | .704 |
| Ib | 36 (24.5) | 9 (13.2) | 27 (34.2) | .004 |
| Ic | 3 (2.0) | 0 | 3 (3.8) | .249 |
| β‐blocker, n (%) | 100 (68.0) | 48 (70.6) | 52 (68.5) | .537 |
| Verapamil, n (%) | 11 (7.5) | 2 (2.9) | 9 (11.4) | .052 |
| Diltiazem, n (%) | 4 (2.7) | 2 (2.9) | 2 (2.5) | .879 |
| Digitalis, n (%) | 7 (4.8) | 3 (4.4) | 4 (5.1) | .853 |
| ACE‐I/ARB, n (%) | 71 (48.3) | 27 (39.7) | 44 (55.7) | .053 |
AAD, antiarrhythmic drug; ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker.
The results of univariate and multivariate analyses using a Cox hazard model for prediction of appropriate ICD/CRT‐D therapy are shown in Table 4. In univariate analysis, prior history of sustained VT (hazard ratio [HR]: 2.86, confidence interval [CI]: 1.77‐4.76, P < .001), lower LVEF (HR: 0.98, CI: 0.97‐1.00, P = .016), use of sotalol (HR: 3.01, CI: 1.49‐5.52, P = .003), and use of class I AAD (HR: 1.91, CI: 1.19‐3.00, P = .007) were significantly associated with appropriate ICD/CRT‐D therapy. In multivariate analysis, prior sustained VT (HR: 2.80, CI: 1.60‐4.46, P = .001) was the only independent predictor for appropriate ICD/CR‐TD therapy.
Table 4.
Univariate and multivariate Cox proportional hazard model for appropriate ICD/CRT‐D therapy
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |||
| Age | 1.00 (per 1 y.o.) | 0.99 | 1.02 | .746 | ||||
| Male (vs Female) | 1.14 | 0.83 | 2.35 | .227 | ||||
| IHD (vs non‐IHD) | 1.19 | 0.73 | 1.89 | .484 | ||||
| Sustained VT (vs CPA) | 2.86 | 1.77 | 4.76 | <.001 | 2.80 | 1.60 | 4.46 | .001 |
| Cardiac death | 1.12 | 0.60 | 2.07 | .724 | ||||
| BNP | 1.00 (per 1 pg/mL) | 0.24 | 3.25 | .988 | ||||
| LVEF | 0.98 (per 1.0%) | 0.97 | 1.00 | .016 | 0.98 (per 1.0%) | 0.97 | 1.01 | .066 |
| Amiodarone | 0.68 | 0.43 | 1.06 | .087 | ||||
| Sotalol | 3.01 | 1.49 | 5.52 | .003 | ||||
| Bepridil | 2.95 | 0.72 | 8.03 | .117 | ||||
| Class I AAD | 1.91 | 1.19 | 3.00 | .007 | 1.56 | 0.96 | 2.48 | .070 |
| β‐blocker | 0.92 | 0.58 | 1.48 | .721 | ||||
| Verapamil | 2.04 | 0.94 | 3.88 | .068 | ||||
| Diltiazem | 0.90 | 0.15 | 2.87 | .885 | ||||
| Digitalis | 1.22 | 0.37 | 2.94 | .709 | ||||
| ACE‐I/ARB | 1.53 | 0.98 | 2.40 | .060 | ||||
AAD, antiarrhythmic drug; ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BNP, brain natriuretic peptide; CPA, cardiopulmonary arrest; CRT‐D, cardiac resynchronization therapy with defibrillator; HR, hazard ratio; ICD, implantable cardioverter‐defibrillator; IHD, ischemic heart disease; LVEF, left ventricular ejection fraction; VT, ventricular tachycardia.
A Kaplan‐Meier curve for the primary endpoint, that is, appropriate ICD/CRT‐D therapy, is shown in Figure 1A. Appropriate therapy‐free rates were 68% at 1 year, 51% at 3 years, and 46% at 5 years. Patients with sustained VT and those with CPA prior to ICD/CRT‐D implantation showed different Kaplan‐Meier curves. Appropriate therapy‐free rates were 83% at 1 year, 72% at 3 years, and 66% at 5 years in patients with CPA and were 56% at 1 year, 34% at 3 years, and 30% at 5 years in patients with sustained VT (log‐rank, P < .001). Kaplan‐Meier curves for appropriate therapy‐free rates in patients with IHD and those with SHD other than IHD were not significantly different (Figure 2). A lower appropriate therapy‐free rate in patients with prior sustained VT than in patients with CPA was found in subgroups of patients with and without IHD (Figure 3A and 3B).
Figure 1.
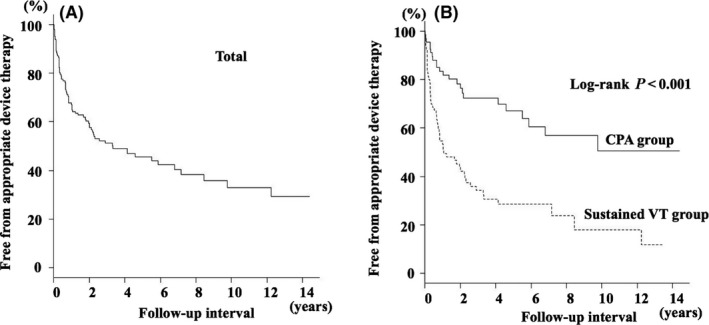
A, Kaplan‐Meier survival curve for being free from appropriate ICD/CRT‐D therapy. The cumulative probabilities for being free from appropriate ICD/CRT‐D therapy were 68% at 1 year, 51% at 3 years, and 46% at 5 years. B, Kaplan‐Meier survival curve for being free from appropriate ICD/CRT‐D therapy according to the presenting arrhythmia before implantation. The cumulative probabilities for being free from appropriate ICD/CRT‐D therapy were 83% at 1 year, 72% at 3 years, and 66% at 5 years in patients with CPA and they were 56% at 1 year, 34% at 3 years, and 30% at 5 years in patients with sustained VT (P < .001)
Figure 2.
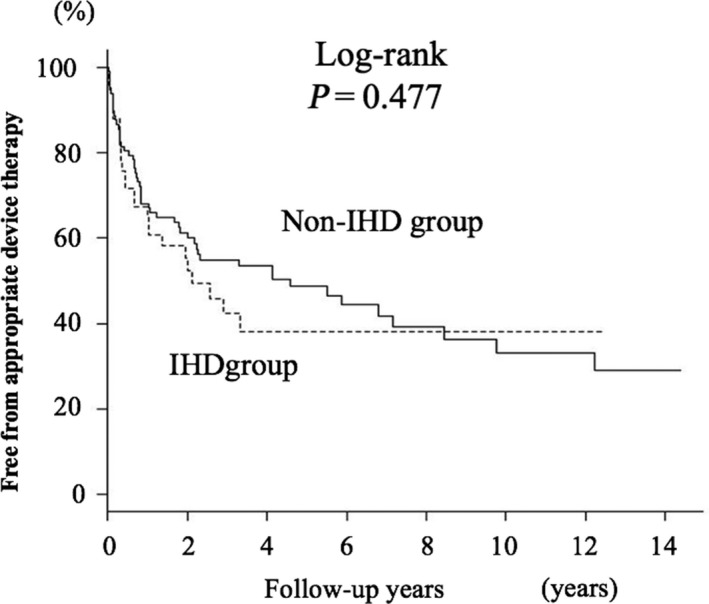
Kaplan‐Meier survival curve for being free from appropriate ICD/CRT‐D therapy according to the type of underlying cardiac etiology. There was no significant difference between patients with IHD and patients with non‐IHD
Figure 3.
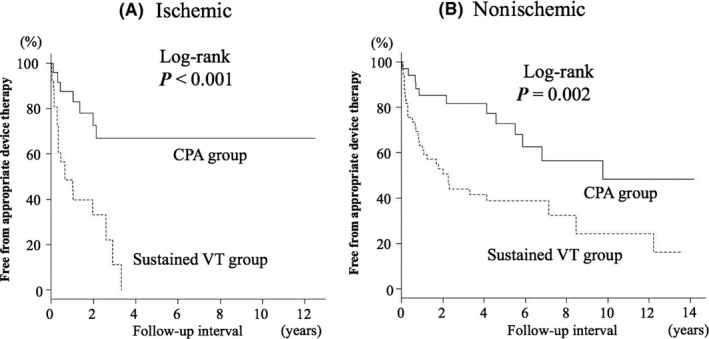
Kaplan‐Meier survival curve for being free from appropriate ICD/CRT‐D therapy according to the presenting arrhythmia before implantation in patients with IHD (A) and patients with non‐IHD (B). The probability of appropriate device therapy was significantly higher in patients with VT than in patients with CPA for both etiologies
Figure 4 shows Kaplan‐Meier curves for freedom from the secondary endpoint (i.e., appropriate therapy or all‐cause death) in the entire cohort (Figure 4A) and in groups of patients with CPA or VT (Figure 4B) before ICD/CRT‐D implantation. Kaplan‐Meier curve analysis showed that the secondary endpoint, like the primary endpoint, was less frequent in patients with CPA than in patients with VT.
Figure 4.
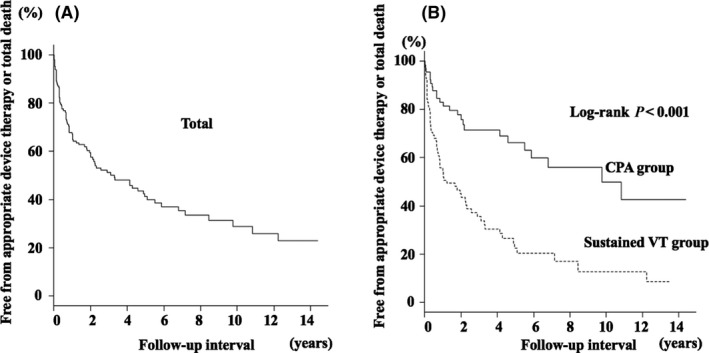
A, Kaplan‐Meier survival curve for being free from cardiac death and/or appropriate ICD/CRT‐D therapy. B, Kaplan‐Meier survival curve for being free from cardiac death and/or appropriate ICD/CRT‐D therapy according to the index arrhythmia between patients with sustained VT and CPA
4. DISCUSSION
4.1. Rates of appropriate ICD/CRT‐D therapy during follow‐up for secondary prevention
It is not clear whether the proportion of patients who receive appropriate ICD/CRT‐D therapy during follow‐up differs depending on the etiology of the underlying heart disease. Several groups of investigators have reported rates of appropriate therapy in patients with ICD implantation for secondary prevention of SCD, but in most of those earlier studies conducted in Western countries, the underlying heart disease in the majority of patients was IHD. In a study by Borleffs et al,8 the rates of appropriate ICD therapy were 52% at 5 years and 61% at 8 years after ICD implantation in patients with IHD. Similar results were reported by Schaer et al9 for patients with heart diseases, 83% of whom had IHD and 17% of whom had cardiomyopathy. In their studies, the rates of appropriate ICD therapy were 59% at 5 years and 65% at 10 years. In Japan, the proportion of patients with IHD in patients implanted with an ICD/CRT‐D was reported to be 31%, and cardiomyopathy and valvular heart disease were etiologies of the heart disease in 66% and 3% of the patients, respectively.13 In the present study, rates of appropriate ICD/CRT‐D therapy were 49% at 3 years and 54% at 5 years (Figure 1), and the rates are similar to the rates in earlier studies despite the difference in the proportion of patients with IHD in the study subjects.8, 9, 10, 11 It is notable that there was no significant difference between the rates of appropriate therapy in patients with IHD and patients with non‐IHD SHD in the present study (Figure 2). Taken together, the findings suggest that the rate of appropriate ICD/CRT‐D therapy for secondary prevention of SCD in Japanese patients is comparable with the rates in the other ethnic groups and that IHD and cardiomyopathy in ICD/CRT‐D therapy recipients have similar impacts on the rate of appropriate therapy.
4.2. Comparison of rates of appropriate therapy in patients with ischemic and nonischemic cardiomyopathy
ICD therapy is effective for primary prevention and secondary prevention of SCD in patients with ischemic heart disease.1, 2, 3, 5, 7 However, conclusive evidence of a survival benefit in patients with nonischemic cardiomyopathy has not been obtained.17 Although favorable data for mortality have not been obtained, our study showed comparable cumulative rates of device therapy in both etiologies, indicating a clinical benefit of ICD/CRT‐D therapy at least in terms of control of ventricular arrhythmia in cardiomyopathy patients. Data reported in the literature are not sufficient for drawing a conclusion regarding a difference between rates of device therapy in patients with ischemic cardiomyopathy and patients with nonischemic cardiomyopathy. However, results of three previous studies18, 19, 20 are consistent with the results of the present study. Darma et al18 compared the rates of appropriate therapy in patients with ischemic heart disease and patients with nonischemic heart disease, including 26% of patients who had an ICD/CRT‐D implantation for secondary prevention. Rates of appropriate therapy in the two groups were comparable during a mean follow‐up period of 19 ± 9 months (29.4% vs 27.0%). Boule et al19 enrolled 239 patients with ICD/CRT‐D for secondary prevention, 28% of whom had nonischemic cardiomyopathy, and they found that IHD was not a significant predictor of appropriate device therapy (hazard ratio, 1.07; confidence interval, 0.73‐1.56). Recurrence rates of ventricular arrhythmia in patients who underwent ICD/CRT‐D implantation were comparable in patients with IHD and patients with nonischemic heart disease.18, 19, 20 Thus, despite a lack of evidence provided by randomized trials, circumstantial evidence to date supports the notion that patients with nonischemic cardiomyopathy also receive benefits of ICD/CRT‐D implantation in terms of control of ventricular arrhythmia.
4.3. Rates of appropriate ICD/CRT‐D therapy: patients with prior sustained VT vs patients with prior CPA
A difference in the rates of appropriate ICD therapy in patients with different clinical backgrounds was reported by two groups of investigators. Borleffs et al8 assessed the rate of ICD therapy for potentially life‐threatening ventricular arrhythmia (>188 bpm) in patients with an ICD implanted for secondary prevention. They found that VT as the presenting arrhythmia was an independent predictor of appropriate therapy, and its hazard ratio was 1.51 compared with VF as the presenting arrhythmia. In a study by Schaer et al,9 patients were divided into VT and VF groups according to the presenting arrhythmias before ICD implantation. During a mean follow‐up period of 6.8 ± 4.4 years, 64% of the patients in the VT group and 44% of the patients in the VF group experienced appropriate ICD therapy, and the hazard ratio for appropriate therapy in the VT group was 1.45. In the present study, the cumulative probability of ICD/CRT‐D therapy was significantly different between patients with sustained VT and CPA (70% vs 34% at 5 years after implantation), and the hazard ratio for appropriate ICD/CRT‐D therapy was 2.8. The hazard ratio for VT prior to ICD implantation in the present study is consistent with those in earlier studies,8, 9 and the consistency supports the notion that the recurrence rate of life‐threatening arrhythmias after ICD implantation is higher in patients with prior VT than in patients with prior VF.
Why rates of appropriate therapy differed depending on indications of ICD implantation, that is, VT vs CPA, in the present and earlier studies is unclear. However, a possible explanation is a stable reentrant substrate in patients with monomorphic VT compared with the substrate in patients with VF. Reentrant ventricular arrhythmia is usually triggered by premature ventricular contraction penetrating into the circuit, and once it has been initiated on a stable circuit, it would sustain for long enough to cause appropriate device therapy. Higher stability of the reentrant substrate may ensure higher reproducibility of ventricular arrhythmia, leading to a higher recurrence rate in the VT group. In fact, ATP therapy was significantly frequent in VT group than CPA group in our analysis. Similarly, higher stability of the substrate and higher reproducibility of ventricular arrhythmia in patients with VT than in those with VF have been demonstrated in previous studies using electrophysiological induction tests and follow‐up data for ventricular arrhythmia stored in an ICD.21, 22, 23, 24, 25, 26 Adhar et al23 performed an electrophysiological study in survivors of cardiac arrest and patients with sustained VT not associated with cardiac arrest. In the cardiac arrest group, VT was induced in 30% and VF in 25% of the patients; however, VT was induced in 69% and VF only in 3% of the patients in the VT group, suggesting higher reproducibility of VT induction in the VT group. Furthermore, the mean cycle length of the induced VT was significantly shorter and most of the induced VT was polymorphic in the cardiac arrest group, whereas most of the induced VT was stable monomorphic type in the VT group, suggesting higher stability of the substrate in the VT group. Raitt et al25 assessed 111 patients, including 55 with only VF and 56 with monomorphic VT, who had ICD implantation for secondary prevention. During a follow‐up period of 14 months, monomorphic VT was detected in 18% of patients in the VF group and in 54% of patients in the VT group. Spontaneous VF was detected in 11% of patients in the VF group but not in any of the patients in the VT group. The mean cycle length of the recurring VT was significantly longer (314 ms vs 279 ms), and the number of VT episodes was greater (20 ± 31 vs 7 ± 7) in the VT group. They also performed an electrophysiological study and found that VT was more frequently induced in patients with VT than in patients with VF (75% vs 25%). These data suggest that there are significant differences in the electrophysiological property of ventricular arrhythmias between patients who present with hemodynamically well‐tolerated VT and survivors of out‐of‐hospital cardiac arrest. Nevertheless, ICD/CRT‐D implantation for VT is associated with a higher rate of recurrence of ventricular arrhythmia, requiring careful medical management.
4.4. Study limitations
There are several limitations in this study. First, the possibility that initial ventricular arrhythmia was fast VT rather than VF, deteriorating into VF before the first documentation by an electrocardiogram in some cases in the CPA group, cannot be excluded as patients with CPA were defined on the basis of VF documented at the time of resuscitation. Electrophysiological study was not performed in most cases to reproduce the initial ventricular arrhythmia. Second, we could not strictly assess the relationship between the index arrhythmia and type of recurrent arrhythmia. Data for the type of recurrent ventricular arrhythmia (VT or VF) and its cycle length during the follow‐up period were not available, and whether appropriate therapies were performed for VF or VT was also unclear though data for the type of appropriate therapy were available in most of the cases. Third, some selection bias was present in patient enrollment in the present study. Our institute is a tertiary medical center, and most of the patients were referred from affiliated hospitals. Although a single first episode of VF is sufficient evidence for indication of ICD therapy, patients who developed monomorphic VT for the first time might have been initially assigned to medical therapy in the affiliated hospitals, being not referred to our institute. Forth, the impact of class I AAD on the rate of ICD/CRT‐D therapy could not be determined because of retrospective nature of this study design. Patients with appropriate ICD/CRT‐D therapy were more frequently treated with class I AAD than those without appropriate therapy. We speculate that the difference in the frequency of class I AAD was result of selection bias due to preferable use of the AAD in those with high‐risk sign such as high burden of nonsustained VT at the time of device implantation. However, we cannot exclude the possibility that class I AAD itself causally related with increase in appropriate therapy. Fifth, ICD/CRT‐D programming was different between CPA and VT group, that is, VF one zone in the CPA group vs VF + VT zone in the VT group. This difference might have contributed to the lower rate of appropriate therapy in the CPA group, but it is unlikely to affect the overall result of this study because only 3 patients in the CPA group had an episode of nonsustained VT which lasted more than 20 beats.
5. CONCLUSIONS
Patients who underwent ICD/CRT‐D implantation due to prior sustained VT have significantly more frequent appropriate ICD/CRT‐D therapies than do patients with CPA. This finding is observed regardless of the cardiac disease etiology.
DISCLOSURE
The protocol for this research project has been approved by a suitably constituted Ethics Committee of the institution and it conforms to the provisions of the Declaration of Helsinki and Committee of Sapporo Medical University Clinical trial center, Approval No. 282‐62.
CONFLICT OF INTEREST
Authors declare no conflict of interests for this article.
Nagahara D, Fujito T, Mochizuki A, Shimoshige S, Hashimoto A, Miura T. Predictors of appropriate ICD therapy in Japanese patients with structural heart diseases: A major role of prior sustained ventricular tachycardia in secondary prevention. J Arrhythmia. 2018;34:527–535. 10.1002/joa3.12086
REFERENCES
- 1. The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators . A comparison of antiarrhythmic‐drug therapy with implantable defibrillators in patients resuscitated from near‐fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576–83. [DOI] [PubMed] [Google Scholar]
- 2. Kuck KH, Cappato R, Siebels J, Rüppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: the Cardiac Arrest Study Hamburg (CASH). Circulation. 2000;102:748–54. [DOI] [PubMed] [Google Scholar]
- 3. Connolly SJ, Gent M, Roberts RS, et al. Canadian implantable defibrillator study (CIDS): a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation. 2000;101:1297–302. [DOI] [PubMed] [Google Scholar]
- 4. Sabbag A, Suleiman M, Laish‐Farkash A, et al. Contemporary rates of appropriate shock therapy in patients who receive implantable device therapy in a real‐world setting: from the Israeli ICD Registry. Heart Rhythm. 2015;12:2426–33. [DOI] [PubMed] [Google Scholar]
- 5. Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. [DOI] [PubMed] [Google Scholar]
- 6. Satake H, Fukuda K, Sakata Y, et al. Current status of primary prevention of sudden cardiac death with implantable cardioverter defibrillator in patients with chronic heart failure–a report from the CHART‐2 Study. Circ J. 2015;79:381–90. [DOI] [PubMed] [Google Scholar]
- 7. Betts TR, Sadarmin PP, Tomlinson DR, et al. Absolute risk reduction in total mortality with implantable cardioverter defibrillators: analysis of primary and secondary prevention trial data to aid risk/benefit analysis. Europace. 2013;15:813–9. [DOI] [PubMed] [Google Scholar]
- 8. Borleffs CJ, van Erven L, Schotman M, et al. Recurrence of ventricular arrhythmias in ischemic secondary prevention implantable cardioverter defibrillator recipients: long‐term follow‐up of the Leiden out‐of‐hospital cardiac arrest study (LOHCAT). Eur Heart J. 2009;30:1621–6. [DOI] [PubMed] [Google Scholar]
- 9. Schaer B, Kühne M, Reichlin T, Osswald S, Sticherling C. Incidence of and predictors for appropriate implantable cardioverter‐defibrillator therapy in patients with a secondary preventive implantable cardioverter‐defibrillator indication. Europace. 2016;18:227–31. [DOI] [PubMed] [Google Scholar]
- 10. van Welsenes GH, van Rees JB, Borleffs CJ, et al. Long‐term follow‐up of primary and secondary prevention implantable cardioverter defibrillator patients. Europace. 2011;13:389–94. [DOI] [PubMed] [Google Scholar]
- 11. Germano JJ, Reynolds M, Essebag V, Josephson ME. Frequency and causes of implantable cardioverter‐defibrillator therapies: is device therapy proarrhythmic? Am J Cardiol. 2006;97:1255–61. [DOI] [PubMed] [Google Scholar]
- 12. Rahmawati A, Chishaki A, Ohkusa T, et al. Influence of primary and secondary prevention indications on anxiety about the implantable cardioverter‐defibrillator. J Arrhythmia. 2016;32:102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Watanabe E, Okajima K, Shimane A, et al. Inappropriate implantable cardioverter defibrillator shocks‐incidence, effect, and implications for driver licensing. J Interv Card Electrophysiol. 2017;49:271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Watanabe E, Kasai A, Fujii E, Yamashiro K, Brugada P. Reliability of implantable cardioverter defibrillator home monitoring in forecasting the need for regular office visits, and patient perspective. Japanese HOME‐ICD study. Circ J. 2013;77:2704–11. [DOI] [PubMed] [Google Scholar]
- 15. Noda T, Kurita T, Nitta T, et al. Appropriate duration of driving restrictions after inappropriate therapy from implantable cardiac shock devices‐interim analysis of the Nippon Storm Study. Circ J. 2014;78:1989–91. [DOI] [PubMed] [Google Scholar]
- 16. JCS Joint Working Group . Guidelines for non‐pharmacotherapy of cardiac arrhythmias (JCS 2011). Circ J. 2013;77:249–74. [DOI] [PubMed] [Google Scholar]
- 17. Desai AS, Fang JC, Maisel WH, Baughman KL. Implantable defibrillators for the prevention of mortality in patients with non‐ischemic cardiomyopathy: a meta‐analysis of randomized controlled trials. JAMA. 2004;292:2874–9. [DOI] [PubMed] [Google Scholar]
- 18. Darma A, Nedios S, Kosiuk J, et al. Differences in predictors of implantable cardioverter‐defibrillator therapies in patients with ischaemic and non‐ischaemic cardiomyopathies. Europace. 2016;18:405–12. [DOI] [PubMed] [Google Scholar]
- 19. Boulé S, Sémichon M, Guédon‐Moreau L, et al. Long‐term outcome of implantable cardioverter‐defibrillator implantation in secondary prevention of sudden cardiac death. Arch Cardiovasc Dis. 2016;109:517–26. [DOI] [PubMed] [Google Scholar]
- 20. Park KH, Lee CH, Jung BC, et al. Effectiveness of implantable cardioverter‐defibrillator therapy for heart failure patients according to ischemic or non‐ischemic etiology in Korea. Korean Circ J. 2017;47:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rüppel R, Schlüter CA, Boczor S, et al. Ventricular tachycardia during follow‐up in patients resuscitated from ventricular fibrillation: experience from stored electrograms of implantable cardioverter‐defibrillators. J Am Coll Cardiol. 1998;32:1724–30. [DOI] [PubMed] [Google Scholar]
- 22. Stevenson WG, Brugada P, Waldecker B, Zehender M, Wellens HJ. Clinical, angiographic, and electrophysiologic findings in patients with aborted sudden death as compared with patients with sustained ventricular tachycardia after myocardial infarction. Circulation. 1985;71:1146–52. [DOI] [PubMed] [Google Scholar]
- 23. Adhar GC, Larson LW, Bardy GH, Greene HL. Sustained ventricular arrhythmias: differences between survivors of cardiac arrest and patients with recurrent sustained ventricular tachycardia. J Am Coll Cardiol. 1988;12:159–65. [DOI] [PubMed] [Google Scholar]
- 24. Fogoros RN, Elson JJ, Bonnet CA, Fiedler SB, Chenarides JG. Long‐term outcome of survivors of cardiac arrest whose therapy is guided by electrophysiologic testing. J Am Coll Cardiol. 1992;19:780–8. [DOI] [PubMed] [Google Scholar]
- 25. Raitt MH, Dolack GL, Kudenchuk PJ, Poole JE, Bardy GH. Ventricular arrhythmias detected after transvenous defibrillator implantation in patients with a clinical history of only ventricular fibrillation. Implications for use of implantable defibrillator. J Am Coll Cardiol. 1991;18:1711–9. [DOI] [PubMed] [Google Scholar]
- 26. Gillis AM, Sheldon RS, Wyse DG, et al. Long‐term reproducibility of ventricular tachycardia induction in patients with implantable cardioverter/defibrillators. Serial noninvasive studies. Circulation. 1995;91:1996–2001. [DOI] [PubMed] [Google Scholar]


