Abstract
Cerebellar ataxia with neuropathy and vestibular areflexia syndrome (CANVAS) is a newly recognized disorder characterized by cerebellar ataxia, nonlength‐dependent sensory impairment, and bilateral vestibular loss. Sudomotor dysfunction has been described in CANVAS; however, the underlying pathology is not well characterized. To describe novel histopathological features of this syndrome, 2 siblings are presented who had CANVAS with unique findings of sweat gland denervation. Skin biopsy testing was performed to assess sudomotor structure and revealed markedly reduced sweat gland nerve fiber density below the 2.5th percentile in both patients. These histopathological findings suggest that postganglionic sudomotor dysfunction is an additional feature of CANVAS.
Keywords: cerebellar ataxia, sweat gland denervation, postganglionic, sudomotor dysfunction, cerebellar ataxia with neuropathy and vestibular areflexia syndrome (CANVAS)
Cerebellar ataxia with neuropathy and vestibular areflexia syndrome (CANVAS) is a recently recognized disorder characterized by cerebellar ataxia, nonlength‐dependent sensory impairment, and bilateral vestibular loss.1, 2 Sudomotor dysfunction is recognized in patients with CANVAS; however, the underlying pathophysiology has not been fully elucidated.3, 4 To describe novel histopathological features of this syndrome, we present 2 siblings who had CANVAS with unique findings of sweat gland denervation.
Case Series
Case 1
A woman aged 63 years presented with a 12‐year history of numbness in the lower extremities, a 10‐year history of slowly progressive gait ataxia, and a 3‐year history of dysarthria. She reported decreased sweating and chronic cough. She had a history of hypertension and seizures. Her examination was notable for spontaneous downbeat nystagmus, impaired vestibulo‐ocular reflex on head impulse testing, dysarthria, moderate to severe appendicular dysmetria, and severe gait ataxia. Sensory examination revealed decreased pinprick sensation in the face and lower extremities. She had 3+ reflexes in the upper and lower extremities except for 1+ Achilles reflexes.
Case 2
The sibling of case 1, a man aged 60 years, presented with a 15‐year history of slowly progressive gait ataxia and a 2‐year history of dysarthria. He reported generalized lack of sweating and chronic cough. He had a history of bradycardia status post pacemaker placement. His examination showed gaze‐evoked horizontal nystagmus, impaired vestibulo‐ocular reflex on head impulse testing, dysarthria, moderate appendicular dysmetria, and markedly ataxic gait. On sensory examination, he had decreased pinprick sensation in the lower extremities. He had 3+ reflexes in the upper and lower extremities except for 2+ Achilles reflexes.
Results
Laboratory tests for several causes of ataxia, including vitamin deficiencies, infectious causes, and autoimmune causes, were negative. The following serum laboratory tests performed in both cases were within normal limits: vitamin B12, folate, serum protein electrophoresis/immunofixation, vitamin E, rapid plasma reagin, lyme antibody, antigliadin antibody, antiendomysial antibody, and antiglutamic acid antibody. Exome sequencing in both patients did not reveal specific polymorphisms associated with the clinical presentation; further genetic studies are ongoing.
In case 1, a magnetic resonance image (MRI) of the brain showed a small incidental meningioma in the left tentorium cerebelli without significant cerebellar atrophy. Case 2 was unable to have a brain MRI because of his pacemaker. Nerve‐conduction studies showed absent sensory responses in the upper and lower limbs. All motor nerves tested in the upper and lower limbs had normal distal latencies, amplitudes, velocities, and F‐waves. This pattern was suggestive of a severe sensory neuronopathy/ganglionopathy. Video head impulse testing, caloric testing, and rotatory chair testing revealed profound bilateral loss of vestibular function in both patients. Skin biopsy samples (3 mm) were taken along the leg and proximal arm. Sections were immunohistochemically stained with the panaxonal marker PGP9.5 (ubiquitin carboxyl‐terminal esterase L1 [ubiquitin thiolesterase]). Sweat gland nerve fiber density was assessed by stereology5 and was markedly reduced at all sites (Fig. 1). Clinically, a diagnosis of CANVAS was made given the characteristic triad of cerebellar ataxia, bilateral vestibulopathy, and somatosensory impairment.4
Figure 1.
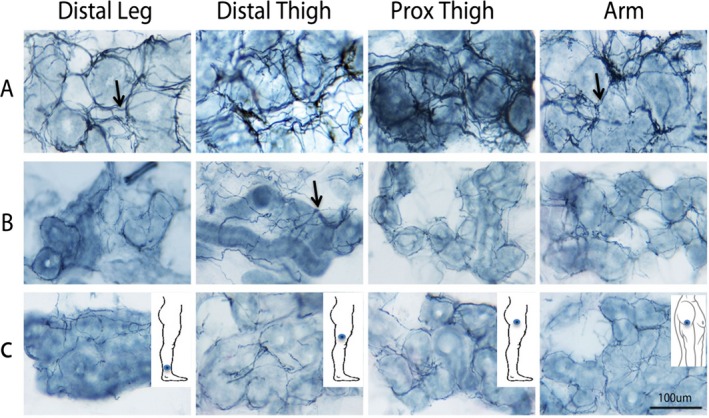
Sweat gland innervation is observed in dermal sweat gland fragments from (A) a representative control and (B,C) the 2 patients who had cerebellar ataxia with neuropathy and vestibular areflexia syndrome (CANVAS). Nerve fibers (arrows) were visualized by immunohistochemistry directed against the panaxonal marker PGP9.5 (ubiquitin carboxyl‐terminal esterase L1 [ubiquitin thiolesterase]). Nerve fibers envelope sweat glands in a net‐like pattern. The sweat gland nerve fiber density (SGNFD) was calculated by stereology and was below the 2.5th percentile at all sites in both CANVAS cases. (A) In the representative control, the SGNFD (from left to right) is 13.6, 15.5, 19.5, and 21.3 m nerve fiber length/mm3 in the distal leg, distal thigh, proximal (Prox) thigh, and arm, respectively. (B) In case 1, the SGNFD is 5.0, 7.6, 9.1, and 11.3 m nerve fiber length/mm3, respectively. (C) In case 2, the SGNFD is 8.0, 5.2, 3.2, and 11.6 m nerve fiber length/mm3, respectively. Intraepidermal nerve fiber density values (not shown) from the distal leg, distal thigh, proximal thigh, and arm were 13.5, 15.6, 19.4, and 32.2 fibers/mm, respectively, in the control; 0.1, 0.0, 0.0, and 2.2 fibers/mm, respectively, in case 1; and 0.1, 0.0, 0.0, and 0.8 fibers/mm, respectively, in case 2.
Discussion
Patients with CANVAS can present with various patterns of symptoms,4 as in both of our patients. However, the clinical triad of cerebellar ataxia, bilateral vestibulopathy, and somatosensory impairment must be established for the diagnosis of CANVAS while excluding other possible causes, and our patients met these criteria. When CANVAS is suspected, other potential causes of cerebellar ataxia, sensory neuronopathy, and vestibulopathy should be excluded. Our patients underwent laboratory testing to exclude infectious, autoimmune, and nutritional causes of neuropathy or ataxia. Genetic testing with exome sequencing in both patients was unrevealing. Familial amyloid polyneuropathy, a rare cause of a length‐dependent sensory polyneuropathy and autonomic dysfunction,6 was considered an unlikely explanation for the siblings’ presentation with cerebellar ataxia, sensory neuronopathy, and vestibulopathy. Patients with familial amyloid polyneuropathy often have small‐fiber neuropathy (painful), symptomatic autonomic dysfunction, and motor neuropathy with relentless progression, a pattern not seen in our patients.
In addition to physical examination, laboratory tests can help diagnose CANVAS. Video‐oculography or rotational chair testing can test for vestibulopathy, and neurophysiological studies can test for sensory neuronopathy.4, 7 A brain MRI may show cerebellar atrophy but can be normal in patients with CANVAS.3
To assess sudomotor function in CANVAS, several tests are available, including a quantitative sudomotor axon reflex test, sympathetic skin responses, a thermoregulatory skin test, and sweat gland innervation with a skin biopsy to measure sweat gland nerve fiber density, which has been correlated with orthostatic hypotension and sympathetic skin responses.8, 9, 10 Skin biopsy testing was done to assess sudomotor structure in our 2 patients.
The pathophysiology of CANVAS is not completely understood; however, this condition is reported as a ganglionopathy involving the sensory, vestibular, autonomic, facial, and trigeminal ganglia.3, 4 Previous pathological studies in CANVAS have demonstrated atrophy of the vestibular, facial, and trigeminal nerves and of the dorsal root ganglia.1, 4, 11 The nonlength‐dependent nature of sensory loss observed on neurophysiological testing in our cases supports a sensory ganglionopathy/neuronopathy rather than a classic length‐dependent axonal sensory neuropathy. This is consistent with neurophysiological testing results in 14 CANVAS patients who were diagnosed with a sensory neuronopathy.7 The underlying pathology for the sensory neuronopathy is dorsal root ganglia neuronal degeneration and secondary loss of posterior column myelinated axons.7, 12, 13 The autonomic pathology of CANVAS is less well understood; and, to our knowledge, this is the first study describing the histopathology of sudomotor nerves in CANVAS. Skin biopsies from our patients revealed markedly reduced sweat gland innervation, suggesting a postganglionic process. Further pathological studies of the autonomic nervous system in CANVAS are needed to characterize the underlying pathology resulting in autonomic dysfunction.
In summary, we describe 2 siblings who had CANVAS with sweat gland denervation suggesting postganglionic sudomotor dysfunction as an additional feature of this syndrome. This pattern of autonomic abnormality may be useful in the differential diagnosis of ataxia.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
C.C.U.: 1A, 1B, 1C, 3A, 3B
M.P.: 1A, 1B, 1C, 3B
V.C.: 1A, 1B, 1C, 3B
D.S.Z.: 1A, 1B, 1C, 3B
Disclosures
Funding Sources and Conflicts of Interest: The authors report no funding sources for this study and have no conflicts of interest.
Financial Disclosures for the previous 12 months: Chizoba C. Umeh has received research support from the Parkinson's Research Fund, Brigham and Women's Hospital. Michael Polydefkis has received grants from the Johns Hopkins School of Medicine and has received research support from the Juvenile Diabetes Research Foundation, ISIS Pharmaceuticals (clinical trial), Alnylam Pharmaceuticals (clinical trial), and Genzyme Pharmaceuticals (clinical trial). Vinay Chaudhry has served as a consultant for Novartis, has provided expert opinion for the Division of Vaccine Injury, and received royalties for a TNS license (Johns Hopkins School of Medicine). David S. Zee has received honoraria from Sun Pharma (teaching course in India), Abbot Pharmaceuticals (teaching courses in Morocco, Egypt, and Mexico), Micromed (teaching courses in Baltimore, MD), the University of Toronto (teaching course in Neuro‐ophthalmology), and for grand rounds presentations at the University of Rochester and Beth Israel, Boston; he also has received royalties from Oxford University Press for The Neurology of Eye Movements.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Szmulewicz DJ, Waterston JA, MacDougall HG, et al. Cerebellar ataxia, neuropathy, vestibular areflexia syndrome (CANVAS): a review of the clinical features and video‐oculographic diagnosis. Ann N Y Acad Sci 2011;1233:139–147. [DOI] [PubMed] [Google Scholar]
- 2. Szmulewicz DJ, Roberts L, McLean CA, MacDougall HG, Halmagyi GM, Storey E. Proposed diagnostic criteria for cerebellar ataxia with neuropathy and vestibular areflexia syndrome (CANVAS). Neurol Clin Pract 2016;6:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu TY, Taylor JM, Kilfoyle DH, et al. Autonomic dysfunction is a major feature of cerebellar ataxia, neuropathy, vestibular areflexia “CANVAS” syndrome. Brain 2014;137:2649–2656. [DOI] [PubMed] [Google Scholar]
- 4. Szmulewicz DJ, McLean CA, MacDougall HG, Roberts L, Storey E, Halmagyi GM. CANVAS an update: clinical presentation, investigation and management. J Vestib Res 2014;24:465–474. [DOI] [PubMed] [Google Scholar]
- 5. Liu Y, Billiet J, Ebenezer GJ, Pan B, Hauer P, Wei J, Polydefkis M. Factors influencing sweat gland innervation in diabetes. Neurology 2015;84:1652–1659. [DOI] [PubMed] [Google Scholar]
- 6. Plante‐Bordeneuve V, Said G. Familial amyloid polyneuropathy. Lancet Neurol 2011;10:1086–1097. [DOI] [PubMed] [Google Scholar]
- 7. Szmulewicz DJ, Seiderer L, Halmagyi GM, Storey E, Roberts L. Neurophysiological evidence for generalized sensory neuronopathy in cerebellar ataxia with neuropathy and bilateral vestibular areflexia syndrome. Muscle Nerve 2015;51:600–603. [DOI] [PubMed] [Google Scholar]
- 8. Gibbons CH, Illigens BM, Wang N, Freeman R. Quantification of sweat gland innervation: a clinical‐pathologic correlation. Neurology 2009;72:1479–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vinik AI, Nevoret ML, Casellini C. The new age of sudomotor function testing: a sensitive and specific biomarker for diagnosis, estimation of severity, monitoring progression, and regression in response to intervention [serial online]. Front Endocrinol (Lausanne) 2015;6:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chao CC, Huang CM, Chiang HH, et al. Sudomotor innervation in transthyretin amyloid neuropathy: pathology and functional correlates. Ann Neurol 2015;78:272–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Szmulewicz DJ, Merchant SN, Halmagyi GM. Cerebellar ataxia with neuropathy and bilateral vestibular areflexia syndrome: a histopathologic case report [serial online]. Otol Neurotol 2011;32:e63–e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Camdessanche JP, Jousserand G, Ferraud K, Vial C, Petiot P, Honnorat J, Antoine JC. The pattern and diagnostic criteria of sensory neuronopathy: a case‐control study. Brain 2009;132(pt 7):1723–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gwathmey KG. Sensory neuronopathies. Muscle Nerve 2016;53:8–19. [DOI] [PubMed] [Google Scholar]


