Abstract
Background
Proprioception has not been examined in the lower limb in people with Parkinson's disease (PD). Impaired proprioception may contribute to activity limitations, including falls in individuals with PD.
Objectives
The aims of this study were to determine whether: (1) people with PD have impaired proprioception in the ankles during active movements; (2) there are correlations between ankle proprioception and history of falls, fear of falling, and parkinsonian symptoms.
Methods
This was a cross‐sectional observational study of ankle proprioception in people with mild to moderate PD and healthy age‐matched controls. Included in the study were thirteen participants with mild to moderate PD, aged 71 SD (31) years, and 14 age‐matched controls, aged 66 SD (21) years. Proprioception of the ankle was measured using the Active Movement Extent Discrimination Apparatus. Symptom severity was measured using the PDQ‐39. Fear of falling was measured using the Falls Efficacy Scale, and participants were questioned about their history of falls during the previous 12 months. All measures were completed on one occasion.
Results
People with PD had significantly worse proprioception in plantarflexion (mean difference 0.045, 95% CI 0.00 to 0.09), inversion (mean difference 0.059, 95% CI 0.02 to 0.10), and overall proprioception (mean difference 0.048, 95% CI 0.00 to 0.10) than control participants. In people with PD, there was a significant moderate negative correlation between impaired proprioception and Parkinson's symptoms (r = −0.441, P = 0.021).
Conclusions
Impaired proprioception of the ankle is evident in people with PD. Further research is warranted to determine whether proprioception can be improved in people with PD.
Keywords: Parkinson's disease, proprioception, kinesthesia, motor control, balance
Parkinson's disease (PD) is a progressive neurological disorder frequently characterized by balance impairment and a decline in motor function. Motor symptoms may include resting tremor, bradykinesia, stooped posture, and freezing of gait. Those suffering from PD may also present with a lack of both limb and trunk position awareness, also known as proprioception, which is served by afferent information arising from a variety of sensory receptors. More specifically, people with PD may present with a lack of kinesthesia, a term used to describe proprioceptive sense during movement. PD is known to affect kinesthesia, and this loss of kinesthetic sensitivity may be linked to common motor deficits that are seen with progression of the disorder.1, 2, 3, 4 Kinesthesia may also contribute to the large number of falls in PD, with two thirds of people living in a PD community having experienced a fall in the previous 12 months.5
The basal ganglia appear to be important for sensorimotor integration, which is the process by which sensory information, like proprioceptive information, is mapped onto the motor command.6 The basal ganglia receive input from visual and proprioceptive receptors, and it is proposed that the dopamine depletion in the basal ganglia characteristic of PD can negatively impact the integration of this information.6 Impaired proprioception has been directly correlated with reduced independence and falls in geriatric populations,1 and it is possible that impaired proprioception may contribute to the postural instability typical of PD. Postural instability in this group is exacerbated when vision is occluded, which may also indicate a problem with integrating proprioceptive information.7
Maschke and colleagues (2003) found that people with PD were significantly impaired in the ability to detect displacements of the forearm when compared with healthy controls.6 However, there is little evidence regarding the integration of proprioceptive information from the lower leg or ankle in people with PD.8 Understanding the impact of PD on proprioception at the ankle is important, as it has been shown that impairments in proprioception can be joint specific, and a loss of proprioception at the ankle could be an important contributor to postural instability and falls.2 Therefore, the research questions for this study were: (1) Do people with Parkinson's disease have impaired proprioception in the ankles during active movements? (2) Is there a correlation between proprioception of the ankle and the history of falls, the fear of falling, and parkinsonian symptoms?
Methods
Design
This was a two‐group comparison study. Participants with PD were compared with age‐matched control participants who did not have any neurological impairment. The Human Research Ethics Committee institution approved the study. All participants gave written informed consent before data collection. All measures were taken during the ON phase of medication (ie, currently using).
Participants
Adults with mild to moderate Parkinson's disease were eligible to participate if they were able to walk independently, with or without an aid, and were stable on current medication. Potential participants were excluded if they had severe cognitive impairment, early‐onset PD, late stages of PD, a history of other neurological disease(s), or a history of severe ankle injury.
Setting
Participants were recruited from a metropolitan community between January and June 2015 and were measured in a university research clinic.
Outcome Measures
The primary outcome measure was ankle proprioception measured on one occasion with the Active Movement Extent Discrimination Assessment (AMEDA).9 Unlike other measures of proprioception, such as the Threshold to Detection of Passive Motion and the Joint Position Reproduction (matching), the AMEDA provides information on both joint movement and joint position.9 The AMEDA was developed to examine proprioception in an environment as close as possible to normal,10 and it measures proprioception with full weight bearing during the midrange of normal active ankle movement, either into inversion or plantarflexion. The AMEDA operates with the participant in a standing position, with one foot on a solid platform, and the other on a platform that is able to tilt in one direction (Fig. 1). There is a mechanism located underneath this platform that moves between 5 different levels, changing the depth to which the platform can tilt. These 5 levels are approximately 1 degree of rotation apart. Depending on the direction of the foot, it is able to measure proprioception during both inversion and plantarflexion movements. The participants were familiarized with each of the 5 levels (1–5) in shallowest to deepest order, and they were instructed to focus on a point on the wall in front of them and to avoid looking down. The familiarization trial consisted of practicing each level 3 times to allow participants to understand the difference between each level. Participants were then tested 50 times, in which each of the 5 levels presented 10 times in a random order. The participants were required to tilt the platform down, return it to the start position, and immediately report the level they sensed the platform to have tilted. Only one movement attempt was provided before participants were required to report the level to which they had moved. No feedback was given throughout the trial as to the accuracy of their estimates.
Figure 1.
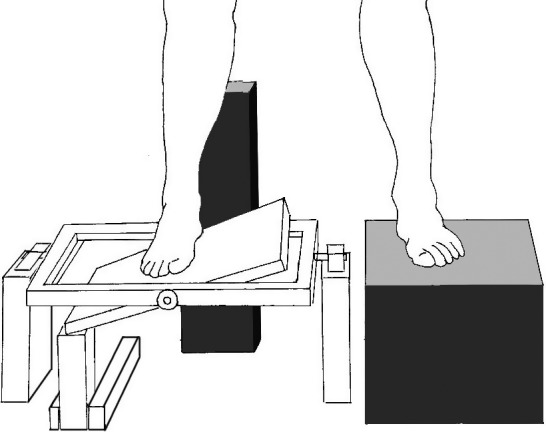
The Active Movement Extent Discrimination Assessment (AMEDA).
The secondary outcomes included Parkinson's disease symptom severity and quality of life using a modified version of the Parkinson's Disease Questionnaire (PDQ‐39), fear of falling using the falls efficacy scale (FES), and self‐reported number of falls during the previous 12 months. The PDQ–39 consists of 39 questions in 8 categories: mobility, activities of daily living, emotional well‐being, stigma, social support, cognition, communication, and bodily discomfort.11 Participants were asked each question in relation to the previous 2 weeks, and they answered by selecting one of the 5 responses: never, occasionally, sometimes, often, and always. The PDQ‐39 has high internal consistency and validity.12
The FES was developed to measure fear of falling, defined as being “low perceived self‐efficacy at avoiding falls during essential, nonhazardous activities of daily living.”13 The FES consisted of 16 questions relating to daily activities or outings. Participants rated how concerned they were about the possibility of falling during these activities as “not at all concerned,” “somewhat concerned,” “fairly concerned,” and “very concerned.” If they had previously discontinued preforming the activity, they answered regarding how they would feel if they were to attempt the activity.
Data Analysis
The area under the receiver operating characteristic (ROC) curve was calculated for each data set generated by the AMEDA. An AMEDA score of 0.5 represented a chance response, whereas an AMEDA score of 1 represented perfect discrimination of the difference among all 5 levels (ie, perfect proprioception). Descriptive statistics (mean and 95% confidence intervals) and unpaired t tests were conducted to determine differences in proprioception among the groups. A Spearman's correlation was performed to test for correlation between the proprioception and falls, proprioception and fear of falling, and proprioception and symptom severity.
Results
Thirty‐six people responded to the request for participants; however 9 of these were excluded (see Fig. 2 for flow of participants through the trial). Thirteen participants with mild to moderate PD, aged 71 SD (7) years, and 14 age‐matched controls, aged 66 SD (8) years, participated. Participant characteristics are presented in Table 1.
Figure 2.
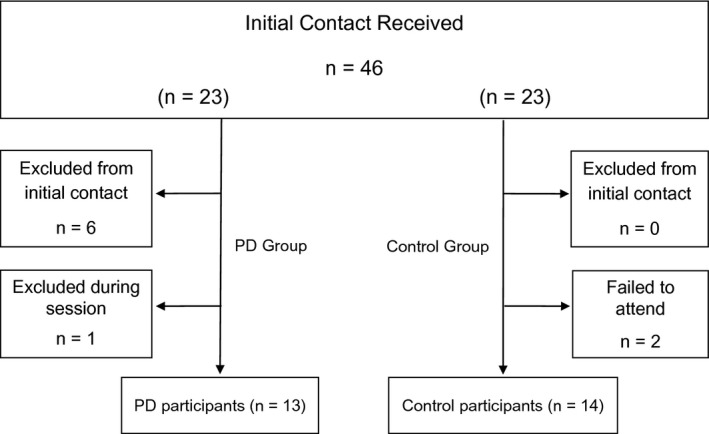
Flow of participants through the trial.
Table 1.
Baseline Characteristics of Participants
| Characteristic | All Participants (n = 27) | |
|---|---|---|
| PD (n = 13) | Con (n = 14) | |
| Participants | ||
| Age (yr), mean (SD) | 71 (7) | 66 (8) |
| Gender, n males (%) | 10 (77) | 9 (64) |
| PDQ‐39 score (0–88), mean (SD) | 15 (14) | 1 (3) |
PD, Affected by Parkinson's disease; Con, control group; PF, plantarflexion; INV, inversion.
Results are presented in Table 2. People with Parkinson's disease had significantly worse proprioception in plantarflexion than control participants, with an average difference of 0.045 (95% CI 0.00 to 0.09). People with Parkinson's disease also had significantly worse proprioception during inversion compared with control participants, with an average difference of 0.059 (95% CI 0.02 to 0.10). Total difference in proprioception (all scores combined, regardless of direction) was also significantly worse in people with Parkinson's disease, with an average difference of 0.048 (95% CI 0.00 to 0.10). The clinically important difference in proprioception when measured using the AMEDA was 0.05.9
Table 2.
Mean (SD) of Groups, Mean (SD) Difference Within Groups, and mean (95% CI) Difference Between Groups
| Outcome | PD (n = 13) | Con (n = 14) | Between Group Difference |
|---|---|---|---|
| PDQ‐39 score (0–88) | 15 (14) | 1 (3) | 14 (6 to 22) |
| FES score (9–90) | 18 (9) | 9 (2) | 9 (4 to 14) |
| Number of falls (12 months) | 4 (7) | 0 (0) | 3 (−1 to 7) |
| Proprioception | |||
| Plantarflexion | 0.636 (0.06) | 0.669 (0.08) | 0.045 (0.00 to 0.09) |
| Inversion | 0.624 (0.05) | 0.683 (0.06) | 0.059 (0.02 to 0.10) |
| Average (PF + Inv) | 0.627 (0.06) | 0.675 (0.07) | 0.048 (0.00 to 0.10) |
PD, affected by parkinson's disease; Con, control group; PDQ, Parkinson's Disease questionnaire; FES, falls efficacy scale; PF, plantarflexion; INV, inversion; AV, average.
In people with PD, there was a significant moderate negative correlation between average proprioception and Parkinson's symptoms reported on the PDQ39 (r = −0.441, P = 0.021). Negative trending correlations were found between number of recent falls and proprioception scores (r = −0.306, P = 0.121) and between FES and proprioception (r = −0.245, P = 0.217). No correlation was found between age and proprioception (r = −0.068, P = 0.737).
Discussion
This study examined proprioception of the ankle in people with mild to moderate PD and healthy older adults. The results demonstrated a significant difference in ankle proprioception between people with PD and healthy control participants, during both inversion and overall proprioception. There was a significant moderate correlation between impairment of proprioception and PD symptom severity, weak correlations between impairment of proprioception and falls, and fear of falling, but no correlation between impairment of proprioception and age.
The impairment in proprioception evident in participants with PD may be a result of reduced central sensory integration, as a consequence of dopamine depletion in the basal ganglia. It has been established that neurons in the basal ganglia have proprioceptive fields and these neuronal responses are joint specific.6 When tested in an animal model with PD, the number of neurons in the basal ganglia responding to proprioceptive information increased compared with animal models without PD.14 This increased response created a large degree of noise within the basal ganglia, which resulted in decreased joint specificity with regard to proprioceptive integration.14 This may account for the impairment in proprioception evident in people with PD in this study.
Proprioception during ankle inversion has been suggested as a more useful measure of proprioception than plantarflexion in healthy populations.15 Symes, Waddington, and Adams (2010) demonstrated that proprioception was increased in joint ranges in which there is greater use, therefore resulting in a practice effect for proprioception.16 It is hypothesized that proprioception is better in plantarflexion because of the potentially greater volume of plantar flexor muscles used in walking and therefore the greater volume of muscle fibers available to receive proprioceptive information.15 Consequently a small loss of proprioception in plantarflexion will not have as much of an impact as a small loss of proprioception in inversion. The participants with PD enrolled in this study were able to walk independently and therefore this practice effect is likely to be relevant. As such, the impairment of proprioception evident during inversion is likely to be highly indicative of a loss of proprioception.
A moderate correlation was found between impaired proprioception and PD symptoms, suggesting that the loss of proprioception was occurring along with the other motor impairments in PD and also with the deterioration of the basal ganglia. Based on the correlation between impaired proprioception and PD symptoms it is possible that impaired proprioception could contribute to activity limitations and participation restrictions. However, only a weak and nonsignificant correlation was found between impaired proprioception and falling in this study, suggesting that other impairments might contribute more substantially to falling in this population, or that the sample size was too small to detect a correlation.
Given that proprioception is impaired in people with PD, future research could examine whether proprioception can be trained in people with PD. Proprioception has been effectively trained in healthy people and people with other neurological conditions.17 Balance and proprioception training has improved lower‐limb motor control in healthy individuals,17 and training proprioception using biofeedback has been demonstrated as effective in improving balance and proprioception18 and also independence and falls risk19 in people with multiple sclerosis. The efficacy of assessing and training proprioception in people with PD, however, needs to be established.
There are several limitations of this study. First, the sample size is small, thereby reducing the power of the study. This may account for the weak correlations evident between proprioception and falling, and proprioception and fear of falling. There was also a large range in the age of participants, however there was no correlation found between age and proprioception in this sample. This may, however, be a reflection of activity levels of the participants, as more active older participants may achieve heightened levels of proprioceptive capacity, meaning that activity levels need to be controlled in future studies. The current study included participants who had mild to moderate PD, so the results cannot be generalized to people with severe PD. Including participants with more severe disease in future studies may provide greater insight into the role of proprioception in symptom development in PD.
Conclusions
The results of this study indicate impaired proprioception in the ankle, in particular during ankle inversion, in people with mild to moderate PD compared with healthy aged‐matched control participants. Impairment in proprioception was correlated with symptom severity in people with PD, but not falling, fear of falling, or age, although the sample size was small. Future studies could examine the efficacy of training proprioception in people with PD.
Author Roles:
1. Research project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript: A. Writing of the first draft, B. Review and Critique.
H.T.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
E.P.: 2C, 3B
G.W.: 2A, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. This study was conducted in accordance with the institution's Human Research Ethics Committee.
Funding Sources and Conflicts of Interest: The University of Canberra, Faculty of Health, funded this research. The authors report no conflicts of interest.
Financial Disclosures for the previous 12 months: The authors report no sources of support and no competing interests.
Acknowledgments
We thank the participants, assessors, and staff who assisted in this study, particularly Andrew Flood and Allyson Flynn.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Suetterlin KJ, Sayer AA. Proprioception: where are we now? A commentary on clinical assessment, changes across the life course, functional implications and future interventions. Age Ageing 2014;43:313–318. [DOI] [PubMed] [Google Scholar]
- 2. Dietz V. Proprioception and locomotor disorders. Neuroscience 2002;3:781–790. [DOI] [PubMed] [Google Scholar]
- 3. Haas C, Buhlmann A, Turbanski S, Schmidtbleicher D. Proprioceptive and sensorimotor performance in Parkinson's disease. Res Sports Med 2006;14:273–287. [DOI] [PubMed] [Google Scholar]
- 4. Tan T, Almeida QJ, Rahmi F. Proprioceptive deficits in Parkinson's disease patients with freezing of gait. Neuroscience 2011;192:746–752. [DOI] [PubMed] [Google Scholar]
- 5. Ashburn A, Fazakarley L, Ballinger C, Pickering R, McLellan LD, Fitton C. A randomised controlled trial of a home based exercise programme to reduce the risk of falling among people with Parkinson's disease. J Neurol Neurosurg Psychiatry 2007;78:678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maschke M, Gomez C, Tuite P, Konzac J. Dysfunction of the basal ganglia, but not the cerebellum, impairs kinaesthesia. Brain 2003;126:2312–2322. [DOI] [PubMed] [Google Scholar]
- 7. Jacobs JV, Horak FB. Abnormal proprioceptive‐motor integration contributes to hypometric postural responses of subjects with Parkinson's disease. Neuroscience 2006;141:999–1009. [DOI] [PubMed] [Google Scholar]
- 8. Khudados E, Cody FWJ, O'Boyle DJ. Proprioceptive regulation of voluntary ankle movements, demonstrated using muscle vibration, is impaired in Parkinson's disease. J Neurol Neurosurg Psychiatry 1999;67:504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han J, Anson J, Waddington G, Adams R, Liu Y. The role of ankle proprioception for balance control in relation to sports performance and injury. BioMed Res Int 2015;2015:842804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schlenstedt C, Brombacher S, Hartwigsen G, Weisser B, Moller B, Deuschl G. Comparing the Fullerton Advanced Balance Scale with the Mini‐BESTest and Berg Balance Scale to assess postural control in patients with Parkinson disease. Arch Phys Med Rehabil 2015;96:218–225. [DOI] [PubMed] [Google Scholar]
- 11. Hagell P, Nilsson M. The 39‐item Parkinson's Disease Questionnaire (PDQ‐39): is it a unidimensional construct? Ther Adv Neurol Disord 2009;2:205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson's Disease Questionnaire (PDQ‐39): development and validation of a Parkinson's disease summary index score. Age Ageing 1997;26:353–357. [DOI] [PubMed] [Google Scholar]
- 13. Tinetti M, Richman D, Powell L. Falls efficacy as a measure of fear of falling. J Gerontol 1990;45:P239–P243. [DOI] [PubMed] [Google Scholar]
- 14. Boraud T, Bezard E, Bioulac B, Gross C. Ratio of inhibited‐to‐activated pallidal neurons decreases dramatically during passive limb movement in the MPTP‐treated monkey. J Neurophysiol 2000;83:1760–1763. [DOI] [PubMed] [Google Scholar]
- 15. Black G, Waddington G, Adams R. Relvative sensitivity of depth discrimiation for ankle inversion and plantar flexion movements. Percept Mot Skills 2014;118:115–125. [DOI] [PubMed] [Google Scholar]
- 16. Symes M, Waddington G, Adams R. Depth of ankle inversion and discrimination of foot positions. Percept Mot Skills 2010;111:475–484. [DOI] [PubMed] [Google Scholar]
- 17. Irrgang J, Whitney S, Cox E. Balance and proprioceptive training for rehabilitation of the lower extremity. J Sport Rehabil 1994;3:68–83. [Google Scholar]
- 18. Prosperini L, Fortuna D, Gianni C, Leonardi L, Marchetti M, Pozzilli C. Home‐based balance training using the Wii balance board: a randomized, crossover pilot study in multiple sclerosis. Neurorehabil Neural Repair 2013;27:516–525. [DOI] [PubMed] [Google Scholar]
- 19. Prosperini L, Leonardi L, De Carli P, Mannocchi M, Pozzilli C. Visuo‐proprioceptive training reduces risk of falls in patients with multiple schlerosis. Mult Scler 2010;16(4):491–499. [DOI] [PubMed] [Google Scholar]


