Abstract
Background
Autonomic dysfunction is common in the later stages of Parkinson's disease (PD), but less is known about its presence and severity in early disease.
Objective
To analyze features of autonomic dysfunction in recent onset PD cases, and their relationship to motor severity, medication use, other nonmotor symptoms (NMS), and quality‐of‐life scores.
Methods
Detailed patient‐reported symptoms of autonomic dysfunction were assessed in a multicenter cohort study in PD cases that had been diagnosed within the preceding 3.5 years.
Results
There were 1746 patients (1132 males, 65.2%), mean age 67.6 years (SD 9.3), mean disease duration 1.3 years (SD 0.9), mean Movement Disorder Society Unified Parkinson's Disease Rating Scale motor score 22.5 (SD 12.1). Orthostatic symptoms were reported by 39.6%, male erectile dysfunction by 56.1%, and female anorgasmia by 57.4%. Sialorrhea was an issue in 51.4% of patients, constipation in 43.6%, and dysphagia in 20.1%. Autonomic features increased with higher modified Hoehn and Yahr stages (P < 0.001). The severity of autonomic dysfunction was associated with the postural instability gait difficulty motor phenotype [β‐coefficient 1.7, 95% confidence interval (CI) 0.7, 2.6, P < 0.001], depression (β‐coefficient 4.1, CI 3.0, 5.2, P < 0.001), and excess daytime sleepiness (β‐coefficient 3.1, CI 1.9, 4.2, P < 0.001). Dopamine agonists were the only drug class associated with greater autonomic dysfunction (P = 0.019). The severity of autonomic dysfunction strongly correlated with the presence of other NMS (ρ = 0.717, P < 0.001), and with poorer quality‐of‐life scores (ρ = 0.483, P < 0.001).
Conclusions
Autonomic dysfunction is common in early PD. Autonomic dysfunction correlates with the presence of other NMS, and with worse quality of life.
Keywords: Parkinson's disease, sexual dysfunction, autonomic dysfunction
Introduction
Autonomic dysfunction is well recognized in Parkinson's disease (PD), and is often multifaceted.1 The symptoms of autonomic dysfunction in PD range from orthostatic intolerance to vasomotor dysfunction, secretomotor problems, gastroparesis, diarrhea, constipation, bladder disturbances, pupillary and focusing abnormalities causing visual blurring, and sexual dysfunction. Dysautonomia is common in the later stages of PD, with disease progression and medication side effects.2 Prominent autonomic features in recent onset cases are classically regarded as a “red flags” for multiple system atrophy (MSA), and are highlighted as raising diagnostic doubt for PD. Specifically, orthostatic hypotension and urinary incontinence in the first 5 years are suggestive of MSA.3 However, the accuracy of differentiating PD from MSA is low in early disease4 and sometimes even in later disease.5 This could reflect a failure to recognize the cardinal distinguishing features, but might also result from significant overlap in the autonomic manifestations of the two disorders, which is likely because the underlying distribution and type of pathology (α‐synuclein accumulation) has significant similarities.6
We explored the prevalence, range, and severity of autonomic symptoms in a cohort of cases recently diagnosed with PD. We also examined the extent to which cases with unusual or atypical features exhibited differences in autonomic function from those without such features. We hypothesized that the presence of atypical features would be reflected in greater levels of autonomic dysfunction.
Methods
The Tracking Parkinson's study is a large prospective, observational, multicenter project in the United Kingdom (UK). Patients were recruited with a clinical diagnosis of PD, fulfilling UK Brain Bank criteria7 and supported by structural and/or functional neuroimaging performed when the diagnosis was not firmly established clinically. Both drug‐naïve and treated patients, aged 18 to 90 years, were eligible. Recent onset cases (diagnosed with PD in the preceding 3.5 years) were recruited between February 2012 and May 2014. Patients were not enrolled if they had severe comorbid illness, other degenerative forms of parkinsonism including clinician‐diagnosed MSA, or parkinsonism attributable to significant cerebrovascular disease. Patients with drug‐induced parkinsonism were excluded, but drug‐unmasked PD was allowed if justified by abnormal functional dopaminergic imaging. Patients whose diagnosis was changed from PD during a 6‐month follow‐up were excluded from analysis.
Any features that were potentially unusual for PD were noted at recruitment and during follow‐up, including atypical signs or symptoms, either a static or a rapidly progressive disease course, and a poor response to dopaminergic medication. Cases with any of these potentially atypical features, and cases with missing data, were excluded from the main analysis (Figure 1). However, an additional analysis was performed to compare autonomic features, in cases with possible atypical features, to the entirely typical PD cases.
Figure 1.
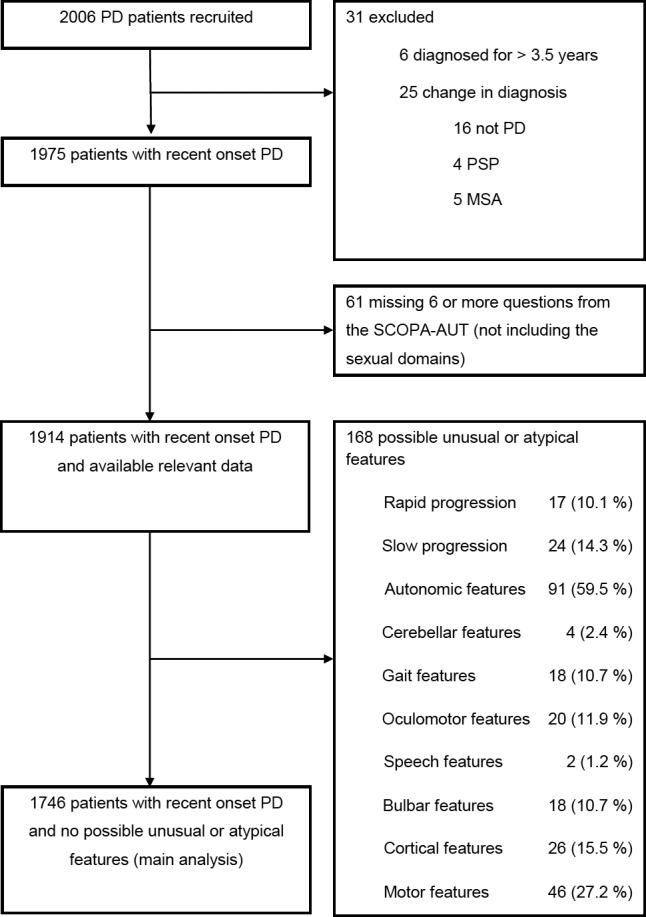
Patient recruitment to this study. The main analysis of autonomic features was performed in cases without features that might be atypical for Parkinson's disease. Additional analysis was undertaken comparing autonomic features in those with and without atypical features. PD, Parkinson's disease; PSP, progressive supranuclear palsy; MSA, multiple system atrophy; SCOPA‐AUT, scales for outcomes in Parkinson's disease—autonomic symptoms.
The study was performed in accordance with the Declaration of Helsinki. Parkinson's UK, the national patient care and research organization, provided research funding for this project.
Seventy‐two sites in the UK, which provide secondary care treatment for PD patients as part of the UK National Health Service (NHS) (or linked academic institutions), participated, with ethics committee and research and development approvals, and written patient informed consent.
Demographic characteristics, diagnostic features at presentation, medication history, dietary history, modified Hoehn and Yahr (H&Y) staging,8 Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS UPDRS) scores,9 and motor “on” or “off” state in the clinic were recorded. The Scale for Outcomes in Parkinson's disease for Autonomic Symptoms (SCOPA‐AUT), a 23‐item validated questionnaire, was used to assess features of autonomic dysfunction10 in six domains: gastrointestinal, urinary, cardiovascular, thermoregulatory, pupillomotor, and sexual, resulting in a total score out of 69. Patients completed this questionnaire on paper. Results were tabulated against the H&Y stage, considering mild disease as grade 1 or 1.5, moderate as grade 2 or 2.5, and more severe as grade 3 or higher. The nonmotor symptom severity scale (NMSS)11 and PD 8‐item quality‐of‐life Questionnaire (PDQ‐8)12 were also scored. Montreal cognitive assessment (MoCA) test scores were adjusted for years of education. Sleep problems were assessed with the PD sleep scale (PDSS, higher PDSS scores are associated with less sleep disturbance),13 Epworth Sleepiness scale (ESS)14 and rapid eye movement sleep (REM) sleep behavior disorder scale.15 Hyposmia was defined as Sniffin' Stick score at or below the 10th centile16 or University of Pennsylvania Smell Identification Test score (British version) at or below the 15th centile, corrected for age and sex.17 Presence of low mood and depression was assessed using the Leeds Anxiety and Depression Scale (LADS) with a cut‐off score >6.18 Daily antiparkinsonian medication doses [in levodopa (l‐dopa) equivalent units] were calculated using an established formula.19 Orthostatic hypotension was defined by 2 established methods, based on a blood pressure fall from lying flat to standing for 3 minutes of (a) ≥20 mm Hg systolic or 10 mm Hg diastolic,20 and (b) ≥30 mm Hg systolic or 15 mm Hg diastolic.3
Statistical Analysis
Spearman rank order correlation was used to assess the relationship between baseline SCOPA‐AUT score (in those answering >75% of questions) and baseline motor, nonmotor and quality‐of‐life scores. Multiple linear regression was performed to determine the influence of H&Y staging on autonomic scores. Logistic regression was used when outcomes were binary, ordinal logistic regression (also called a proportional odds model) when outcomes were ordinal, and multinomial logistic regression when the motor subtype was outcome with tremor dominant as the baseline category. When exposures are categorical, as in the H&Y stage, we calculated both heterogeneity P values across all groups and a linear trend P value. For examining SCOPA‐AUT domains a linear regression approach was used when there were >10 categories and ordinal logistic regression was used for ≤10 categories. The proportional odds assumption in ordinal logistic regression was tested and relaxed to a partial proportional odds model in which a variable did not meet the assumption. The linearity of continuous confounders (such as age and disease duration) was tested using fractional polynomials in univariate models and then was transformed if they showed evidence of nonlinearity. P values were 2‐tailed, significance was set at P < 0.05, and STATA (version 13, StataCorp, College Station, TX) was used.
Results
Of 2006 cases recruited, 31 were excluded because of a change in diagnosis or disease duration greater than 3.5 years, 61 had missing detail for autonomic features, and 168 had one or more possible unusual or atypical features for PD (Figure 1). The main analysis dataset was therefore 1746 patients (1132 males, 65.2%), mean age 67.6 years (SD 9.3), mean disease duration 1.3 years (SD 0.9), mean MDS UPDRS 3 score 22.5 (SD 12.1), mean LEDD 294 mg (SD 205), and mean SCOPA‐AUT score 11.7 (SD 7.0). Considering motor score assessments, 1624 patients had their “on–off” state recorded, of whom 1494 (92.0%) were in the “on” state, and 44 of the 130 in the “off” state (33.8%) were drug naïve, meaning that 86 of the 1624 cases (5.3%) were in an “off” state when assessed and while on prescribed antiparkinsonian medication. The SCOPA‐AUT score was significantly higher in relation to more advanced H&Y motor stages (P < 0.001; Table 1). Other demographic characteristics are shown in Table 1.
Table 1.
Demographic and Clinical Features in Recent Onset Parkinson's Disease
| Variable | Total (N = 1738) | Hoehn and Yahr Stage | Heterogeneity (P Value) | Trend (P Value) | ||
|---|---|---|---|---|---|---|
| 1 (N = 855) | 2 (N = 783) | 3 + (N = 100) | ||||
| Age (y) | 67.6 (9.3) | 66.1 (9.2) | 68.7 (9.0) | 71.8 (9.1) | <0.001a | <0.001a |
| Age at diagnosis (y) | 66.3 (9.2) | 64.9 (9.2) | 67.3 (9.0) | 70.3 (9.1) | <0.001a | <0.001a |
| Duration (y) | 1.3 (0.9) | 1.2 (0.9) | 1.4 (0.9) | 1.4 (1.0) | <0.001b | <0.001b |
| Male sex | 1132 (65.1%) | 539 (63.0%) | 540 (69.0%) | 53 (53.0%) | 0.001c | 0.88c |
| Drug naïve | 168 (9.7%) | 108 (12.6%) | 56 (7.2%) | 4 (4.1%) | 0.007 d | 0.001d |
| LEDD (mg) | 294 (205) | 264 (208) | 320 (202) | 353 (158) | <0.001d | <0.001d |
| MDS UPDRS 3 | 22.5 (12.1) | 16.8 (8.8) | 26.4 (11.3) | 40.1 (13.7) | <0.001d | <0.001d |
| Motor Subtype | ||||||
| Tremor dominant | 763 (46.9%) | 414 (51.7%) | 339 (46.2%) | 10 (10.8%) | ||
| PIGD | 652 (40.1%) | 273 (34.1%) | 306 (41.7%) | 73 (78.5%) | <0.001d | <0.001d |
| Indeterminate | 212 (13.0%) | 114 (14.2%) | 88 (12.0%) | 10 (10.8%) | 0.018d | 0.45d |
| SCOPA‐AUT total | 11.7 (7.0) | 10.7 (6.2) | 12.7 (7.6) | 13.5 (6.9) | <0.001d | <0.001d |
Eight cases with missing H&Y status are not shown; data are presented as mean and standard deviation or percentage.
LEDD, l‐dopa equivalent daily dose; MDS UPDRS 3, Movement Disorder Society Unified Parkinson's disease rating scale Part 3; PIGD, postural instability and gait difficulty; SCOPA‐AUT, scales for outcomes in Parkinson's disease—autonomic symptoms.
Adjusted for sex and disease duration.
Adjusted for sex and age.
Adjusted for age and disease duration.
Adjusted for sex, age, and disease duration.
Symptoms of autonomic dysfunction were present in all six domains of the SCOPA‐AUT questionnaire, but to varying degrees (Table 2). Those with more advanced motor stages of disease reported significantly more of the following features, whether considering the heterogeneity or trend P values: sialorrhea, constipation, incomplete bladder emptying, lightheaded when standing up, lightheaded after standing for some time, nighttime sweating, and cold intolerance (Tables 2 and 3). Hyperhidrosis during the day was less likely (<20% of cases) than hyperhidrosis during the night (nearly 30%). Only 11.1% of patients who reported syncope had an objective drop in blood pressure exceeding 30/15 mm Hg, whereas 24.4% of those who reported syncope had a blood pressure drop exceeding 20/10 mm Hg. There was a weakly positive correlation of sialorrhea with both dysphagia (ρ = 0.126, P < 0.001) and with swallowing/choking (ρ = 0.157, P < 0.001).
Table 2.
Gastrointestinal and Urinary Disturbance in Relation to Motor Severity Grading in Recent Onset Parkinson's Disease (% with an Item Score ≥1)
| Variable | Total (N = 1738) (%) | Hoehn and Yahr Stage | Heterogeneity (P Value)a | Trend (P Value)a | ||
|---|---|---|---|---|---|---|
| 1 (N = 855) (%) | 2 (N = 783) (%) | 3 + (N = 100) (%) | ||||
| Gastrointestinal domain | 1437 (83.7) | 687 (81.1) | 659 (85.6) | 91 (91.0) | 0.091 | 0.033 |
| Swallowing/choking | 421 (24.3) | 199 (23.3) | 190 (24.3) | 32 (32.0) | 0.22 | 0.21 |
| Sialorrhea | 891 (51.4) | 387 (45.3) | 448 (57.5) | 56 (56.0) | 0.003 | 0.002 |
| Dysphagia | 349 (20.1) | 168 (19.7) | 156 (19.9) | 25 (25.0) | 0.62 | 0.74 |
| Early abdominal fullness | 361 (20.8) | 164 (19.2) | 168 (21.5) | 29 (29.0) | 0.095 | 0.035 |
| Constipation | 755 (43.6) | 335 (39.2) | 362 (46.5) | 58 (58.0) | 0.003 | <0.001 |
| Straining for defecation | 1017 (58.7) | 470 (55.1) | 486 (62.4) | 61 (61.0) | 0.12 | 0.12 |
| Fecal incontinence | 125 (7.2) | 59 (6.9) | 56 (7.2) | 10 (10.0) | 0.78 | 0.75 |
| Urinary domain | 1654 (96.6) | 815 (96.6) | 746 (96.8) | 93 (95.9) | 0.85 | 0.74 |
| Urinary urgency | 707 (40.8) | 329 (38.6) | 333 (42.7) | 45 (45.5) | 0.72 | 0.48 |
| Urinary incontinence | 500 (28.8) | 234 (27.4) | 228 (29.2) | 38 (38.0) | 0.57 | 0.41 |
| Incomplete emptying | 739 (42.6) | 342 (40.0) | 340 (43.5) | 57 (57.6) | 0.011 | 0.016 |
| Weak stream of urine | 717 (41.4) | 334 (39.1) | 339 (43.5) | 44 (44.9) | 0.59 | 0.30 |
| Frequency | 1371 (79.1) | 679 (79.6) | 617 (79.0) | 75 (75.0) | 0.71 | 0.69 |
| Nocturia | 1518 (87.5) | 736 (86.5) | 693 (88.5) | 89 (89.0) | 0.85 | 0.63 |
Eight cases with missing H&Y status are not shown.
Adjusted for age, sex, and disease duration.
Table 3.
Cardiovascular, Thermoregulatory, Pupillomotor, and Sexual Disturbance in Relation to Motor Severity Grading in Recent Onset Parkinson's Disease (% with an Item Score ≥1)
| Variable | Total (N = 1738) (%) | Hoehn and Yahr Stage | Heterogeneity (P Value)a | Trend (P Value)a | ||
|---|---|---|---|---|---|---|
| 1 (N = 855) (%) | 2 (N = 783) (%) | 3 + (N = 100) (%) | ||||
| Cardiovascular domain | 680 (39.6) | 315 (37.1) | 313 (40.4) | 52 (54.7) | 0.007 | 0.005 |
| Lightheaded when standing up | 580 (33.6) | 265 (31.1) | 270 (34.8) | 45 (46.4) | 0.007 | 0.004 |
| Lightheaded when standing for some time | 414 (23.9) | 187 (21.9) | 191 (24.4) | 36 (36.7) | 0.011 | 0.006 |
| Syncope | 48 (2.8) | 18 (2.1) | 26 (3.3) | 4 (4.0) | 0.30 | 0.14 |
| Thermoregulatory domain | 1174 (68.0) | 563 (66.1) | 539 (69.5) | 72 (72.0) | 0.19 | 0.078 |
| Hyperhidrosis during the day | 333 (19.2) | 162 (19.0) | 149 (19.1) | 22 (22.0) | 0.21 | 0.088 |
| Hyperhidrosis during the night | 516 (29.7) | 242 (28.3) | 236 (30.2) | 38 (38.0) | 0.04 | 0.016 |
| Cold intolerance | 804 (46.4) | 360 (42.2) | 395 (50.7) | 49 (49.0) | 0.003 | 0.006 |
| Heat intolerance | 487 (28.0) | 244 (28.6) | 204 (26.1) | 39 (39.0) | 0.059 | 0.33 |
| Pupillomotor domain | ||||||
| Oversensitive to bright light | 485 (28.0) | 226 (26.5) | 229 (29.2) | 30 (30.3) | 0.57 | 0.40 |
| Sexual domain | 711 (63.0) | 365 (61.9) | 315 (63.6) | 31 (72.1) | 0.76 | 0.73 |
| Men: erection problem | 513 (56.1) | 238 (51.5) | 254 (60.3) | 21 (65.6) | 0.21 | 0.085 |
| Men: ejaculation problem | 393 (45.9) | 181 (41.6) | 197 (50.4) | 15 (50.0) | 0.14 | 0.099 |
| Women: vaginal lubrication | 144 (48.5) | 88 (53.0) | 48 (41.4) | 8 (53.3) | 0.081 | 0.12 |
| Women: problem with orgasm | 166 (57.4) | 99 (61.9) | 59 (51.3) | 8 (57.1) | 0.17 | 0.12 |
Eight cases with missing H&Y status are not shown.
Adjusted for age, sex, and disease duration (except gender‐specific sexual domain not adjusted for sex).
A substantial number of patients (33.9%) marked “not applicable” for one or both of the sexual items (in 24.9% of males and 50.8% of females), and such cases were older at the time of assessment and had an older age at diagnosis compared to the other patients (P < 0.001 for age and both genders).
The presence of depression (P < 0.001), REM sleep behavior disorder (P < 0.001), and the postural instability gait difficulty phenotype (P < 0.001) correlated with SCOPA‐AUT scores (Table 4).
Table 4.
Autonomic Severity in Relation to Key Motor and Nonmotor Categorized Variables in Recent Onset Parkinson's Disease
| Variable | Number (%) | SCOPA‐AUT Score | Adjusted P Valuea | |||
|---|---|---|---|---|---|---|
| Mean (SD) | Unadjusted β (95% CI) | P value | Adjusteda β (95% CI) | |||
| Hyposmia | 699 (75.3) | 11.6 (6.8) | −0.0 (−1.1, 1.0) | 0.95 | 0.0 (−1.0, 1.1) | 0.94 |
| Depression | 227 (21.3) | 16.0 (8.2) | 5.5 (4.6, 6.5) | <0.001 | 4.1 (3.0, 5.2) | <0.001 |
| RBD | 485 (46.3) | 13.5 (7.4) | 3.5 (2.7, 4.4) | <0.001 | 1.9 (1.0, 2.8) | <0.001 |
| Epworth sleepiness | 197 (18.5) | 15.7 (8.4) | 4.9 (3.9, 6.0) | <0.001 | 3.1 (1.9, 4.2) | <0.001 |
| Motor subtype | ||||||
| Tremor dominant | 487 (48.0) | 10.1 (6.5) | 0 (reference) | 0 (reference) | ||
| PIGD | 386 (38.0) | 13.8 (7.4) | 3.7 (2.8, 4.6) | <0.001 | 1.7 (0.7, 2.6) | <0.001 |
| Indeterminate | 142 (14.0) | 11.5 (6.1) | 1.4 (0.1, 2.7) | 0.030 | 0.3 (−1.1, 1.6) | 0.71 |
SCOPA‐AUT, scales for outcomes in Parkinson's disease—autonomic symptoms; SD, standard deviation; CI, confidence interval; RBD, REM sleep behavior disorder; PIGD, postural instability gait difficulty.
Adjusted for age, sex, disease duration, and each of the nonmotor features.
Patients who were diagnosed with PD at study entry but who had atypical features had significantly higher MDS UPDRS 3 scores (P < 0.001) compared to those without any atypical features, but there were no significant differences in age or disease duration (Table S1). Patients with atypical features were less likely to have the tremor dominant subtype (P = 0.020), more likely to report gastrointestinal symptoms (P = 0.016) as well as urinary dysfunction (P < 0.001), and they had higher total SCOPA‐AUT scores (P = 0.008) (Table S1). Orthostatic hypotension with a drop of more than 30/15 mm Hg was present in 6.8% of cases with entirely typical PD, whereas an additional 10% of these typical PD cases fulfilled the less stringent criteria (20/10 mm Hg) (Table S1). There was no significant difference in the proportion of cases with orthostatic hypotension by either criterion, when comparing typical PD cases to the PD cases with possibly atypical features. There was a no correlation of orthostatic hypotension with ESS total score (ρ = 0.032, P = 0.20) or PDSS total scores (ρ = −0.018, P = 0.47), and the correlation with MoCA total score was negligible (ρ = −0.053, P = 0.037).
The majority of our patients were using l‐dopa–based medication (61.1%), 34.2% were using dopamine agonists, and 27.3% were on monoamine oxidase type B inhibitors. Very few (1.3%) were on central anticholinergics. Other medications with potential to cause postural hypotension, including diuretics, β‐blockers, calcium channel blockers, angiotensin converting enzyme inhibitors, α‐blockers, nitrates, peripheral vasodilators, centrally acting antihypertensives, and angiotensin receptor blockers, were used by 38.6%. Among the antiparkinsonian drug classes, only the use of dopamine agonists was associated with higher SCOPA‐AUT scores (β coefficient 1.3, CI 0.2, 2.4, P = 0.019) after appropriate adjustment for age, sex, and disease duration (Table S2).
There was a positive correlation between the presence of autonomic symptoms and motor severity based on MDS UPDRS part 3 scores (ρ = 0.207, P < 0.001) as well as the presence of sleep disturbance based on PDSS scores (ρ = −0.487, P < 0.001) and other nonmotor symptoms (NMS) based on NMSS scores (ρ = 0.717, P < 0.001). SCOPA‐AUT scores also correlated positively with quality‐of‐life scores based on PDQ 8 (ρ = 0.483, P < 0.001). Correlation of SCOPA‐AUT total score against disease duration was weak (ρ = 0.114, P < 0.001). Cognitive scores showed a weakly negative correlation with SCOPA‐AUT scores (ρ = −0.119, P < 0.001) (Table S3).
In the 168 drug‐naïve cases, SCOPA‐AUT scores were lower at 9.5 (5.8), and were higher with more advanced H&Y stage, P < 0.001 (Table S4). Depression was significantly correlated (P = 0.003) with SCOPA‐AUT scores (Table S5), and there were also significant relationships between total SCOPA‐AUT scores and NMSS (ρ = 0.682, P < 0.001), PDSS (ρ = −0.588, P < 0.001), PDQ8 (ρ = 0.474, P < 0.001), and MDS UPDRS 3 (ρ = 0.230, P = 0.021) scores (Table S6).
Discussion
In this study, we show that symptoms of autonomic dysfunction are common at an average PD duration of slightly more than 1 year. The total SCOPA‐AUT scores at this early stage were around seven points lower than in cases with an average disease duration of around 10 years, and around three points higher than that of matched controls.10, 21 Within our cohort, a more advanced motor stage was accompanied by a higher autonomic symptom burden, which was also found in two previously published studies of PD at a longer disease duration.2, 10 One previous study did not document such differences in autonomic disturbance between mild and moderate motor PD stages, but did show greater autonomic disturbance at more advanced stages (H&Y 4 and 5).21 However, the size of that study was a quarter of our study size, and patients were approximately 6.5 years younger, which could explain the differences.
The relative proportions of symptoms in different domains of autonomic function in our study are similar to the PROPARK study.21 In that study, syncope affected 4% of 420 patients at around 10 years of disease duration, being only 2% among H&Y 1 and 2 patients,21 which is similar to the 2.8% figure in our series at 1.3 years' duration, suggesting that this feature is partly related to stage and disease duration, but generally the prevalence of this symptom is low. In contrast, urinary incontinence was present in 51% of patients at 10 years' duration (and 43% at H&Y 1 and 2),21 compared to 28.8% in our study, suggesting that this problem becomes more common with increasing PD disease duration.
One recurrent aspect in assessing autonomic symptoms relates to sexual dysfunction, which, as in our study, is often the main source of missing data.21 Whether or not the choice of the “not applicable” option for sexual items by patients reflects earlier loss of autonomic function, relates to the personal and sensitive nature of the subject, or is for reasons of sexual inactivity as a result of other influences, the reason is difficult to determine from the questionnaire, and this deserves further study.
Sleep disorders are common in PD and some sleep disorders such as REM sleep behavior disorder can predate the presenting motor symptoms of PD. The correlation we identified between sleep problems and autonomic symptoms confirms previous findings among 135 PD patients at a mean disease duration of 5.3 years, in which worse SCOPA‐AUT scores were also associated with worse sleep quality.2 Our finding that the ESS and the RBDSQ were both significant independent predictors of autonomic severity (even after adjusting for age, sex, and disease duration) is also consistent with this.2
We found that the use of dopamine agonists was associated with more autonomic dysfunction, but there was no such association with other antiparkinsonian drug classes, or when comparing treated to untreated patients. Comparison with a previous study, which reported greater autonomic symptom severity in “treated” compared to “untreated” patients, is difficult because our definition was of entirely drug‐naïve patients whereas their patients were defined as not taking dopamine replacement therapy (but this definition allowed selegiline and amantadine).21 Similarly, comparisons with another study that showed no correlation between SCOPA‐AUT and antiparkinsonian medication are difficult, because that study of 154 patients included around one‐fifth of cases with either MSA or progressive supranuclear palsy (PSP),22 and included patients that were on average 9 years younger than our patients, with disease duration that was 5 years longer. Our results are therefore novel in identifying an association of autonomic dysfunction and dopamine agonists, which appears to be independent from blood pressure–lowering effects, as we did not find any linkage between autonomic scores and the use of other medications known to lower blood pressure.
Specifically addressing the issue of orthostatic hypotension, another study showed a much higher rate (58% of 91 patients had a systolic blood pressure fall of 20 mm Hg) than we did, but their disease duration was longer (7 years), all their patients were receiving l‐dopa, and they were receiving it at higher doses (mean daily dose 682 mg) than our cases.23 Our finding that less than a quarter of patients reporting syncope had an objective drop in blood pressure is in keeping with a previous study.24 This presumably relates to transient drops in blood pressure, which are not captured at every clinic visit, because they can be remote in timing from previous syncopal events. This can make it difficult to establish a consistent relationship between syncope (identified by history) and orthostatic hypotension (confirmed on examination). However, both are important risk factors for fall‐related physical injuries.25 Further, orthostatic hypotension can be a key marker of autonomic dysfunction. In one study of patients with PD (and dementia with Lewy bodies), those with persistent orthostatic hypotension had a significantly shorter survival compared to those with no or nonpersistent orthostatic hypotension. Patients with constipation and/or urinary incontinence, in addition to persistent orthostatic hypotension, had a poorer prognosis compared to those with isolated persistent orthostatic hypotension or no orthostatic hypotension.26 The presence of orthostatic hypotension is reported to influence certain domains of cognitive function in PD such as attention.27 Although exact pathological correlations have not been established, α‐synuclein accumulation in the central and peripheral autonomic nervous systems as well as in neocortical areas is generally believed to be a link between autonomic dysfunction and cognitive decline in PD.28 The negative correlation between the presence of orthostatic hypotension and cognitive scores that we found is consistent with that hypothesis, and the associations with both daytime sleepiness and nighttime sleep disturbance, are of potential significance. We intend to analyze these prognostic indicators in longer‐term follow‐up of our cohort.
Sweating dysfunction was more than twice as common in another small study (64% of 77 cases), compared to our series, but their cases had a mean disease duration of more than 12 years, and the relationship was strongest between motor fluctuations (both “off” periods and dyskinesia) and sweating episodes,29 in contrast with our study at a much earlier disease stage when motor fluctuations are uncommon.
Constipation is a recognized prodromal feature of PD30 but more than half of our cases did not report this, which implies limited sensitivity in the prodromal phase, for this is as an early nonmotor feature.31 Gastrointestinal symptoms that occur more frequently in PD than controls are sialorrhea, dysphagia, nausea, constipation, and defecatory dysfunction.32 This is likely to reflect direct involvement of the myenteric plexus with Lewy body pathology. Comparison of our findings to previous reports is inexact because of longer disease duration in earlier reports,32 but the rank order and frequency of key symptoms such as sialorrhea, constipation, dysphagia, and fecal incontinence in our study was similar to one large study at around 7 years' disease duration.33 This raises the question as to whether or not such gastrointestinal features evolve significantly with time, or alternatively reflect long‐standing pathological changes that emerge long before motor features of PD appear, and have a very slow progression rate.34
The severity of autonomic dysfunction in our study of recently diagnosed cases of PD correlated with motor severity, other nonmotor features, and quality‐of‐life scores. This is not surprising, as previous studies assessing various subdomains of autonomic function also found positive correlations with disease severity and quality‐of‐life measures.21, 23, 29, 32 In a study to evaluate the relative frequency and comparative impact of all NMS on health‐related quality‐of‐life scores, not only were autonomic symptoms the most common NMS reported by patients, but the health‐related quality of life correlated most strongly with these symptoms.35 The strongest correlation we found between the SCOPA‐AUT and other clinimetric variables was with NMSS scores. Although both SCOPA‐AUT and NMSS are global scales for assessing autonomic symptoms, when more detailed measures are required for assessing specific domains of autonomic function, such as sialorrhea, dysphagia, or constipation, more specific scales for individual symptoms might be required.36
Diagnostic accuracy is a key consideration, and it is particularly relevant to the assessment of autonomic features related to PD, as the inclusion of MSA cases would exaggerate the findings. A greater autonomic symptom burden is expected in MSA than in PD, and it is reflected by higher SCOPA‐AUT scores, which are based on more bowel and urinary problems.22For this reason, we defined our main analysis group as PD cases without any unusual or atypical features, and we examined the extent to which clinicians appeared to be influenced by autonomic features in their categorization of the cases that were excluded from the main analysis. Such atypical cases showed significantly higher autonomic scores, both “total” and in the gastrointestinal and urinary domains, and they also had greater motor severity and were more likely to have postural instability and a gait difficulty phenotype. This suggests that the practicing clinician must recognize a mix of motor and nonmotor features that are potentially challenging to the diagnosis of PD. If a significant proportion of these “possibly atypical” cases are correctly diagnosed as PD, then we will have somewhat underestimated the autonomic system components of early PD. On the other hand, cases may later turn out to be MSA may be present in our group without atypical features at the time of assessment. In considering the balance of these issues, 4.8% of our cohort (91 of 1914 cases) had possibly unusual or atypical clinical features. Inclusion of a qualitative component regarding diagnostic accuracy may be useful in helping to understand the findings from pathological studies that consistently report a proportion of MSA cases among patients labeled as PD in life.4, 5, 7, 37 Early diagnostic accuracy in both PD and MSA is critical to research advances,4 but this may require the discovery of biomarkers rather than the more rigid application of clinical diagnostic criteria, noting the overlap in autonomic and other features between the “entirely typical” and “less typical” PD cases. Although our study predated the most recent consensus regarding PD diagnosis,38 our follow‐up assessments will encompass these criteria.
The present report represents the largest study (n > 1700) to date examining the issue of autonomic dysfunction in PD at an early PD stage. The large study size increased our ability to detect small differences between groups, and some of these differences may not be clinically significant. Considering the potential bias of motor state (“on” versus “off” when motor scoring was assessed at clinic visits), the proportion of treated patients in an “off” state was 5.3%, so this is unlikely to have influenced our interpretation significantly.
In addition, a limitation of most studies performed at an early stage of PD is the lack of autopsy data. In the current study the diagnosis of PD was based on clinical expert opinion without histopathological confirmation given the early stage at which patients were recruited to the study. The validity of our interpretation will be reassessed as we continue to follow these cases, which includes consent for autopsy. Another limitation was the use of questionnaire‐based diagnostic criteria for RBD, hyposmia, and depression without the use of laboratory testing or psychiatric assessment.
In conclusion, autonomic symptoms are present in a majority of people with early PD, and these correlate with other markers of disease severity and an impaired quality of life. Our study highlights the extent to which we may expect to see autonomic features in early PD, and the relationship between autonomic features and diagnostic certainty, highlighting the importance of the balanced diagnostic judgment approach advocated in the recent consensus guidelines.38
Author Roles: 1. Research Project: A. Conception, B. Organization, C. Data Collection, D. Execution; 2. Statistical Analysis: A. Study Design, B. Data Analysis, C. Execution, D. Review and Critique; 3. Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
N.M.: 1C, 2B, 3A, 3B
M.A.L.: 2B, 3A, 3B
K.A.G.: 1A, 1C, 2A, 2B, 3A, 3B
N.B.: 1C, 2A, 3B
R.A.B.: 1C, 2A, 3B
D.J.B.: 1C, 2A, 3B
T.F.: 1C, 2A, 3B
J.H.: 2A, 3B
H.R.M.: 1C, 2A, 3B
N.M.W.: 2A, 3B
Y.B.‐S.: 1B, 2A, 2B, 3B
N.W.W.: 2A, 3B
D.G.G.: 1A, 1C, 2A, 2B, 3A, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: The research was funded by Parkinson's UK and was supported by the National Institute for Health Research (NIHR) DeNDRoN network, the NIHR Newcastle Biomedical Research Unit based at Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University, and the NIHR funded Biomedical Research Centre in Cambridge. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. The authors report no commercial conflicts of interest. Full financial disclosures are documented in the next section.
Financial Disclosures for the previous 12 months: Naveed Malek, Michael A. Lawton, Katherine A. Grosset, Nigel M. Williams, and Yoav Ben‐Shlomo report no financial disclosures. Nin Bajaj received support from Bial Pharmaceuticals to attend an educational meeting. Roger A. Barker received grants from Parkinson's UK, NIHR, Cure Parkinson's Trust, Rosetrees Trust, MRC, Birax and EU, and received payment for advisory board attendance from Oxford Biomedica, Biogen and LCT, and honoraria from Wiley and Springer. David J. Burn received grants from NIHR, MRC, Wellcome Trust, and Parkinson's UK. He has acted as consultant for Bial Pharmaceuticals and received honoraria from Profile Pharma. John Hardy received honoraria from Eisai, and grant support from MRC/Wellcome, Parkinson's UK, and the Michael J. Fox Foundation. Huw R. Morris received grants from Medical Research Council UK, Wellcome Trust, Parkinson's UK, Ipsen Fund, Motor Neurone Disease Association, Welsh Assembly Government, PSP Association, CBD Solutions, and Drake Foundation, and payment for advisory board attendance and lectures from Teva, AbbVie, Boehringer Ingelheim, and GSK. Donald G. Grosset received grants from Parkinson's UK, and Michael's Movers, and honoraria from Acorda Inc. Tom Foltynie received grants from the Michael J. Fox Foundation, Brain Research Trust, John Black Charitable Foundation, and European Union FP7. He received honoraria from Medtronic, BIAL, Profile Pharma and Britannia Pharmaceuticals.
Supporting information
Table S1. Demographic and Autonomic Features of Parkinsonian Patients According to the Presence of One or More Possible Unusual or Atypical Diagnostic Features
Table S2. Autonomic Symptom Severity in Relation to Medication Use in Recent Onset Parkinson's Disease
Table S3. Correlation Between Various Domains of SCOPA‐AUT and Motor, Nonmotor, and Quality‐of‐Life Data.
Table S4. Demographic and Clinical Features in Recent Onset Untreated Parkinson's Disease.
Table S5. Autonomic Severity in Relation to Key Motor and Nonmotor Categorized Variables in Recent Onset Untreated Parkinson's Disease.
Table S6. Correlation Between Various Domains of SCOPA‐AUT and Motor, Nonmotor, and Quality‐of‐Life Data in Recent Onset Untreated Parkinson's Disease.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Kaufmann H, Goldstein DS. Autonomic dysfunction in Parkinson disease. Handb Clin Neurol 2013;117:259–278. [DOI] [PubMed] [Google Scholar]
- 2. Arnao V, Cinturino A, Valentino F, et al. In patient's with Parkinson disease, autonomic symptoms are frequent and associated with other nonmotor symptoms. Clin Auton Res 2015;25(5):301–307. [DOI] [PubMed] [Google Scholar]
- 3. Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008;71(9):670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adler CH, Beach TG, Hentz JG, et al. Low clinical diagnostic accuracy of early vs advanced Parkinson disease: clinicopathologic study. Neurology 2014;83(5):406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson's disease. Arch Neurol 1993;50(2):140–148. [DOI] [PubMed] [Google Scholar]
- 6. McCann H, Stevens CH, Cartwright H, Halliday GM. Alpha‐synucleinopathy phenotypes. Parkinsonism Relat Disord 2014;20(Suppl 1):S62–S67. [DOI] [PubMed] [Google Scholar]
- 7. Hughes AJ, Daniel SE, Lees AJ. Improved accuracy of clinical diagnosis of Lewy body Parkinson's disease. Neurology 2001;57(8):1497–1499. [DOI] [PubMed] [Google Scholar]
- 8. Goetz CG, Poewe W, Rascol O, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord 2004;19(9):1020–1028. [DOI] [PubMed] [Google Scholar]
- 9. Goetz CG, Fahn S, Martinez‐Martin P, et al. Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS): process, format, and clinimetric testing plan. Mov Disord 2007;22(1):41–47. [DOI] [PubMed] [Google Scholar]
- 10. Visser M, Marinus J, Stiggelbout AM, Van Hilten JJ. Assessment of autonomic dysfunction in Parkinson's disease: the SCOPA‐AUT. Mov Disord 2004;19(11):1306–1312. [DOI] [PubMed] [Google Scholar]
- 11. Chaudhuri KR, Martinez‐Martin P, Brown RG, et al. The metric properties of a novel non‐motor symptoms scale for Parkinson's disease: results from an international pilot study. Mov Dis 2007;22(13):1901–1911. [DOI] [PubMed] [Google Scholar]
- 12. Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The PDQ‐8: development and validation of a short‐form parkinson's disease questionnaire. Psychol Health 1997;12(6):805–814. [Google Scholar]
- 13. Chaudhuri KR, Pal S, DiMarco A, et al. The Parkinson's disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson's disease. J Neurol Neurosurg Psychiatry 2002;73(6):629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14(6):540–545. [DOI] [PubMed] [Google Scholar]
- 15. Stiasny‐Kolster K, Mayer G, Schafer S, Moller JC, Heinzel‐Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire—a new diagnostic instrument. Mov Disord 2007;22(16):2386–2393. [DOI] [PubMed] [Google Scholar]
- 16. Hummel T, Kobal G, Gudziol H, Mackay‐Sim A. Normative data for the “Sniffin' Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol 2007;264(3):237–243. [DOI] [PubMed] [Google Scholar]
- 17. Doty RL, Bromley SM, Stern MB. Olfactory testing as an aid in the diagnosis of Parkinson's disease: development of optimal discrimination criteria. Neurodegeneration 1995;4(1):93–97. [DOI] [PubMed] [Google Scholar]
- 18. Snaith RP, Bridge GW, Hamilton M. The Leeds scales for the self‐assessment of anxiety and depression. Br J Psychiatry 1976;128:156–165. [DOI] [PubMed] [Google Scholar]
- 19. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25(15):2649–2653. [DOI] [PubMed] [Google Scholar]
- 20. Bradley JG, Davis KA. Orthostatic hypotension. Am Fam Physician 2003;68(12):2393–2398. [PubMed] [Google Scholar]
- 21. Verbaan D, Marinus J, Visser M, van Rooden SM, Stiggelbout AM, van Hilten JJ. Patient‐reported autonomic symptoms in Parkinson disease. Neurology 2007;69(4):333–341. [DOI] [PubMed] [Google Scholar]
- 22. Berganzo K, Tijero B, Somme JH, et al. SCOPA‐AUT scale in different parkinsonisms and its correlation with (123) I‐MIBG cardiac scintigraphy. Parkinsonism Rel Disord 2012;18(1):45–48. [DOI] [PubMed] [Google Scholar]
- 23. Senard JM, Rai S, Lapeyre‐Mestre M, et al. Prevalence of orthostatic hypotension in Parkinson's disease. J Neurol Neurosurg Psychiatry 1997;63(5):584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ha AD, Brown CH, York MK, Jankovic J. The prevalence of symptomatic orthostatic hypotension in patients with Parkinson's disease and atypical parkinsonism. Parkinsonism Rel Disord 2011;17(8):625–628. [DOI] [PubMed] [Google Scholar]
- 25. Mussi C, Ungar A, Salvioli G, et al. Orthostatic hypotension as cause of syncope in patients older than 65 years admitted to emergency departments for transient loss of consciousness. J Gerontol 2009;64(7):801–806. [DOI] [PubMed] [Google Scholar]
- 26. Stubendorff K, Aarsland D, Minthon L, Londos E. The impact of autonomic dysfunction on survival in patients with dementia with Lewy bodies and Parkinson's disease with dementia. PLoS ONE 2012;7(10):e45451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peralta C, Stampfer‐Kountchev M, Karner E, et al. Orthostatic hypotension and attention in Parkinson's disease with and without dementia. J Neural Transm (Vienna) 2007;114(5):585–588. [DOI] [PubMed] [Google Scholar]
- 28. Poewe W. Dysautonomia and cognitive dysfunction in Parkinson's disease. Mov Dis 2007;22(Suppl 17):S374–S378. [DOI] [PubMed] [Google Scholar]
- 29. Swinn L, Schrag A, Viswanathan R, Bloem BR, Lees A, Quinn N. Sweating dysfunction in Parkinson's disease. Mov Disord 2003;18(12):1459–1463. [DOI] [PubMed] [Google Scholar]
- 30. Pont‐Sunyer C, Hotter A, Gaig C, et al. The onset of nonmotor symptoms in Parkinson's disease (the ONSET PD study). Mov Disord 2015;30(2):229–237. [DOI] [PubMed] [Google Scholar]
- 31. Swallow DM, Lawton MA, Grosset KA, et al. Variation in recent onset Parkinson's disease: implications for prodromal detection. J Parkinson's Dis 2016;6(2):289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Edwards LL, Pfeiffer RF, Quigley EM, Hofman R, Balluff M. Gastrointestinal symptoms in Parkinson's disease. Mov Disord 1991;6(2):151–156. [DOI] [PubMed] [Google Scholar]
- 33. Martinez‐Martin P, Schapira AH, Stocchi F, et al. Prevalence of nonmotor symptoms in Parkinson's disease in an international setting; study using nonmotor symptoms questionnaire in 545 patients. Mov Disord 2007;22(11):1623–1629. [DOI] [PubMed] [Google Scholar]
- 34. Liepelt‐Scarfone I, Behnke S, Godau J, et al. Relation of risk factors and putative premotor markers for Parkinson's disease. J Neural Transm (Vienna 1996) 2011;118(4):579–585. [DOI] [PubMed] [Google Scholar]
- 35. Gallagher DA, Lees AJ, Schrag A. What are the most important nonmotor symptoms in patients with Parkinson's disease and are we missing them? Mov Disord 2010;25(15):2493–2500. [DOI] [PubMed] [Google Scholar]
- 36. Evatt ML, Chaudhuri KR, Chou KL, et al. Dysautonomia rating scales in Parkinson's disease: sialorrhea, dysphagia, and constipation—critique and recommendations by movement disorders task force on rating scales for Parkinson's disease. Mov Disord 2009;24(5):635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jankovic J, Rajput AH, McDermott MP, Perl DP. The evolution of diagnosis in early Parkinson disease: Parkinson study group. Arch Neurol 2000;57(3):369–372. [DOI] [PubMed] [Google Scholar]
- 38. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2015;30(12):1591–1601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Demographic and Autonomic Features of Parkinsonian Patients According to the Presence of One or More Possible Unusual or Atypical Diagnostic Features
Table S2. Autonomic Symptom Severity in Relation to Medication Use in Recent Onset Parkinson's Disease
Table S3. Correlation Between Various Domains of SCOPA‐AUT and Motor, Nonmotor, and Quality‐of‐Life Data.
Table S4. Demographic and Clinical Features in Recent Onset Untreated Parkinson's Disease.
Table S5. Autonomic Severity in Relation to Key Motor and Nonmotor Categorized Variables in Recent Onset Untreated Parkinson's Disease.
Table S6. Correlation Between Various Domains of SCOPA‐AUT and Motor, Nonmotor, and Quality‐of‐Life Data in Recent Onset Untreated Parkinson's Disease.


