Abstract
S-1 is an oral fluoropyrimidine agent used for the treatment of non-small-cell lung cancer (NSCLC). Although S-1 monotherapy has been reported to exhibit lesser hematotoxicity compared with other third-generation chemotherapeutics, digestive toxicity was also frequently observed. Alternate-day administration of S-1 has shown a lower rate of severe digestive toxicity than the daily standard administration in patients with NSCLC. However, the safety of alternate-day S-1 therapy in elderly patients aged 75 years or older has not been investigated. The present study was a multi-center and prospective feasibility study aimed to evaluate the safety of alternate-day S-1 therapy in elderly patients with NSCLC. The patients received S-1 orally twice daily for 4 days (Monday, Wednesday, Friday, and Sunday) every week until disease progression or unacceptable toxicity. The primary endpoint was safety, which was evaluated as the number of grade ≥3 adverse events, and the secondary endpoints were progression-free survival (PFS), 1-year survival, and disease control rate (DCR). A total of 10 patients were enrolled, but 2 patients failed to initiate the treatment protocol. Finally, 8 patients were treated with the study protocol regimen. No grade 3 or higher adverse events were observed. Four (50%) and 1 (12.5%) patient had grade 2 or lower digestive symptoms such as anorexia, diarrhea, or stomatitis and grade 1 lacrimation, respectively. Moreover, 2 (25%), 1 (12.5%), and 1 (12.5%) patients had grade 2 renal dysfunction, grade 2 ileus, and elevated blood bilirubin, respectively. The median PFS was 1.5 months (95% confidence interval: 0.9–1.8), and the 1-year survival rate was 42.9%. The DCR was 12.5%. In conclusion, alternate-day S-1 administration can be a safe treatment regimen for elderly patients with NSCLC, but its therapeutic efficacy and safety for elderly patients with NSCLC should be compared against the standard S-1 administration in a large-scale study.
Keywords: S-1, elderly patient, non-small-cell lung cancer, prospective feasibility study, alternative day administration, chemotherapy
Introduction
Lung cancer is the most common cause of cancer-related death worldwide (1), and the number of elderly patients aged 75 years or older with lung cancer is increasing. Previous studies have demonstrated that the incidence of chemotherapy-related adverse events is higher in elderly patients with lung cancer than in non-elderly patients (2,3). Discontinuing chemotherapy due to the adverse events would shorten the prognosis of the elderly patients with lung cancer. Thus, a chemotherapy drug with limited adverse effects should be selected for such patients.
Kinase inhibitors are the standard first-line treatment for elderly patients with advanced non-small-cell lung cancer (NSCLC) and driver mutations. Moreover, a programmed death-1 antibody is considered in patients with high programmed death-ligand 1 expression (tumor proportion score of ≥50%) and also without driver mutation (4). Meanwhile, in patients without these conditions, monotherapy using third-generation chemotherapy drugs, such as docetaxel (DTX), gemcitabine, and vinorelbine, is recommended (5–7).
S-1 is an oral fluoropyrimidine agent containing the 5-fluorouracil prodrug tegafur and 2 enzyme inhibitors, namely, 5-chloro-2, 4-dihydroxypyridine and potassium oxonate, which can reduce the adverse effect of tegafur. S-1 is approved for patients with gastric cancer in 7 Asian countries and 15 European countries. It is also approved for patients with 8 type of cancers including NSCLC in Japan. A phase-3 study compared the efficacy of S-1 monotherapy with that of DTX for NSCLC patients previously treated with platinum-based chemotherapy. The results revealed the non-inferiority of S-1 to DTX in terms of overall survival (OS) (8). Therefore, S-1 monotherapy can be considered for first-line chemotherapy of elderly patients with NSCLC.
S-1 monotherapy has also showed lesser hematotoxic adverse events than other third-generation chemotherapy drugs. Among NSCLC patients, the incidence of febrile neutropenia and grade 3 or higher neutropenia was lower in those administered with S-1 than those who received DTX monotherapy (18.2% vs. 37.2%) (8). Thus, S-1 can be safely administered to elderly patients. However, digestive toxicity was frequently observed (8,9). Diarrhea and oral mucositis have been shown to occur more frequently in patients receiving S-1 than those receiving DTX (23.9% vs. 14.5%) (8), indicating that the digestive toxicity of S-1 should be reduced. As such, several studies have been conducted to this end.
The standard regimen for S-1 monotherapy is 4 weeks of continuous oral administration followed by a 2-week off period. A clinical study comprising previously treated NSCLC patients who underwent at least one chemotherapy regimen showed that the rates of severe diarrhea and appetite loss were significantly lower in the alternate-day administration than the standard S-1 administration (9.7% vs. 0% and 19.4% vs. 0%, respectively). By contrast, no significant difference in the chemotherapeutic effect for NSCLC was observed. The median progression-free survival (PFS) for the alternate-day and standard administration groups was 2.1 vs. 2.7 months (log-rank test: P=0.49), and the median OS was 11 vs. 12 months (log-rank test: P=0.35) (10). In addition, alternate-day administration of S-1 showed lesser adverse events than did the standard administration in Japanese patients with unresectable advanced pancreatic cancer in a multi-center, randomized, phase II study (11).
In this study, we hypothesized that alternate-day administration of S-1 to elderly patients aged 75 years or older with previously treated advanced NSCLC will have fewer adverse events than the standard administration. To investigate this hypothesis, we conducted a multi-center prospective feasibility study to evaluate the rate of adverse events in elderly patients with NSCLC treated with alternate-day S-1 administration.
Materials and methods
Study design and eligibility criteria
The present study was designed as a multi-center and prospective feasibility study. We enrolled the patients from Hiroshima Prefectural Hospital, Hiroshima City Asa Citizens' Hospital, and Hiroshima University Hospital between January 2014 and January 2016 according to the following eligibility criteria: Histologically or cytologically confirmed diagnosis of NSCLC; Stage IIIB and IV disease according to the Union for International Cancer Control TNM Classification of Malignant Tumors 7th edition (12), an Eastern Cooperative Oncology Group, performance status (ECOG PS) of 0–1, an age of ≥75 years, ability to take drugs orally, and an estimated life expectancy of at least 3 months. The patients had received one or two regimens of chemotherapy and two or three regimens if epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors were used. Physical examinations within 14 days after the registration showed all included patients had adequate organ functions according to the following parameters: Leukocyte count ≥3,500/mm3; neutrophil count ≥1,500/mm3; platelet count ≥100,000/mm3; hemoglobin ≥9.0 g/dl; total bilirubin ≤1.5 mg/dl; aspartate aminotransferase and alanine aminotransferase ≤2.5 × upper limit of normal; serum creatinine <1.5 mg/dl; and creatinine clearance (CCr) ≥50 ml/min. Although the eligibility criteria included stage IIIB and IV, incidentally only patients with stage IV were enrolled in this study.
Patients were excluded if they had radiographically confirmed interstitial pneumonia or pulmonary fibrosis, a massive pleural or pericardial effusion or ascites requiring drainage, active double cancer, severe complications, ileus, poorly controlled diabetes, poorly controlled myocardial infraction within 6 months, symptomatic brain metastasis, concomitant treatment with flucytosine, psychiatric disorder, previous severe drug allergy (≥grade 3), previous treatment with fluoropyrimidine, pregnant or possibly pregnant, active hepatitis B virus infection, and if the physician concluded that the patient's participation in this trial was inappropriate.
Treatment schedule
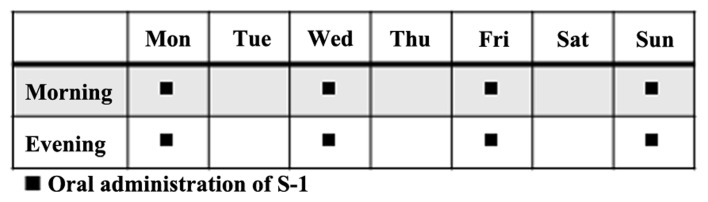
The patients received S-1 orally twice daily for 4 days (Monday, Wednesday, Friday, and Sunday) a week (Fig. 1). The dose of S-1 administered per day was based on the patient's body surface area as follows: <1.25 m2, 40×2 mg; 1.25–1.5 m2, 50×2 mg; >1.5 m2, 60×2 mg. The regimen was continued until progressive disease (PD) or unacceptable toxicity was observed. The criteria of dose reduction were as follows: Leukocyte count ≤1,000/mm3; neutrophil count ≤500/mm3; platelet count ≤25,000/mm3; total bilirubin ≥2.0 mg/dl; serum creatinine ≥ upper limit of normal; and grade 3 or higher non-hematologic toxicity. Meanwhile, the criteria to continue administration were as follows: Leukocyte count ≥2,000/mm3; neutrophil count ≥1,000/mm3; platelet count ≥50,000/mm3; total bilirubin ≤2.0 mg/dl; CCr ≥50 ml/min and grade1 or lower diarrhea or stomatitis, grade 2 or lower other non-hematologic toxicity.
Figure 1.
S-1 administration schedule.
Evaluation of efficacy and toxicity
Tumor response was evaluated every 8 weeks according to the Response Evaluation Criteria in Solid Tumors guideline version 1.1. PFS was defined as the period from enrollment until the date of confirmation of PD or death as a result of any cause. Toxicity was evaluated based on the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 Japanese edition, Japan Clinical Oncology Group version (13). Disease control rate (DCR) was defined as the percentage of patients who obtained complete response, partial response, and stable disease (SD) from the treatment.
Study endpoints and statistical analysis
The primary endpoint was safety and was evaluated by calculating the proportion of ≥grade 3 adverse events. The secondary endpoints were PFS, DCR, and 1-year survival rate. This was a pilot feasibility study to estimate only the safety of alternate-day administration, and the number of enrolled patients was inadequate for evaluating the efficacy of such method of administration. Kaplan-Meier method was used to draw the survival curve. A P-value of <0.05 was considered significant. All data analyses were performed using JMP PRO statistical software version 12.2.0 (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
A total of 10 patients were enrolled, but 2 patients failed to initiate the treatment protocol due to renal dysfunction or intolerance of oral ingestion after enrollment. Thus, 8 patients were observed until March 2017. Table I shows the patients' background characteristics. The cohort comprised 6 men and 2 women, and the median age was 79 years. Of the 8 patients, 2 had a PS score of 0, while the other 6 had a PS score of 1. All patients were diagnosed with Stage IV disease and receiving second-line therapy. EGFR gene mutations and ALK gene translocation were negative in all patients.
Table I.
Patient characteristics.
| Variable | n=8 |
|---|---|
| Sex | |
| Male | 6 |
| Female | 2 |
| Age, years | |
| Range (median) | 75–85 (79) |
| ECOG PS | |
| 0 | 2 |
| 1 | 6 |
| Histology | |
| Ad | 5 |
| Sq | 2 |
| NOS | 1 |
| Clinical stage | |
| IIIB | 0 |
| IV | 8 |
| EGFR mutation | |
| Positive | 0 |
| Negative | 8 |
| ALK translocation | |
| Positive | 0 |
| Negative | 8 |
| The number of prior chemotherapy regimen | |
| 1 | 8 |
| 2 | 0 |
| First-line regimen | |
| CBDCA+PEM | 2 |
| CBDCA+PTX | 2 |
| PEM (+ BEV) | 3 (2) |
| DTX | 1 |
ECOG PS, Eastern Cooperative Oncology Group performance status; Ad, adenocarcinoma; Sq, squamous cell carcinoma; NOS, not otherwise specified; CBDCA, carboplatin; PEM, pemetrexed; PTX, paclitaxel; BEV, bevacizumab; DTX, docetaxel; EGFR, epidermal growth factor receptor.
Toxicity and treatment delivery
No grade 3 or higher adverse events were observed. There were 4 cases of ≤grade 2 digestive symptoms such as anorexia, diarrhea, or stomatitis, and grade 1 lacrimation was observed in 1 case. In addition, grade 2 renal dysfunction was observed in 2 cases, and ileus and elevated total bilirubin were observed in 1 case each (Table II).
Table II.
Hematologic and non-hematologic toxicities.
| Adverse event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Any (%) | Grade 3/4 |
|---|---|---|---|---|---|---|
| All | 4 | 5 | 0 | 0 | 0 | |
| Hematologic | ||||||
| Neutropenia | 0 | 0 | 0 | 0 | 0 (0) | 0 |
| Anemia | 0 | 0 | 0 | 0 | 0 (0) | 0 |
| Thrombocytopenia | 0 | 0 | 0 | 0 | 0 (0) | 0 |
| Febrile neutropenia | 0 | 0 | 0 | 0 | 0 (0) | 0 |
| Non-hematologic | ||||||
| Anorexia | 1 | 1 | 0 | 0 | 2 (25) | 0 |
| Diarrhea | 1 | 0 | 0 | 0 | 1 (12.5) | 0 |
| Stomatitis | 1 | 0 | 0 | 0 | 1 (12.5) | 0 |
| Lacrimation | 1 | 0 | 0 | 0 | 1 (12.5) | 0 |
| Ileus | 0 | 1 | 0 | 0 | 1 (12.5) | 0 |
| Increased serum creatinine | 0 | 2 | 0 | 0 | 2 (25) | 0 |
| Increased total bilirubin | 0 | 1 | 0 | 0 | 1 (12.5) | 0 |
The S-1 administration period ranged from 0.7 to 1.5 months (median: 1.1 months). Treatment was suspended in 2 patients due to grade 2 renal dysfunction. In 1 patient, treatment was also suspended due to grade 2 ileus. The median duration of treatment suspension in these 3 patients was 11 days. Table III shows a summary of the baseline characteristics of these 3 patients.
Table III.
Baseline characteristics of the patients in whom S-1 administration was discontinued.
| Case | Cause of discontinuation | Sex | Age | ECOG PS | Histology | Clinical stage | 1st line regimen | Ccr |
|---|---|---|---|---|---|---|---|---|
| 1 | Renal dysfunction | Female | 84 | 1 | Ad | IV | PEM | 53 |
| 2 | Renal dysfunction | Male | 81 | 1 | Ad | IV | PEM + BEV | 63 |
| 3 | Ileus | Male | 75 | 1 | Sq | IV | DTX | 82 |
ECOG PS, Eastern Cooperative Oncology Group performance status; Ad, adenocarcinoma; Sq, squamous cell carcinoma; PEM, pemetrexed; BEV, bevacizumab; DTX, docetaxel; Ccr, creatinine clearance.
Efficacy
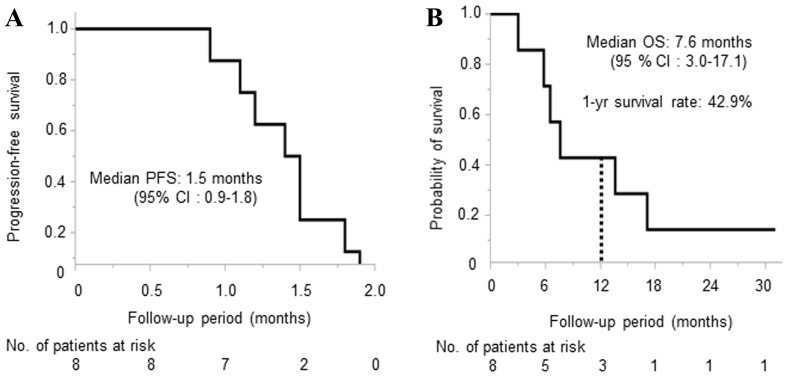
The median PFS and OS was 1.5 months (95% confidence interval: 0.9–1.8) and 7.6 months (95% confidence interval: 3.0–17.1), respectively (Fig. 2), and the 1-year survival rate was 42.9%. Regarding antitumor effect, 1 case reached stable disease, and 7 cases reached PD. DCR was 12.5% (Table IV). The individual characteristics of the 8 patients including the treatment administered after this study are shown in Table V.
Figure 2.
Kaplan-Meier Curve of (A) progression-free survival and (B) overall survival.
Table IV.
Overall response.
| Tumor response | n=8 (%) |
|---|---|
| CR | 0 (0) |
| PR | 0 (0) |
| SD | 1 (12.5) |
| PD | 7 (87.5) |
| DCR (CR + PR + SD) | 1 (12.5) |
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; DCR, disease control rate.
Table V.
Individual characteristics of the patients (n=8).
| Patient number | Sex | Age (years) | ECOG PS | Histology | T | N | M | Overall response | PFS (months) | Treatment after S.1 | OS (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 84 | 1 | Ad | 3 | 3 | 1a | SD | 1.2 | GEM→VNR→RT→DTX | 13.6 |
| 2 | Male | 75 | 1 | Ad | 1 | 0 | 1b | PD | 1.8 | PEM+BEV→DTX | 17.1 |
| 3 | Male | 81 | 1 | Ad | 4 | 3 | 1b | PD | 1.9 | DTX+BEV | 7.6 |
| 4 | Male | 75 | 1 | Ad | 1b | 0 | 1a | PD | 1.5 | DXT→nab-PTX→VNR | 31.1 |
| 5 | Male | 82 | 0 | Sq | 4 | 1 | 1a | PD | 0.9 | WBRT | 5.8 |
| 6 | Male | 76 | 0 | Ad | 4 | 3 | 1b | PD | 1.1 | DTX→VNR→DTX | 6.5 |
| 7 | Male | 75 | 1 | Sq | 4 | 2 | 1a | PD | 1.4 | Erlotinib | 2.8 |
| 8 | Female | 85 | 1 | NOS | 2a | 3 | 1b | PD | 1.5 | RT | 3.0 |
ECOG PS, Eastern Cooperative Oncology Group performance status; Ad, adenocarcinoma; Sq, squamous cell carcinoma; NOS, not otherwise specified; SD, stable disease; PD, progressive disease; PFS, progression free survival; OS, overall survival; T, tumor; N, node; M, metastasis; GEM, gemcitabine; VNR, vinorelbine; RT, radiation therapy; nab-PTX, nab-paclitaxel; WBRT, whole-brain radio therapy; PEM, pemetrexed; BEV, bevacizumab; DTX, docetaxel.
Discussion
We conducted a multi-center and prospective feasibility study to evaluate the safety of alternate-day S-1 therapy in elderly patients with NSCLC. In this study, no severe grade 3 or higher adverse events was observed, and the major adverse events were gastrointestinal toxicity, consistent with previous reports (10).
The mechanism by which alternate-day S-1 administration reduces toxicity has been studied. Previous studies showed differences in the cell cycle between normal and malignant cells. Normal cells regenerate in 0.5–1.5 days, whereas cancer cells regenerate in 3 to 5 days, with the S-phase lasting more than 24 h (14,15). Five-fluorouracil (5-FU), a metabolite included in S-1, acts on S-phase cells and suppresses cell proliferation. Based on this biological mechanism, the alternate-day S-1 administration would allow growth and reproduction of normal cells, while maintaining its anticancer effect. Using gastric cancer cell lines in vitro and in vivo, a previous study showed that alternate-day S-1 administration had lower toxicity while yielding similar antitumor effect compared with standard daily administration (16). In addition, a retrospective study showed that alternate-day S-1 administration decreases gastrointestinal toxicity in the patients with advanced gastric cancer (17). These results indicate that alternate-day administration is reasonable for decreasing the toxicity while maintaining the efficacy of S-1.
Severe gastrointestinal toxicities were not observed during this study, while up to grade 2 anorexia, diarrhea, and stomatitis were observed in 50% of the patients. This rate of adverse event was similar to that in a previous study in which alternate-day S-1 was administrated to previously treated NSCLC patients including 13.3% elderly patients (10). By contrast, in a study of standard S-1 administration as first-line treatment for elderly NSCLC patients, severe anorexia and nausea/vomiting were observed in 4.3% of patients (18). In addition, in a study of 2-week S-1 monotherapy treatment followed by a 1-week interval as a first-line treatment of elderly NSCLC patients, severe neutropenia and anorexia occurred in 5.0 and 7.5% of patients, respectively (19). These results indicate that alternate-day S-1 administration can be safer than the standard S-1 administration and would be a possible treatment regimen for elderly patients with NSCLC.
Although no hematological toxicities were observed during this study, grade 2 renal dysfunction was observed in 2 cases, and grade 2 ileus was observed in 1 case. Drug administration was suspended in these patients in accordance with the study protocol. The patient who developed ileus had a history of abdominal surgery. S-1 monotherapy was resumed in this patient after the ileus had improved, and no recurrence was observed. Therefore, we considered that the ileus was not related with S-1 administration. The 2 patients who discontinued S-1 due to grade 2 kidney dysfunction had low CCr before S-1 administration. We considered that S-1 administration may be associated with renal dysfunction. However, no severe renal dysfunction was reported in a previous study in which alternate-day or standard daily S-1 was administered to NSCLC patients including 14.8% elderly patients (10). In addition, another study of standard S-1 administration as first-line treatment for the elderly NSCLC patients also did not report any severe renal dysfunction (18). These results show that alternate-day S-1 administration does not induce severe renal dysfunction in elderly patients with NSCLC.
In the present study, severe adverse events were not observed. However, we should recognize that even mild diarrhea and stomatitis due to S-1 would induce kidney dysfunction, which increases the toxicity of S-1 in elderly patients. The renal function of elderly patients might be overestimated because their serum creatinine is decreased due to muscle atrophy.
While this study design was not for investigating the efficacy of alternate-day S-1 administration, the median PFS period was 1.5 months, indicating poor outcomes. However, a previous study reported a median PFS of 2.1 months in the alternate-day S-1 monotherapy for previously treated NSCLC patients including approximately 15% elderly participants (10). The present study only included elderly patients, and 6 of the 8 patients had PS score of 1. Taking these into consideration, the efficacy of the treatment regimen in this study may have been reasonable.
In conclusion, the alternate-day administration of S-1 can be safer than the standard daily administration and would be a possible treatment regimen for elderly patients with NSCLC. However, the current study was a pilot feasibility study, and the number of patients was inadequate to draw conclusive results; thus, the findings should be interpreted cautiously. In the future, the therapeutic safety and efficacy of alternate-day S-1 administration in elderly NSCLC patients should be compared against that of the standard S-1 administration in a large-scale study.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- NSCLC
non-small-cell lung cancer
- PFS
progression-free survival
- DTX
docetaxel
- OS
overall survival
- ECOG PS
Eastern Cooperative Oncology Group performance status
- EGFR
epidermal growth factor receptor
- CCr
creatinine clearance
- PD
progressive disease
- DCR
disease control rate
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
TS, KF and NH were involved in the study conception and design. TM, MW, KF, KH, NI, MD, SK, YH, SM, TN, HI and NH were involved in the acquisition of data. TM, MW, KF, KY, SS and NH analyzed and interpreted the data. TM, MW, KF, KH, NI, MD, SK, KY, SS, YH, SM, TN, TS, HI, HH and NH drafted the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study protocol was approved by the ethics committee of each hospital prior to implementation. Written informed consent was obtained from all patients prior to enrollment. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Quoix E, Zalcman G, Oster JP, Westeel V, Pichon E, Lavolé A, Dauba J, Debieuvre D, Souquet PJ, Bigay-Game L, et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet. 2011;378:1079–1088. doi: 10.1016/S0140-6736(11)60780-0. [DOI] [PubMed] [Google Scholar]
- 3.Santos FN, de Castria TB, Cruz MR, Riera R. Chemotherapy for advanced non-small cell lung cancer in the elderly population. Cochrane Database Syst Rev: CD010463. 2015 doi: 10.1002/14651858.CD010463.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Coprehensive Cancer Network, corp-author. Non-small cell lung cancer. https://www.nccn.org/professionals/physician_gls/default.aspx. [Mar 1;2018 ];NCCN Guidelines for clinical practice in oncology. Version 2. 2018 [Google Scholar]
- 5.Gridelli C, Perrone F, Gallo C, Cigolari S, Rossi A, Piantedosi F, Barbera S, Ferraù F, Piazza E, Rosetti F, et al. Chemotherapy for elderly patients with advanced non-small-cell lung cancer: The multicenter italian lung cancer in the elderly study (MILES) phase III randomized trial. J Natl Cancer Inst. 2003;95:362–372. doi: 10.1093/jnci/95.5.362. [DOI] [PubMed] [Google Scholar]
- 6.Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small-cell lung cancer. The Elderly Lung Cancer Vinorelbine Italian Study Group. J Natl Cancer Inst. 1999;91:66–72. doi: 10.1093/jnci/91.1.66. [DOI] [PubMed] [Google Scholar]
- 7.Kudoh S, Takeda K, Nakagawa K, Takada M, Katakami N, Matsui K, Shinkai T, Sawa T, Goto I, Semba H, et al. Phase III study of docetaxel compared with vinorelbine in elderly patients with advanced non-small-cell lung cancer: Results of the west Japan thoracic oncology group trial (WJTOG 9904) J Clin Oncol. 2006;24:3657–3663. doi: 10.1200/JCO.2006.06.1044. [DOI] [PubMed] [Google Scholar]
- 8.Nishio M, Mok TSK, Nakagawa K, et al. EAST-LC: Randomized controlled phase III trial of S-1 versus docetaxel in patients with non-small-cell lung cancer who had received a platinum-based treatment. Ann Oncol. 2017;28:x124–x143. doi: 10.1093/annonc/mdx419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshioka H, Okamoto I, Morita S, Ando M, Takeda K, Seto T, Yamamoto N, Saka H, Atagi S, Hirashima T, et al. Efficacy and safety analysis according to histology for S-1 in combination with carboplatin as first-line chemotherapy in patients with advanced non-small-cell lung cancer: Updated results of the west Japan oncology group LETS study. Ann Oncol. 2013;24:1326–1331. doi: 10.1093/annonc/mds629. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki A, Maemondo M, Sugawara S, Nakagawa T, Taima K, Inoue A, Matsuno K, Usui K, Yokoi T, Kanbe M, et al. Randomized phase II trial of daily administration versus alternate-day administration of S-1 in patients with advanced non-small cell lung cancer. Cancer Treat Res Commun. 2017;12:56–61. doi: 10.1016/j.ctarc.2017.05.004. [DOI] [Google Scholar]
- 11.Yamaue H, Shimizu A, Hagiwara Y, Sho M, Yanagimoto H, Nakamori S, Ueno H, Ishii H, Kitano M, Sugimori K, et al. Multicenter, randomized, open-label Phase II study comparing S-1 alternate-day oral therapy with the standard daily regimen as a first-line treatment in patients with unresectable advanced pancreatic cancer. Cancer Chemother Pharmacol. 2017;79:813–823. doi: 10.1007/s00280-017-3250-8. [DOI] [PubMed] [Google Scholar]
- 12.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L, International Association for the Study of Lung Cancer International Staging Committee; Participating Institutions The IASLC lung cancer staging project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 13.National Cancer institute, corp-author. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. [Mar 1;2018 ];Common terminology criteria for adverse events (CTCAE) [Google Scholar]
- 14.Lipkin M, Sherlock P, Bell B. CELL proliferation kinetics in the gastrointestinal tract of man. II. Cell renewal in stomach, ileum, colon and rectum. Gastroenterology. 1963;45:721–729. [PubMed] [Google Scholar]
- 15.Clarkson B, Ota K, Ohkita T, O'Connor A. Kinetics of proliferation of cancer cells in neoplastic effusions in man. Cancer. 1965;18:1189–1213. doi: 10.1002/1097-0142(196510)18:10<1189::AID-CNCR2820181002>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 16.Arai W, Hosoya Y, Haruta H, Kurashina K, Saito S, Hirashima Y, Yokoyama T, Zuiki T, Sakuma K, Hyodo M, et al. Comparison of alternate-day versus consecutive-day treatment with S-1: Assessment of tumor growth inhibition and toxicity reduction in gastric cancer cell lines in vitro and in vivo. Int J Clin Oncol. 2008;13:515–520. doi: 10.1007/s10147-008-0780-4. [DOI] [PubMed] [Google Scholar]
- 17.Sakuma K, Hosoya Y, Arai W, Haruta H, Ui T, Kurashina K, Saito S, Hirashima Y, Yokoyama T, Zuiki T, et al. Alternate-day treatment with S-1 in patients with gastric cancer: A retrospective study of strategies for reducing toxicity. Int J Clin Oncol. 2010;15:166–171. doi: 10.1007/s10147-010-0036-y. [DOI] [PubMed] [Google Scholar]
- 18.Shiroyama T, Kijima T, Komuta K, Yamamoto S, Minami S, Ogata Y, Okafuji K, Imamura F, Hirashima T, Tachibana I, et al. Phase II tailored S-1 regimen study of first-line chemotherapy in elderly patients with advanced and recurrent non-small cell lung cancer. Cancer Chemother Pharmacol. 2012;70:783–789. doi: 10.1007/s00280-012-1958-z. [DOI] [PubMed] [Google Scholar]
- 19.Goto H, Okano Y, Machida H, Hatakeyama N, Ogushi F, Haku T, Kanematsu T, Urata T, Kakiuchi S, Hanibuchi M, et al. Phase II study of tailored S-1 monotherapy with a 1-week interval after a 2-week dosing period in elderly patients with advanced non-small cell lung cancer. Respir Investig. 2018;56:80–86. doi: 10.1016/j.resinv.2017.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.