Abstract
Background
Although numerous prescription drugs are available to treat Parkinson's disease (PD), little is known about national use in clinical practice and which factors may influence variability in care. The objectives of this study were to describe the prevalence of anti‐Parkinson drug use among Medicare beneficiaries with PD and to identify demographic and clinical factors associated with drug use.
Methods
This retrospective study was based on a random sample of annual 5% Medicare Part A and B claims linked with Medicare Part D drug files from 2007 through 2010. The study sample included fee‐for‐service Medicare beneficiaries with continuous stand‐alone Part D enrollment who had been diagnosed with PD in the given year. First, any PD drug use and drug use by class (levodopa, dopamine agonist, anticholinerigc, monoamine oxidase B inhibitors, catechol‐O‐methyltransferase inhibitors, and amantadine) were described. Using generalized estimating equation regressions, patient and provider characteristics associated with anti‐Parkinson drug use and choice were examined.
Results
Over 81% of patients with PD were treated with anti‐Parkinson drugs, and this proportion was stable over the 4 years of the study. The majority were treated with levodopa (90%); followed by dopamine agonists (29–31%); then monoamine oxidase B inhibitors, anticholinergics, amantadine, and catechol‐O‐methyltransferase inhibitors (all between 5% and 11%). Holding all else equal, patients who were not seen by a neurologist (odds ratio, 0.41; 95% confidence interval, 0.38–0.44; P < 0.001) and African‐American patients (odds ratio, 0.80; 95% confidence interval, 0.69–0.93; P = 0.003) were significantly less likely to be treated.
Conclusions
Among a national sample of Medicare beneficiaries with PD, the majority received anti‐Parkinson drugs. However, there was relative under‐treatment of African‐Americans and patients who were not seen by a neurologist for care.
Keywords: Parkinson's disease/parkinsonism, medications, outcomes research, Medicare
Parkinson's disease (PD) affects at least 1 million people in the United States and increases both their morbidity and their mortality.1 However, effective symptomatic treatment exists. Under‐utilization of appropriate pharmacologic treatment is associated with greater disability, decreased survival, and lowered health‐related quality of life.2
Evidence‐based reviews of PD management recommend treatment with levodopa (l‐dopa), dopamine agonists, monoamine oxidase B (MAOB) inhibitors, catechol‐O‐methyltransferase inhibitors (COMTIs), and amantadine to improve clinical outcomes.3, 4, 5, 6 However, with incident disease, only from one‐third to two‐thirds of patients with PD start therapy.7, 8, 9 In those with moderate to advanced disease, while most patients should be on anti‐Parkinson drugs, medication management varies widely, and there is no clear consensus on the optimal regimen.
The choice and utilization of anti‐Parkinson drugs may vary for numerous reasons unrelated to clinical factors. Researchers have documented that patient characteristics, such as race and socioeconomic status, are independently associated with treatment differences in numerous conditions.10 There is preliminary evidence that this may be true in PD as well.8 Provider characteristics, such as specialty type, may also contribute to the choice and use of anti‐Parkinson drugs. Care provided by neurologists for PD is associated with improved survival compared with primary care providers; however, women and minorities are less likely to be seen by neurologists.11 It remains unknown whether at least some differences in health outcomes are attributable to different patterns of medications prescribed to patients.
In 2006, the US government introduced Medicare Part D, which is the prescription drug benefit program to increase access to prescription drugs for adults over the age of 65 years and for those who are severely disabled. However, little has been published about the prevalence and correlates of anti‐Parkinson drug use in this population, which has the highest prevalence of PD.12 The purpose of this study was to estimate the prevalence of anti‐Parkinson drug use and choice among Medicare beneficiaries and to identify patient and provider characteristics associated with drug use and choice in order to elucidate potential mechanisms of high‐quality care and disparities in treatment.
Patients and Methods
Study Design, Data Source, and Sample
This was a retrospective analysis of prevalent cases of PD using annual cross‐sections of Medicare beneficiaries from 2007 to 2010. Medicare is a US government‐financed health insurance program for the elderly and disabled. It supports the care of 98% of Americans older than 65 years of age. The data source for the study was the 5% Medicare Chronic Condition Warehouse, which includes Medicare inpatient, outpatient, and carrier claims files; Medicare Part D prescription drug‐event and plan characteristics files; and a personal summary file with demographic and enrollment information for a 5% random sample of Medicare beneficiaries. The study sample included fee‐for‐service Medicare beneficiaries who had at least 12 months of continuous, stand‐alone Part D enrollment and a diagnosis of PD in the given year. To be considered a PD case, beneficiaries must have had at least 1 Medicare‐reimbursed inpatient claim (as either the primary or secondary diagnosis) or 2 outpatient claims (physician visits only) for PD (International Classification of Diseases, 9th Revision [ICD‐9], code 332.0). We excluded individuals who were enrolled in a health maintenance organization due to incomplete data availability. There was no requirement that these claims had to be separated by a minimum time frame. This method optimizes sensitivity (range, 64–89%) over specificity (range, 28–99%) based on prior studies that have evaluated the diagnostic accuracy of administrative claims compared with the gold standard of either self‐reported diagnoses or review of clinical information.13, 14, 15, 16 However, to further improve the specificity of our case definition, and to decrease the risk of misclassification of secondary parkinsonian syndromes in our cohort, we also excluded individuals with a history of schizophrenia (ICD 295) or secondary/atypical parkinsonism (ICD codes 332.1 and 333.X). Similar case ascertainment methods have been used in previous health care utilization studies of Medicare populations.11, 12
Dependent Variables
First, we examined the use of any anti‐Parkinson drug in each year. We defined any anti‐Parkinson drug use as having 1 or more prescription claims for at least 1 PD medication for greater than 30 days. Only anti‐Parkinson drugs approved by the US Food and Drug Administration for the treatment of PD were included. Next, we categorized anti‐Parkinson drug use into the following classes: (1) l‐dopa, (2) dopamine agonists, (3) anticholinergics, (4) MAOB inhibitors, (5) COMTIs, and (6) amantadine. Finally, we counted the number of unique anti‐Parkinson drugs (individual agents) that were used per year by each patient.
Independent Variables
Patient characteristics, including age, sex, race and ethnicity, Part D low‐income subsidy status, and county of residence, were abstracted from the Medicare files. We extrapolated measures of county‐level socioeconomic status, such as education, income, and unemployment, from the Area Resource File. County‐level estimates of neurologist availability (the number of neurologists per 100,000 nondisabled Medicare beneficiaries) were generated using Medicare physician data and beneficiary summary file data. Measures of PD‐related comorbidities that could affect PD medication utilization and/or choice were recorded based on ICD‐9 codes for dementia, anxiety, depression, psychosis/hallucinations, falls, fractures, leg edema, orthostatic hypotension and syncope, and deep brain stimulation implantation. In addition, the burden of overall non‐PD–related comorbidity was measured using the prescription drug hierarchical condition category (RxHCC) risk score. This risk score is created using the Center for Medicare and Medicaid Services’ RxHCC model, which is designed to determine payment for prescription drugs under Part D and is based on the presence of 197 medical conditions recorded on Medicare diagnostic claims.17 Finally, the specialty of the treating provider was abstracted based on Medicare provider specialty codes and was categorized as neurologist or nonneurologist.
Statistical Analysis
The prevalence of any anti‐Parkinson drug use and the number of unique anti‐Parkinson drugs used were described individually and according to different drug classes. Generalized estimating equation logistic regression models were used to account for clustering of patients across multiple years and to estimate the adjusted association between patient and provider characteristics and the following variables: (1) any anti‐Parkinson drug use, (2) the number of unique PD drugs used (ordinal logistic regression), and (3) the type of anti‐Parkinson drug class used. Bonferroni correction was used to adjust for the multiple comparisons made across the 6 different regression models of anti‐Parkinson drug class.18 A P < 0.0083 was used as the threshold for statistical significance. Coefficients are reported as odds ratios (OR) with 95% confidence intervals (CIs) and 2‐sided P values. All analyses were performed using the SAS software package (SAS Institute, Inc., Cary, NC).
Ethics
The Institutional Review Board at the University of Pennsylvania approved the study protocol with a waiver of written informed consent.
Results
For the years from 2007 through 2010, the sample sizes ranged from 9482 to 9626 individuals. This represents a random sample from almost 200,000 Medicare beneficiaries with PD in stand‐alone Part D plans in each year. Table 1 describes the sample according to demographic and clinical characteristics for each annual cohort. The majority of patients with PD were white (range, 87.2–88.1%), and African Americans represented from 5.3% to 5.8% of the cohort. Approximately 60% of all patients were seen by neurologists during the year.
Table 1.
Demographic and clinical characteristics of patients with Parkinson's disease from a 5% Medicare sample, 2007–2010
| Characteristic | Year | |||
|---|---|---|---|---|
| 2007 | 2008 | 2009 | 2010 | |
| Sample size, no. | 9482 | 9626 | 9566 | 9503 |
| Demographics | ||||
| Age, % | ||||
| <65 y | 5.3 | 5.0 | 5.4 | 5.9 |
| 65–69 y | 7.8 | 7.7 | 7.8 | 8.3 |
| 70–74 y | 13.9 | 14.5 | 14.6 | 13.9 |
| 75–79 y | 19.8 | 19.5 | 19.3 | 20.1 |
| 80–84 y | 24.5 | 24.8 | 23.7 | 23.2 |
| >85 y | 28.7 | 28.5 | 29.1 | 28.7 |
| Sex: Men, % | 39.0 | 39.5 | 40.3 | 41.6 |
| Race, % | ||||
| White | 88.1 | 88.1 | 87.7 | 87.2 |
| African‐American | 5.6 | 5.3 | 5.4 | 5.8 |
| Hispanic | 2.7 | 2.6 | 2.9 | 2.8 |
| Other | 3.7 | 4.0 | 4.0 | 4.2 |
| Low‐income subsidy (LIS), % | ||||
| Full | 45.6 | 44.2 | 43.8 | 43.5 |
| Partial | 0.9 | 0.9 | 0.9 | 0.9 |
| Non‐LIS | 50.4 | 52.1 | 52.4 | 52.9 |
| County‐level socioeconomic status measures | ||||
| Education: less than high school, % | 14.3 | 13.9 | 13.9 | 13.9 |
| Median income, US$ | 34,921 | 36,665 | 36,574 | 37,352 |
| Unemployment, % | 4.8 | 5.9 | 9.4 | 9.7 |
| County‐level measure of access to neurologists | ||||
| No. of neurologists in county per 100,000 population | 69.8 | 70.0 | 69.6 | 71.9 |
| PD‐related comorbidity, % | ||||
| Anxiety | 16.7 | 18.6 | 19.8 | 20.0 |
| Depression | 30.5 | 32.4 | 32.4 | 32.9 |
| Psychosis or hallucinations | 18.9 | 19.0 | 19.4 | 19.0 |
| Dementia | 11.2 | 12.2 | 12.9 | 13.2 |
| Falls | 5.8 | 6.0 | 9.8 | 10.9 |
| Fractures | 9.3 | 8.5 | 8.4 | 7.7 |
| Deep brain stimulation | 1.3 | 1.3 | 1.8 | 2.0 |
| Leg edema | 18.8 | 19.2 | 19.8 | 20.7 |
| Orthostatic hypotension | 11.7 | 12.1 | 12.0 | 12.9 |
| Syncope | 12.1 | 12.6 | 12.2 | 12.5 |
| Other comorbidities | ||||
| RxHcc, mean | 1.19 | 1.22 | 1.22 | 1.24 |
| Measure of specialty care | ||||
| Sees neurologist, % | 58.8 | 60.3 | 60.5 | 61.7 |
PD, Parkinson's disease; RxHcc, prescription drug hierarchical condition category.
The majority of PD patients (range, 81–82%) were prescribed at least 1 anti‐Parkinson drug each year. On average, individuals with PD were on from 1.55 to 1.59 unique anti‐Parkinson drugs. Figure 1 shows the proportion of patients who were on 1, 2, 3, 4, or ≥5 unique anti‐Parkinson drugs. Of the treated patients, 90% were on l‐dopa therapy. This was followed by therapy with dopamine agonists (29–31%), MAOB inhibitors (9–11%), amantadine (7–8%), COMTIs (6–8%), and anticholinergics (5–6%) (Table 2).
Figure 1.
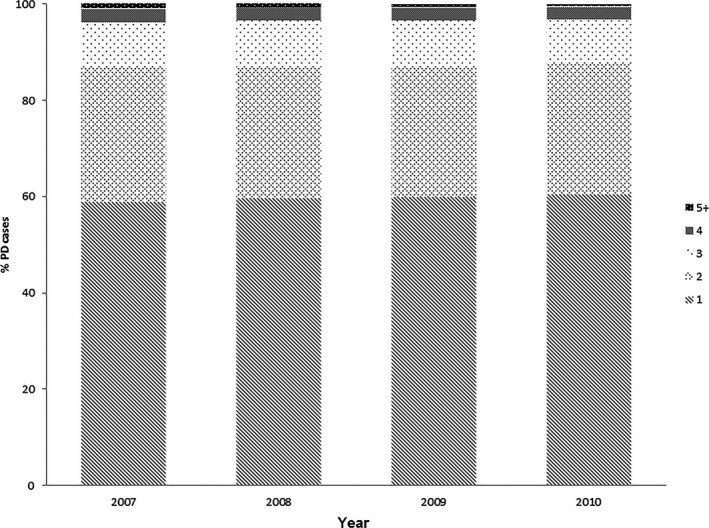
Distribution of the number of unique anti‐Parkinson drugs used per year.
Table 2.
Patterns of anti‐Parkinson drug use and choice among Medicare beneficiaries, 2007–2010
| Variable | Year | |||
|---|---|---|---|---|
| 2007 | 2008 | 2009 | 2010 | |
| Total no. of patients with PD cases | 9482 | 9626 | 9566 | 9503 |
| No. of anti‐Parkinson drug users | 7721 | 7872 | 7835 | 7725 |
| Use of any anti‐Parkinson drug, % | 81 | 82 | 82 | 81 |
| Levodopa use, % | 90 | 90 | 90 | 90 |
| Dopamine agonist use, % | 31 | 31 | 30 | 29 |
| MAOB inhibitor use, % | 9 | 9 | 10 | 11 |
| Anticholinergic use, % | 6 | 6 | 6 | 5 |
| Amantadine use, % | 8 | 7 | 7 | 7 |
| COMT inhibitor use, % | 8 | 7 | 7 | 6 |
PD, Parkinson's disease; MAOB, monoamine oxidase‐B; COMT, catechol‐O‐methyltransferase.
In multivariate regression analyses controlling for patient demographics, county‐level socioeconomic status measures, specialty data, overall and PD‐related comorbidity, being seen by a neurologist for care (OR, 2.42; 95% CI, 2.26–2.60) was significantly associated with an increased odds of anti‐Parkinson drug use, whereas greater overall comorbidity was associated with a decreased odds of anti‐Parkinson drug use (OR, 0.54; 95% CI, 0.50–0.59). Being seen by a neurologist also was significantly associated with being treated with multiple anti‐Parkinson drugs (OR, 2.40; 95% CI, 2.33–2.57), and greater overall comorbidity was associated with a decreased odds of treatment with multiple anti‐Parkinson drugs (OR, 0.52; 95% CI, 0.49–0.57). Overall, younger patients were more likely to be on multiple PD medications compared with older patients. In addition, nonclinical factors, including African‐American race (with white race as the reference category; OR, 0.80; 95% CI, 0.69–0.93), and partial low‐income subsidy (with full low‐income subsidy as the reference category; OR, 0.61; 95% CI, 0.44–0.85), were associated with a significantly decreased odds of any anti‐Parkinson drug use. African‐American race was also associated with decreased odds of being on multiple anti‐Parkinson drugs compared with whites (OR, 0.69; 95% CI, 0.59–0.80). Although there was no significant association between sex and any anti‐Parkinson drug use (OR, 1.03; 95% CI, 0.95–1.10), among those who were treated, women were significantly less likely to be on multiple anti‐Parkinson drugs (OR, 0.91; 95% CI, 0.85–0.98) (Table 3).
Table 3.
Independent predictors of any anti‐Parkinson drug use and the number of unique anti‐Parkinson drugs useda
| Covariate | Any anti‐Parkinson drug use | Greater no. of unique anti‐Parkinson drugs used | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Demographics | ||||||
| Age, y | ||||||
| 65–69 (Ref) | ||||||
| <65 | 0.81 | 0.68–0.97 | 0.025 | 1.20 | 1.02–1.41 | 0.029 |
| 70–74 | 1.09 | 0.94–1.26 | 0.261 | 0.84 | 0.75–0.95 | 0.004 |
| 75–79 | 1.05 | 0.91–1.22 | 0.467 | 0.70 | 0.63–0.79 | <0.001 |
| 80–84 | 0.96 | 0.83–1.10 | 0.534 | 0.55 | 0.49–0.61 | <0.001 |
| ≥85 | 0.88 | 0.77–1.0 | 0.078 | 0.40 | 0.36–0.45 | <0.001 |
| Women | 1.03 | 0.95–1.10 | 0.500 | 0.91 | 0.85–0.98 | 0.008 |
| Race | ||||||
| White (Ref) | ||||||
| African‐American | 0.80 | 0.69–0.93 | 0.003 | 0.69 | 0.59–0.80 | <0.001 |
| Hispanic | 0.89 | 0.72–1.10 | 0.287 | 1.00 | 0.81–1.25 | 0.989 |
| Other | 1.11 | 0.92–1.35 | 0.280 | 1.32 | 1.10–1.58 | 0.003 |
| Low‐income subsidy (LIS) | ||||||
| Full LIS (Ref) | ||||||
| Non‐LIS only | 1.01 | 0.93–1.09 | 0.819 | 0.95 | 0.88–1.03 | 0.194 |
| Partial LIS | 0.61 | 0.44–0.85 | 0.003 | 0.75 | 0.53–1.05 | 0.090 |
| Other comorbidities | ||||||
| RxHcc | 0.54 | 0.50–0.59 | <0.001 | 0.53 | 0.49–0.57 | <0.001 |
| Measure of specialty care | ||||||
| Sees neurologist | 2.42 | 2.26–2.60 | <0.001 | 2.40 | 2.23–2057 | <0.001 |
Models control for all variables listed and for county‐level measures of socioeconomic status and access as well as PD‐related comorbidity.
OR, odds ratio; CI, confidence interval; Ref, reference category; RxHcc, prescription drug hierarchical condition category.
In a multivariate examination of specific drug class choice (Table S1), older age groups were associated with greater odds of being on l‐dopa and lower odds of receiving a dopamine agonist, an MAOB inhibitor, amantadine, or anticholinergic therapy. Patients with PD who were seen by a neurologist were significantly more likely to be on dopamine agonists, MAOB inhibitors, amantadine, or COMTIs, but not l‐dopa or anticholinergics. African Americans were significantly less likely to be treated with dopamine agonists (OR, 0.68; 95% CI, 0.57–0.81) than whites. Women had a significantly lower odds of being on l‐dopa (OR, 0.80; 95% CI, 0.71–0.89), MAOB inhibitors (OR, 0.76; 95% CI, 0.67–0.85,) and COMTIs (OR, 0.73; 95% CI, 0.64–0.84) than men.
Discussion
In a nationally representative sample of Medicare beneficiaries, most patients with PD were treated with anti‐Parkinson drugs, and the overwhelming majority was on l‐dopa. Overall, this reflects a cost‐sensitive and effective approach to treatment. However, several groups were identified that were vulnerable to disparate treatment: African Americans, women, and patients who were not seen by a neurologist. This suggests that there may be potential areas for continued improvement in the pharmacological management of patients with PD in clinical practice, as discussed below.
An older study that investigated the prevalence of anti‐Parkinson drug use in the United States, but was limited only to nursing home residents, found that only 44% of patients with PD were treated.19 A subsequent study using national survey data from the Medicare Current Beneficiary Survey (2000–2003) reported anti‐Parkinson drug use at a higher rate of 58%, but this was still lower than our results.20 However, the same study also noted that having prescription drug coverage was significantly associated with higher odds of anti‐Parkinson drug use. Several years later, our results indicating that from 81% to 82% of patients were treated might reflect several factors. Implementation of the Medicare Part D prescription drug program in 2006 has resulted in greater utilization of standard therapies for many common disorders (such as heart failure and diabetes). It is likely that our results also reflect an increase in access to medications for the PD population. In addition, earlier suspicions that anti‐Parkinson drug therapy might be harmful are being refuted. There is now increasing scientific evidence that PD medication therapy, particularly l‐dopa, is not toxic.21 Thus, there is growing comfort with its prescription by providers and acceptance by patients.
Our finding that l‐dopa is the most frequently prescribed anti‐Parkinson drug aligns with several other studies in different samples. Among incident cases of PD in the United States, l‐dopa is prescribed as initial therapy in greater than 70% of individuals.22, 23 In population‐based studies in Italy and France, the results are similar24, 25; and, in a sample of hospitalized patients with PD, 85% are treated with l‐dopa.26
However, our study findings highlight that racial disparities in PD treatment remain pervasive. In a study of state Medicaid claims, African Americans with incident PD were significantly less likely to be started on medication therapy than whites.8 Similarly, African‐American nursing home residents with PD are less likely to receive anti‐Parkinson drugs.19 African Americans with PD are also less likely to receive therapeutic surgery,27, 28, 29 receive appropriate management of comorbid depression,30 or be represented in relevant clinical trials.31 Our findings that African Americans are relatively undertreated for PD compared with whites is consistent with these earlier results. Although the prevalence and incidence of PD may be lower in those of African descent,32 1 study demonstrated that the response to standard therapy did not differ by race or ethnicity.33 Furthermore, reports indicate that African Americans with treated PD have greater disability and disease severity when they present for care at PD centers compared with whites.34, 35 This prior evidence suggests that the need for anti‐Parkinson drugs should be even greater among African Americans relative to whites. However, the extent to which our observed racial differences in treatment are due to clinical utility, physician preference/bias, or patient beliefs or adherence is unknown.
Women with PD were another group that experienced disparate treatment. They were less likely to be on multiple anti‐Parkinson drugs or to receive l‐dopa, MAOB, or COMTI therapy. This may reflect differences in clinical symptoms or response to medications. Overall, women with PD had fewer symptoms than men in 1 clinic‐based study36 although another report demonstrated that women had greater disability.37 This could translate into different types and amounts of anti‐Parkinson drug prescriptions; however, to our knowledge, there is no evidence that this practice results in improved health outcomes. In addition, there is some evidence that women with PD may be more likely to experience dyskinesias, although studies have been limited by small samples and multiple comparisons.37, 38 An increased risk of l‐dopa–induced dyskinesias may be related to the reduced metabolism of l‐dopa in individuals with lower body weight,39 which, in turn, may explain the lower likelihood for women in this sample to be treated with l‐dopa to limit side effects. Finally, it is possible that the disparate treatment observed is related to nonclinical factors, such as patient preference, physician bias, or other unmeasured socioeconomic confounders.
Another important finding from this study is that individuals with PD who were treated by a neurologist were significantly more likely to receive anti‐Parkinson drug therapy as well as more complicated medication regimens. There is evidence that neurological care in PD is associated with improved health outcomes.11 This raises the intriguing possibility that the improved outcomes seen with neurological care may be due at least in part to greater use of anti‐Parkinson drugs. Similarly, a study of US veterans demonstrated that movement disorder specialists were significantly more likely to adhere to various quality indicators in the management of PD compared with general neurologists and nonneurologists.40 These quality indicators included treatment of wearing‐off and assessments of falls, depression, and hallucinations. It is important to understand the sources of variability in processes of care and outcomes between specialists and nonspecialists so that we can implement clinical pathways or practice guidelines to improve the quality of care for all PD patients.
There were several limitations of this study. First, we relied on administrative claims to identify patients with PD. This method could lead to misclassification errors due to (1) inaccurate PD diagnosis, (2) missing PD cases, and (3) coding errors.16 Because our goal was to describe prescription drug utilization and patterns, we chose a PD case definition that optimized sensitivity first and then specificity. Prior studies have compared the accuracy of Medicare claims in the identification of PD; however, they employed slightly different methodologies.13, 15 Noyes and colleagues compared PD diagnosis by self‐report (“Have you ever been told you have Parkinson's disease?”) against physician‐only claims for ICD code 332.0 and found that the highest positive predictive value was 74%. However, in a smaller sample (N = 28), Jain and colleagues compared physician review of medical records against any 2 Medicare claims and found a lower positive predictive value at 48% (95% CI, 0.30–0.68). It is possible that the inclusion of nonphysician claims in that prior study led to a greater false‐positive rate. Taken together, it seems likely that there are still PD cases that are being missed when relying on diagnostic claims alone, which would lead to an underestimate of the number of detected cases of PD. It is also likely that cases identified as PD by their diagnostic code had another related condition (e.g., atypical or vascular parkinsonism) or no parkinsonism at all, which would bias our findings on the proportion of patients with PD who receive treatment. Furthermore, if misclassification of cases differed between groups (e.g., African Americans and whites), then it might confound observed differences. Also, the use of administrative data does not allow for the examination of more detailed clinical information, which could influence drug use and choice, such as motor disability or functional measures. However, we did control for possible PD‐related comorbidities captured in the data set, such as comorbid dementia or psychosis, in an attempt to account for clinical differences that may affect treatment. It is also important to note that these data only capture prescription fills, not what a provider prescribed or what a patient actually consumed, although, claims for prescription fills are widely used and validated measures of drug use in the literature.
This study provides an important description of anti‐Parkinson drug use among Medicare beneficiaries in real‐world clinical practice at a national level. We find that a large majority of patients with PD, when provided prescription drug coverage under Medicare Part D, receive treatment. However, several areas for continued improvement and investigation still remain. The need for and response to PD treatment in women and minorities requires much additional study to develop effective strategies that will improve the appropriate adoption of PD therapy in these groups. In addition, increased access to neurological expertise for patients with PD may raise the number of treated individuals. Public policy strategies to improve access to expert PD care include adoption of telehealth consultations; increasing the supply of neurologists, particularly in underserved areas; and providing financial incentives for the provision of high‐value, evidence‐based care.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
N.D.: 1A, 1B, 1C, 2A, 2C, 3A
A.W.W.: 1A, 1B, 2C, 3B
P.L.: 1B, 1C, 2A, 2B, 3B
J.A.D.: 1B, 1C, 2A, 2B, 2C, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position in issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: This study was supported by Teva Pharmaceuticals, the National Institute of Neurological Disease and Stroke (K23 NS081087; Allison W. Willis), and NIA K23 AG034236 (Nabila Dahodwala). The authors report no conflicts of interest.
Financial Disclosures for the previous 12 months: Nabila Dahodwala reports research support from the National Institutes of Health (NIH), the Parkinson Council, the National Parkinson Foundation, and Teva Pharmaceuticals and honoraria for speaking at the Movement Disorders Society meeting. Allison W. Willis is an associate editor of Pharmacoepidemiology and Drug Safety; she receives research support from the NIH. Pengxiang Li reports consulting fees from Avalon Health Economics, LLC, and Robert Oshfeldt, LLC. Jalpa A. Doshi reports personal/consulting fees from Alkermes Inc., Boehringer Ingelheim, Forest Laboratories, Ironwood Pharmaceuticals, Merck & Company Inc., and Shire; research support from Teva Pharmaceuticals, Amgen Inc., Humana, Merck & Company Inc., Pfizer Inc., Pharmaceutical Research and Manufacturers of America (PhRMA), and the National Pharmaceutical Council, all outside the submitted work; and holds stock in Merck & Company Inc., Pfizer Inc., and Gilead.
Supporting information
Table S1. Independent predictors of choice of drug class.
Acknowledgments
We thank Fei Fei Yang, Xinyan Yu, and Vrushabh Ladage for their invaluable technical assistance.
Supporting information may be found in the online version of this article.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007;68:384–386. [DOI] [PubMed] [Google Scholar]
- 2. Gallagher DA, Schrag A. Impact of newer pharmacological treatments on quality of life in patients with Parkinson's disease. CNS Drugs 2008;22:563–586. [DOI] [PubMed] [Google Scholar]
- 3. Miyasaki JM, Marting W, Suchowersky O, Weiner WJ, Lang AE. Practice parameter: initiation of treatment for Parkinson's disease: an evidence‐based reviews. Neurology 2002;58:11–17. [DOI] [PubMed] [Google Scholar]
- 4. Practice parameters: initial therapy of Parkinson's disease (summary statement). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 1993;43:1296–1297. [PubMed] [Google Scholar]
- 5. Pahwa R, Factor SA, Lyons KE, et al. Practice parameter: treatment of Parkinson's disease with motor fluctuations and dyskinesias (an evidence‐based review). Neurology 2006;66:983–995. [DOI] [PubMed] [Google Scholar]
- 6. Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA 2014;311:1670–1683. [DOI] [PubMed] [Google Scholar]
- 7. Grosset D, Taurah L, Burn DJ, et al. A multicentre longitudinal observational study of changes in self‐reported health status in people with Parkinson's disease left untreated at diagnosis. J Neurol Neurosurg Psychiatry 2007;78:465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dahodwala N, Xie M, Noll E, Siderowf A, Mandell DS. Treatment disparities in Parkinson's disease. Ann Neurol 2009;66:142–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Asimakopoulos P, Caslake R, Harris CE, Gordon JC, Taylor KS, Counsell C. Changes in quality of life in people with Parkinson's disease left untreated at diagnosis. J Neurol Neurosurg Psychiatry 2008;79:716–718. [DOI] [PubMed] [Google Scholar]
- 10. Geiger HJ. Racial and ethnic disparities in diagnosis and treatment: a review of the evidence and a consideration of causes In: Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Healthcare. Washington, DC: The National Academies Press; 2003:417–454. [PubMed] [Google Scholar]
- 11. Willis AW, Schootman M, Evanoff BA, Perlmutter JS, Racette BA. Neurologist care in Parkinson disease: a utilization, outcomes, and survival study. Neurology 2011;77:851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wei YJ, Palumbo FB, Simoni‐Wastila L, et al. Antiparkinson drug use and adherence in Medicare part D beneficiaries with Parkinson's disease. Clin Ther 2013;35:1513–1525.e1. [DOI] [PubMed] [Google Scholar]
- 13. Noyes K, Liu H, Holloway R, Dick AW. Accuracy of Medicare claims data in identifying parkinsonism cases: comparison with the Medicare current beneficiary survey. Mov Disord 2007;22:509–514. [DOI] [PubMed] [Google Scholar]
- 14. Butt DA, Tu K, Young J, et al. A validation study of administrative data algorithms to identify patients with parkinsonism with prevalence and incidence trends. Neuroepidemiology 2014;43:28–37. [DOI] [PubMed] [Google Scholar]
- 15. Jain S, Himali J, Beiser A, et al. Validation of secondary data sources to identify Parkinson disease against clinical diagnostic criteria. Am J Epidemiol 2015;18:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Szumski NR, Cheng EM. Optimizing algorithms to identify Parkinson's disease cases within an administrative database. Mov Disord 2009;24:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Robst J, Levy JM, Ingber MJ. Diagnosis‐based risk adjustment for Medicare prescription drug plan payments. Health Care Financ Rev 2007;28:15–30. [PMC free article] [PubMed] [Google Scholar]
- 18. Bonferroni CE. Teoria Statistica delle Classi e Calcolo delle Probabilita. Firenze, Italia: Libreria Internazionale Seeber; 1936. [Google Scholar]
- 19. Lapane KL, Fernandez HH, Friedman JH; the SAGE Study Group . Prevalence, clinical characteristics, and pharmacologic treatment of Parkinson's disease in residents in long‐term care facilities. Pharmacotherapy 1999;19:1321–1327. [DOI] [PubMed] [Google Scholar]
- 20. Wei YJ, Stuart B, Zuckerman IH. Use of antiparkinson medications among elderly Medicare beneficiaries with Parkinson's disease. Am J Geriatr Pharmacother 2010;8:384–394. [DOI] [PubMed] [Google Scholar]
- 21. Fahn S, Oakes D, Shoulson I, et al. Levodopa and the progression of Parkinson's disease. N Engl J Med 2004;351:2498–2508. [DOI] [PubMed] [Google Scholar]
- 22. Huse DM, Castelli‐Haley J, Orsini LS, Lenhart G, Abdalla JA. Patterns of initial pharmacotherapy for Parkinson's disease in the United States. J Geriatr Psychiatry Neurol 2006;19:91–97. [DOI] [PubMed] [Google Scholar]
- 23. Swarztrauber K, Koudelka C, Brodsky MA. Initial pharmacotherapy in a population of veterans with Parkinson disease. Neurology 2006;66:1425–1426. [DOI] [PubMed] [Google Scholar]
- 24. Trifiro G, Savica R, Morgante L, et al. Prescribing pattern of anti‐Parkinson drugs in Southern Italy: cross‐sectional analysis in the years 2003–2005. Parkinsonism Relat Disord 2008;14:420–425. [DOI] [PubMed] [Google Scholar]
- 25. Fayard C, Bonaventure A, Benatru I, et al. Impact of recommendations on the initial therapy of Parkinson's disease: a population‐based study in France. Parkinsonism Relat Disord 2011;17:543–546. [DOI] [PubMed] [Google Scholar]
- 26. Crispo JA, Fortin Y, Thibault DP, et al. Trends in inpatient antiparkinson drug use in the USA, 2001–2012. Eur J Clin Pharmacol 2015;71:1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eskandar EN, Flaherty A, Cosgrove GR, Shinbou LA, Barker FG. Surgery for Parkinson's disease in the United States, 1996 to 2000: practice patterns, short‐term outcomes, and hospital charges in a nationwide sample. J Neurosurg 2003;99:863–871. [DOI] [PubMed] [Google Scholar]
- 28. Willis AW, Schootman M, Kung N, Wang XY, Perlmutter JS, Racette BA. Disparities in deep brain stimulation surgery among insured elders with Parkinson disease. Neurology 2014;82:163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chan AK, McGovern RA, Brown LT, et al. Disparities in access to deep brain stimulation surgery for Parkinson disease: interaction between African American race and Medicaid use. JAMA Neurol 2014;71:291–299. [DOI] [PubMed] [Google Scholar]
- 30. Cheng EM, Siderowf AD, Swarztrauber K, et al. Disparities of care in veterans with Parkinson's disease. Parkinsonism Relat Disord 2008;14:8–14. [DOI] [PubMed] [Google Scholar]
- 31. Schneider MG, Swearingen CJ, Shulman LM, Ye J, Baumgarten M, Tilley BC. Minority enrollment in Parkinson's disease clinical trials. Parkinsonism Relat Disord 2009;15:258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McInerney‐Leo A, Gwinn‐Hardy K, Nussbaum RL. Prevalence of Parkinson's disease in populations of African ancestry: a review. J Natl Med Assoc 2004;96:974–979. [PMC free article] [PubMed] [Google Scholar]
- 33. Parkinson Study Group . Pramipexole in levodopa‐treated Parkinson disease patients of African, Asian and Hispanic heritage. Clin Neuropharmacol 2007;30:72–85. [DOI] [PubMed] [Google Scholar]
- 34. Hemming JP, Gruber‐Baldini AL, Anderson KE, et al. Racial and socioeconomic disparities in parkinsonism. Arch Neurol 2011;68:498–503. [DOI] [PubMed] [Google Scholar]
- 35. Dahodwala N, Karlawish J, Siderowf A, Duda JE, Mandell DS. Delayed Parkinson's disease diagnosis among African‐Americans: the role of reporting of disability. Neuroepidemiology 2011;36:150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scott B, Borgman A, Engler H, Johnels B, Aquilonius SM. Gender differences in Parkinson's disease symptom profile. Acta Neurol Scand 2000;102:37–43. [DOI] [PubMed] [Google Scholar]
- 37. Baba Y, Putzke JD, Whaley NR, Wszolek ZK, Uitti RJ. Gender and the Parkinson's disease phenotype. J Neurol 2005;252:1201–1205. [DOI] [PubMed] [Google Scholar]
- 38. Accolla E, Caputo E, Cogiamanian F, et al. Gender differences in patients with Parkinson's disease treated with subthalamic deep brain stimulation. Mov Disord 2007;22:1150–1156. [DOI] [PubMed] [Google Scholar]
- 39. Zappia M, Crescibene L, Arabia G, et al. Body weight influences pharmacokinetics of levodopa in Parkinson's disease. Clin Neuropharmacol 2002;25:79–82. [DOI] [PubMed] [Google Scholar]
- 40. Cheng EM, Swarztrauber K, Siderowf AD, et al. Association of specialist involvement and quality of care for Parkinson's disease. Mov Disord 2007;22:515–522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Independent predictors of choice of drug class.


