Abstract
Background
Parkinson's disease (PD) and the skin are related in a number of ways, including clinical abnormalities of the disease itself and skin‐related side effects of dopaminergic medication, pumps, and surgical therapies. Recent advances in understanding the role of α‐synuclein suggest skin biopsies as a potential diagnostic or even a premotor marker of PD.
Methods
The PubMed database was searched for publications up to October 2015, and the current evidence on skin‐related issues in PD was comprehensively summarized.
Results
The evidence was summarized on the prevalence, etiology, and management of seborrheic dermatitis, sweating dysfunctions, bullous pemphigoid, and malignant melanoma, as well as therapy‐related skin disorders, especially those observed in amantadine, rotigotine, apomorphine, and levodopa/carbidopa intestinal gel therapies and deep‐brain stimulation. Skin biopsies evaluating the presence of α‐synuclein, the density and morphology of cutaneous nerves, and skin fibroblast functions also are discussed.
Conclusions
Skin disorders are a common manifestation of PD. However, the exact pathophysiology and prevalence of these disorders are not well understood, and more systematic research is needed in this regard. Peripheral tissue biopsies as a diagnostic marker of PD are an exciting avenue in future PD research, although multiple caveats and pending issues need to be solved before they can be used in routine clinical practice.
Keywords: α‐synuclein, melanoma, Parkinson's disease, skin, therapy
Parkinson's disease (PD) is a progressive neurodegenerative disease characterized by the presence of multiple motor and nonmotor symptoms. Skin disorders, such as seborrheic dermatitis (SD) and hyperhidrosis, are well recognized and frequent—but often overlooked—nonmotor symptoms of PD; they have been associated with PD for nearly a century, since the first reports of seborrheic facies in PD by Krestin.1 Given the interest in biomarkers that precede the motor features of PD, it is worth noting that skin affection may precede the onset of typical motor symptoms by years.2 Other skin disorders, such as malignant melanoma and bullous pemphigoid (BP), are significantly more prevalent in PD patients compared with the general population.3, 4 Recent advances in the understanding of the role of α‐synuclein (SNCA) in the pathophysiology of PD, its prion‐like mechanism of spreading, and findings of typical Lewy body pathology in the peripheral nervous system, suggest skin biopsies as a potential diagnostic marker of PD. Unlike the peripheral melanins produced in skin melanocytes, neuromelanin is produced in specific populations of catecholaminergic neurons in the brain. It is believed that neuromelanin interacts with transition metals, especially iron, and mediates intracellular oxidative mechanisms. This function seems to be compromised in PD, thus rendering pigmented neurons vulnerable to oxidative damage.5 The skin can be affected by antiparkinsonian medication as well. Specific drug‐delivery methods, such as skin patches, subcutaneous application of apomorphine, levodopa (l‐dopa)/carbidopa intestinal gel (LCIG) application via percutaneous endoscopic gastrostomy (PEG), and implantation of deep‐brain stimulation (DBS) hardware, can cause iatrogenic skin lesions. The aim of this review is to summarize current evidence on clinical, diagnostic, and therapeutic issues of skin disorders in PD.
Patients and Methods
We searched the PubMed database for all relevant articles until October 2015 using the main search terms “Parkinson's disease” or “parkinsonism” combined with the following terms: “skin,” “seborrhea,” “dermatitis,” “hyperhidrosis,” “sweating,” “pemphygoid,” “melanoma,” “sensory,” “autonomic,” “α‐synuclein,” “skin biopsy,” “fibroblasts,” “iPSC” (induced pluripotent stem cells), “livedo reticularis,” “amantadine,” “dopamine agonist,” “rotigotine,” “skin patches,” “apomorphine,” “nodules,” “skin necrosis,” “Duodopa,” “LCIG,” “PEG,” “DBS,” “skin erosion,” and their combinations. The references from relevant articles were also checked. Only articles in English that were related to human studies were included. Relevant articles were related to clinical and therapeutic issues, including seborrhea, hyperhidrosis, BP, malignant melanoma, sensory and autonomic skin functions, skin SNCA as a potential biomarker of PD, amantadine‐related livedo reticularis (LR), skin reactions related to rotigotine patches, apomorphine‐related skin nodules, PEG issues related to LCIG therapy, and skin erosions caused by DBS therapy. Due to the complexity of the issue, fibroblast enzymatic and metabolic activity evaluations, as well as fibroblast‐derived iPSCs, are mentioned only briefly in this review.
Seborrheic Dermatitis
SD is a chronic skin disease that affects approximately 3% to 5% of the general population.6 Seborrheic facies was first described as a manifestation of PD in 1927.1 It presents as typical sharply demarcated red patches and plaques with greasy scales in areas that have an increased density of sebaceous glands, namely, the scalp, face, hairline, eyebrow, glabella, nasolabial folds, ears, upper trunk, and flexures. It usually affects multiple body areas, occurring on the face in 88% of patients with SD, on the scalp in 70%, on the chest in 27%, on the arms and legs in 1% to 2%.7
With the discovery of l‐dopa, several authors noticed an improvement of SD in patients with PD; and, in the small studies published, this seemed to correlate with the overall dose of l‐dopa administered.8, 9 Kohn et al.10 demonstrated a statistically significant decrease in the amount of sebum on the foreheads of 21 patients with PD during l‐dopa treatment, although considerable variation in responsiveness was observed. No correlation between the dose of l‐dopa or the duration of treatment and the degree of sebaceous gland inhibition could be established. The quantitative reduction of sebum could not be related to the degree of neurologic improvement. Also, in a later report, Martignoni et al.11 found no correlation of sebum excretion rates with the duration of l‐dopa treatment, the presence of dopamine agonists, or the severity of disease. The prevalence of SD in PD has been reported previously in 18.6% to 59% of patients,12 and it is common in early and even premotor stages of PD. In a retrospective study in this regard, Tanner et al.13 observed that SD was associated with an increased risk of PD development, suggesting that it may represent a premotor feature of PD related to the autonomic nervous system. SD is more common in male parkinsonian patients.11, 12 However, there are multiple caveats in estimations of SD prevalence, and more systematic studies are needed; because the absence of standardized diagnostic criteria, coupled with variability of disease expressiveness, is likely to introduce significant variation into the estimates of SD prevalence. Moreover, some of the prevalence studies included only patients who had “significant skin pathologies,” e.g., conditions that were judged significant enough to warrant at least 1 physician visit.14, 15 There has been only a single report of SD in atypical parkinsonism, in a patient who had pathologically confirmed corticobasal degeneration.16 However, no further prevalence data on SD in atypical parkinsonian syndromes were found in the literature.
The etiology of SD in PD is not fully understood. Some of the contradictory causes include autonomic dysfunction,17 although this was not confirmed in other studies11; the role of androgens or testosterone relating to higher incidence of SD in male populations11; and the systemic effect of melanocyte‐stimulating hormone.18 SD appears to result from a combination of the 3 following factors: sebaceous gland secretion, the presence of Malassezia yeast, and the host‐immune response.19 In a recent study, Arsenijevic et al.20 observed a positive correlation between SD, PD, Malassezia globosa incidence, high yeast density, and high skin phosphatase and lipase activity. Treatment options for nonscalp and scalp SD include topical agents and shampoos that contain antifungal agents (such as ketokonazol), anti‐inflammatory agents, keratolytic agents, and calcineurin inhibitors.21
Other types of dermatitis related to PD are transient acantholytic dermatitis,22 and especially debilitating perioral dermatitis, associated with severe and intractable drooling.23 Various symptomatic treatments are available for drooling that aim either to reduce saliva production (botulinum toxin injections into the salivary glands, anticholinergics, or radiotherapy over the salivary glands) or to improve the quality and frequency of swallowing.23
Sweating Dysfunction
Dyshidrosis, including hyperhidrosis and hypohidrosis, is a common feature of PD and affects from 30% to 60% of patients. In 7 studies in which the prevalence of hyperhidrosis and hypohidrosis was reported separately, hyperhidrosis was identified in from 10% to 100% of patients, whereas hypohidrosis was identified in from 0% to 40% of patients.24, 25, 26, 27, 28, 29, 30 However, only 2 of those studies reported a higher prevalence of hyperhidrosis compared with hypohidrosis.27, 30 Dyshidrosis is considered as part of the spectrum of autonomic dysfunction in PD and is often associated with other autonomic symptoms, especially orthostatic hypotension, sialorrhea, and urinary symptoms.28, 30, 31
Sweating may have a considerable impact on a patient's physical, psychological, and social well‐being.28 It may already be present in the premotor PD period, and results from the recent ONSET‐PD study indicated that excessive sweating preceded the onset of typical motor symptoms usually by 2 to 10 years.2 Sweating can be often asymmetrical27 and is not necessarily correlated with the side of worse motor symptoms. “Drenching sweats” also have been described.32, 33 The areas predominantly affected by localized sweating include the head and trunk, and it is believed that such sweating is a compensatory thermoregulatory mechanism for loss of sweating in the extremities.28, 34, 35 Sweating was reported more commonly in younger onset versus to late‐onset patients.36 It was also more commonly reported in patients with camptocormia.37 Hyperhidrosis seems to be more prevalent with progression of the disease.38
Most of the studies published to date indicate a higher occurrence of sweating during off‐medication (off) periods and dyskinesias.28, 32, 39 Off period sweating may result from insufficient central dopaminergic stimulation,32 whereas sweating associated with dyskinesias may be related to the associated excess physical activity.28 In some studies, sweating has been reported among the most frequent nonmotor off symptoms.40 On the other hand, a study by Storch et al.41 identified excessive sweating, dysphagia, and bladder urgency as the only 3 of 10 nonmotor symptoms studied that were not associated with motor fluctuations.
There are no randomized controlled studies targeting excessive sweating specifically in patients with PD. However, the use of botulinum toxin therapy for the treatment of axillary essential hyperhidrosis did receive a Level A recommendation.42 Future research is needed to validate the efficacy and safety of botulinum toxin therapy for PD‐related hyperhidrosis. Multiple studies suggest that an improvement in motor fluctuations may alleviate sweating. These include apomorphine pump therapy,43 LCIG,44 and DBS.30, 33, 45
Bullous Pemphigoid
BP is the most common autoimmune blistering disease (Fig. 1). BP occurs especially in the elderly and predominantly on the flexural aspects of limbs and on the trunk.46 Multiple previous studies have reported increased prevalence of neurological diseases, including stroke, dementia, PD, multiple sclerosis, and amyotrophic lateral sclerosis, among patients with BP. The prevalence of PD in 13 identified cohorts of patients with BP ranged from 2.2% to 17.9%.3, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58 Four of 9 studies identified demonstrated a statistically significant association between BP and PD (odds ratio [OR], 2.16; 95% confidence interval [CI], 1.09–4.27) and PD (OR, 5.0; 95% CI, 1.57–15.94),3, 47, 50, 55 whereas the 5 remaining studies did not find a statistically significant correlation.48, 52, 54, 57, 58 Only in a single study was PD associated significantly with mortality in BP patients (OR, 1.85; 95% CI, 1.11–2.95), whereas this association was not observed in other neurological diseases, including stroke and dementia.49 Another study identified increased incidence of PD after diagnosis of BP (OR, 8.56; 95% CI, 1.55–47.25; P = 0.01).48
Figure 1.
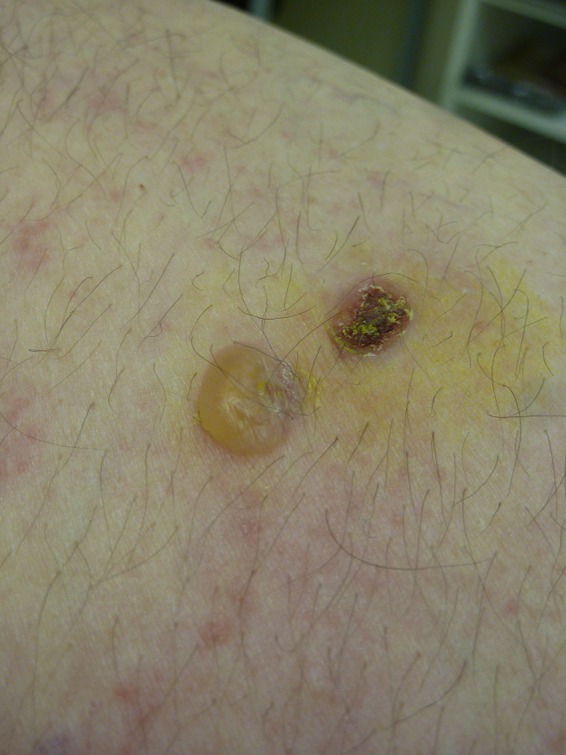
Bullous pemphigoid (courtesy of Dr. Zuzana Hrabovska, Department of Dermatology, University Hospital of L. Pasteur, Kosice, Slovakia).
The correlation between PD and BP is not clearly understood. BP is mediated by autoimmunity to the hemidesmosomal proteins BP antigen 1 (BPAg1) (230 kD) and BPAg2 (180 kD; type XVII collagen). The 2 isoforms of BPAg1—the endothelial isoform (BPAg1‐e), which anchors intermediate filaments to the hemidesmosomes, and the neuronal isoform (BPAg1‐n), which organizes the neuronal cytoskeleton by binding the neuronal filament triplet proteins to the microfilaments, share high homology.59 Because of neurodegeneration, uncovering the neuronal isoform of BPAg1, which normally is protected from antibody recognition, may cause cutaneous damage due to cross‐reactivity.60, 61
Melanoma
Multiple population‐based studies have confirmed a decreased risk in patients with PD for most types of cancer, except melanomas, which are significantly more common in patients with PD than in the general population.62, 63, 64 A recent meta‐analysis, which included 24 studies and 292,275 patients, reported an OR of 1.83 (95% CI, 1.46–2.30) in this regard.4 The authors of the study concluded that the risk of melanoma was significantly higher after a diagnosis of PD (OR, 2.43; 95% CI, 1.77–3.32) but not before a diagnosis of PD (OR, 1.09; 95% CI, 0.78–1.54). The risk of melanoma was increased among patients with PD in Europe (OR, 1.44; 95% CI, 1.22–1.70) and in North America (OR, 2.64; 95% CI, 1.63–4.28), and no significant differences were found when comparing the incidence between men (OR, 1.64; 95% CI, 1.27–2.13) and women (OR, 1.38; 95% CI, 1.04–1.82). The authors also observe a slightly increased risk of nonmelanoma skin cancer in patients with PD than in the general population (OR, 1.20; 95% CI, 1.11–1.29). In a recent study of patients with malignant melanoma without a definite PD diagnosis, an association was observed with a higher frequency of ultrasound substantia nigra hyperechogenicity along with prodromal motor and nonmotor features of PD, especially asymmetric motor slowing and apathy.65 Greater substantia nigra hyperechogenicity in patients with melanoma was correlated with low serum iron levels and with lighter skin pigmentation. An association between light skin pigmentation phenotype and increased substantia nigra hyperechogenicity also was confirmed in another recent study.66
The association between PD and melanoma is not fully understood. It has been suggested that shared biochemical pathways between PD and melanoma and l‐dopa treatment may underlie this association.67 The relationship between l‐dopa and the risk of developing malignant melanoma was suggested in some studies,68 whereas others found no apparent relationship.69, 70 Recent epidemiological studies, however, have refuted a causal association.67, 71 Opponents have argued that, given the relatively long latency between initiation and clinical manifestation of melanoma (generally believed to be greater than 10 years and, in some patients, as long as 40 years),72 it is unlikely that l‐dopa stimulated the growth of melanoma in a short period of a few months or years, as often was described in earlier case reports. Multiple patients with PD and melanoma have been described in the literature who were safely treated with l‐dopa.73 Moreover, a recent meta‐analysis was not able to evaluate the association between PD and melanoma according to l‐dopa use, because few observational or cohort studies have focused on it.4 However, according to the current European Commission's Summary of Product Characteristics, the presence of malignant melanoma is considered a contraindication for l‐dopa therapy.74 We suggest that decisions should be made on a case‐by‐case basis.
Alterations in melanin and melanin‐synthesizing enzymes, genetic factors, and abnormal autophagy, along with the fact that both melanocytes and neurons share their embryological origins from the neural crest, have all been proposed to underlie the increased risk of melanoma in PD.75 Regarding genetic associations, a recent investigation did not identify any significant associations between genome‐wide association studies in single nucleotide polymorphisms of pigmentation or melanoma and the risk for PD.76 Two other genetic studies reported that the melanocortin‐1 receptor (MC1R) gene, which plays a key role in hair and skin pigmentation as well as malignant melanoma predisposition, was associated with an increased risk of PD development.77, 78 An additional study did not report such an association.79 Another recent study examined 11 single nucleotide polymorphisms identified from previous genome‐wide association studies of pigmentation and melanoma, as well as colors of hair, eyes, or skin, and melanoma in relation to PD.80 In that study, no significant associations were identified between genome‐wide association studies in single nucleotide polymorphisms of pigmentation or melanoma and the risk for PD.
An interesting finding comes from a recent study, which cross‐referenced somatic mutations in metastatic cutaneous melanoma (CM) detected by whole‐exome sequencing with the 15 know PD (PARK) genes and applied identical analysis also to adenocarcinoma and squamous cell carcinoma of lungs as controls.81 Forty‐eight percent of CM samples carried ≥1 PARK mutation and 25% carried multiple PARK mutations, which was significantly more compared to the lung carcinoma groups. The overrepresentation of somatic PARK mutations in CM suggests shared dysregulation pathways for CM and PD.
Therapy‐Related Skin Disorders
Amantadine
LR is a rare complication seen with amantadine treatment that has been reported only in case reports or small case series.82, 83, 84 It is characterized by patchy mottling of the skin and can be described as following a “fishnet pattern.”85 It is caused by increased prominence of the venous beds in the skin, arising from impediments to arterial flow, venous dilation, or obstruction of venous flow. The blood supply to the skin is through arterioles, each of which supplies a cone‐shaped area with a base measuring from 1 to 4 cm across. The vein‐rich skin between these well‐perfused areas takes on a reddish‐blue reticular pattern whenever slow flow causes increased oxygen extraction and deepening of the color of venous blood.86 The exact pathophysiologic mechanism of LR in PD is not fully understood, but it has been suggested that amantadine leads to an interruption of the peripheral blood redistribution.87 LR is a rare but well documented side effect of amantadine. It usually appears in the lower extremities but also may affect the upper extremities.83, 84, 85, 86, 87, 88 Rarely, LR even may develop years after amantadine is prescribed.83, 84, 85, 86, 87, 88, 89 Patient perceptions of this condition may play a significant role in the decision to stop amantadine therapy. In this regard, LR in the upper limbs especially may lead to discontinuation of therapy.83
Rotigotine
The nonergolinic dopamine agonist rotigotine has been formulated in a once‐daily transdermal patch for 24‐hour application, which ensures continuous rotigotine release. An integrated analysis of 3 open‐label, randomized, phase I, multiple‐dose studies90 demonstrated that the rate of rotigotine released to the skin was 31% to 62% of the total drug content in the patch. Furthermore, results from that analysis indicated that the variability of rotigotine exposure was low among participants (15%) compared with the variability observed between participants (54%). Plasma concentrations at steady state were stable over the 24‐hour patch‐on period. Delivery by a single, large patch, compared with a combination of smaller patches, did not appear to influence exposure to rotigotine.
A review of phase II and III trials published between 2003 and 2011 indicated that rotigotine has a similar adverse event (AE) profile, although with varying frequency, compared with other nonergolinic dopamine agonists, such as pramipexole and ropinirole.91 In addition to these, local skin reactions were the most common AEs observed in rotigotine treatment. In general, local skin reactions take the form of localized erythema, edema, or pruritus limited to the patch area. Generalized skin reactions (i.e., allergic rash, including erythematous, macular–papular rash, or pruritus) were reported at lower rates than local skin reactions during the development of rotigotine.92 Local skin reactions are generally mild and tolerable and may lead to discontinuation of treatment in up to 8% of patients.91 In another systematic review, up to 3% of patients had severe skin reactions.93 In a clinical study of 221 healthy participants that was designed to investigate the cumulative skin irritation of rotigotine, daily rotation of rotigotine application sites reduced local skin reactions incidence compared with repetitive application to the same site.94 Thus, daily rotation of the application site may reduce the incidence of local skin reactions. The same site should not be used more than once every 14 days. Also, to reduce the possibility of local skin reactions, patients should wash the application site with soap and water after patch removal to remove any drug or adhesive. Baby or mineral oil may be used to remove any excess residue. Alcohol and other solvents (such as nail polish remover) may cause skin irritation and should not be used. Direct sunlight on the area where a skin rash or irritation develops should be avoided until the skin heals, because this exposure could lead to a change of skin color.92, 94
Other Oral Medications
Allergic skin reactions to dopaminergic therapy are very rare.95 Erythromelalgia, a rare neurovascular skin disorder characterized by erythema, swelling, and painful burning sensations, was associated with ergolinic dopamine agonists,96, 97 which are used only rarely today. Skin edema that does not satisfy the criteria for erythromelalgia, however, is more common, especially with dopamine agonist therapy, and may lead to its discontinuation. Other skin disorders, such as bullous eruptions associated with entacapone,98 are very uncommon.
Apomorphine
Subcutaneous application of dopamine agonist apomorphine is a well‐established and effective treatment for PD. Due to its speed of onset, apomorphine injections are a suitable option for patients who require rapid and reliable relief from both predictable and unpredictable off periods. Apomorphine infusion is a suitable option for patients who have advanced PD and more complex motor fluctuations, which cannot be adequately controlled with standard oral medication.99 The subcutaneous administration of apomorphine is generally well tolerated. However, skin nodules at the site of drug infusion are a common and sometimes bothersome complication and typically develop over time (Fig. 2). Skin nodules were reported in up to 92% of patients who received treatment with subcutaneous apomorphine.100 These are usually of mild or moderate severity; in severe cases, the nodules may result in skin abscesses and necrotic ulcers.101 On a pathologic level, skin nodules were associated with panniculitis.102 Recent expert consensus recommendations summarize the potential options for managing skin nodules (Table 1).99 None of these have been proven by means of formal clinical studies, with the exception of ultrasound, which has been shown to be effective for the treatment of skin nodules and can make the treated area suitable for further injections.103
Figure 2.
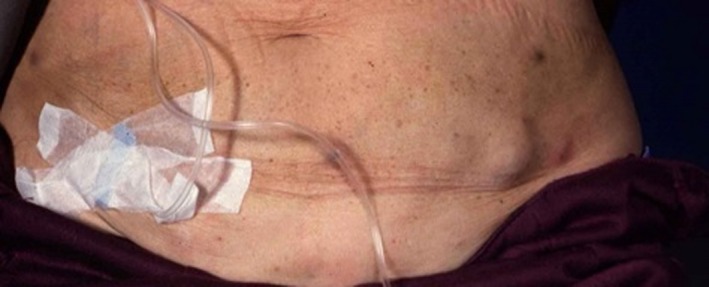
Subcutaneous nodules associated with apomorphine pump therapy.
Table 1.
Management of apomorphine therapy‐related skin nodules, expert consensus recommendationa
| • Rotation of the choice of infusion sites |
| • Use of Teflon® needles |
| • Adjusting delivery through the skin to an optimal angle (i.e., 45–90°) |
| • Maintaining skin hygiene and using emollients at the infusion site |
| • Choosing a lower concentration, e.g., 5 mg/mL; lower concentrations have been used successfully |
| • Massaging the infusion site (using a spiky rubber massage ball or vibrating device) |
| • Applying ultrasound treatment |
| • Use of silicone gel dressings |
See Monk et al.97
LCIG Therapy
Therapy LCIG (Duodopa; AbbVie Inc., North Chicago, IL) through a PEG is a efficacious, long‐term treatment for patients in advance stages of PD.104 AEs in LCIG therapy are most commonly related to the device or surgical procedure. AEs related to the stoma include secretion from the stoma, infection, abdominal cellulite, proud flesh around the stoma, and pain (Fig. 3).104, 105 It is estimated in the general literature that, in the general population, the rate of complications after endoscopic placement of enteral feeding tubes is in the range from 8% to 30%. Serious complications requiring treatment occur in approximately 1% to 4% of individuals. Acute and severe complications, such as perforation, serious abdominal hemorrhage, or peritonitis requiring surgical intervention, occur in far fewer than 0.5% of cases.106 In 2 large LCIG studies that included 91 and 72 patients with PD, PEG‐related AEs led to 18 (18%) and 7 (9.5%) complications, respectively, with subsequent discontinuation of therapy in 2 patients overall.104, 107
Figure 3.
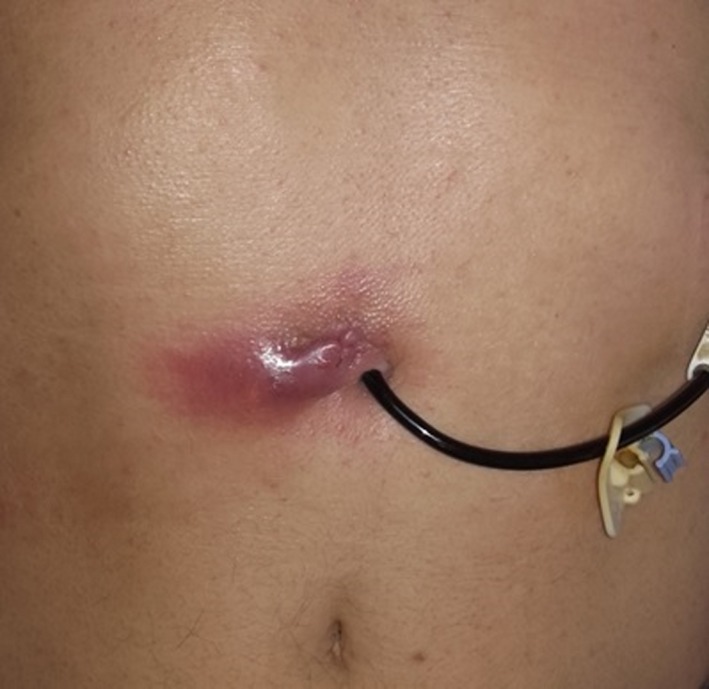
Local infection and subcutaneous abscess associated with percutaneous endoscopic gastrostomy stoma.
General prophylaxis with a single dose of an antibiotic (e.g., 2 g cephazolin intravenously) before PEG insertion as protection against inflammatory complications is debated.107 The rate of infectious complications without antibiotic prophylaxis may be up to 32%, and antibiotic prophylaxis is recommended especially for patients with diabetes, malignancies, and immunosuppression and for the geriatric population.108 After PEG insertion, the PEG should be under moderate tension for 24 to 72 hours. After that, the tube should be loosened and, for long‐term maintenance, should allow 0.5 to 1.0 cm of free movement. If the PEG is pulled too tight, then pressure‐related lesions in the skin area with subsequent ischemia, dermatitis around the stoma, pressure ulcer, and buried bumper syndrome may be induced.109 On the other hand, an outer bumper that is too loose is among the most common reasons for stoma infections.
Deep‐Brain Stimulation
DBS is an efficacious treatment for PD. However, it may be associated with skin complications, such as skin erosions and infections over the implanted foreign material. Skin erosions are most commonly seen in the area of the burr hole cap, at the connection site between the DBS lead and the extensions cable, and in the area of an implantable pulse generator (IPG) (Fig. 4).110 They may be commonly associated with infection of the site of erosion.111 For patients in whom bacteria are not isolated and who do not respond to antibiotics, foreign‐body reactions should be considered in the differential diagnosis. Skin complications after DBS have been reported in up to 24.7% of patients, and this risk seemed to be highest in the first year after implantation.112 The risk of skin erosions is increased especially in thin patients, and implanting smaller (e.g., rechargeable) IPGs may reduce this risk. Surgical techniques, such as sine‐wave–shaped incisions,112 a double C‐shaped incision with dual‐floor burr hole technique,113 isolating the hardware from the skin incision (e.g., burying the lead and the Stimloc below the pericranium), or implanting the IPG within the muscle in thin patients, also may reduce the risk of skin erosions.111 For skin erosions that occur on the scalp, free flaps seem to be superior to scalp rotation flaps.114
Figure 4.
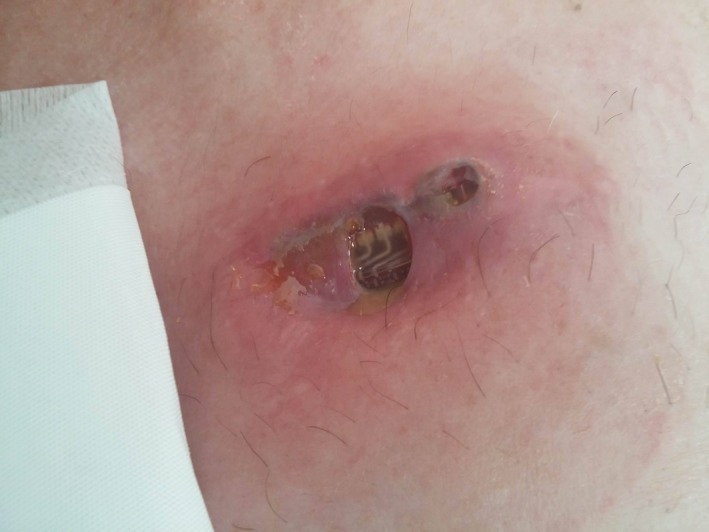
Skin infection and wound dehiscence over a deep‐brain stimulation implantable pulse generator.
Skin Biopsies and SNCA as Diagnostic Markers of PD
The role of SNCA was first described in 1997, when it was established that the SNCA gene caused autosomal dominant PD.115 Subsequently, SNCA was identified as the main component of Lewy bodies.116 Historically, Lewy bodies were considered as a major pathologic hallmark of PD but were not believed to have a role in the etiology of PD. A major breakthrough came with a publication by Kordower et al.,117 who identified Lewy body‐like inclusions in relatively young grafted nigral neurons from a patient with PD 14 years after transplantation and raised the possibility of prion‐like spreading of SNCA in PD. SNCA in PD misfolds from an α‐helix to a β‐sheet–rich conformation and polymerizes to form toxic fibrils and aggregates.118 Cell‐to‐cell spreading of SNCA pathology has been demonstrated in vitro in cell cultures119 as well as in vivo in different animal models.120, 121 Pan‐Montojo et al.122 demonstrated that chronic intragastric administration of low doses of rotenone in rodents could induce typical SNCA pathology in the enteric nervous system, with subsequent time‐dependent spread to the dorsal motor nucleus of the vagus nerve and then to the substantia nigra. This spread of SNCA may be stopped by vagotomy.123 Importantly, SNCA pathology has been identified in different peripheral human tissues, including the olfactory mucosa/bulb,124 salivary glands,125 gastrointestinal tract,126 and the skin.127 These findings suggest the potential for peripheral tissue biopsy as a diagnostic marker or even as a premotor biomarker of PD.
Eleven in vivo studies128, 129, 130, 131, 132, 133, 134, 135, 136, 137 and 3 postmortem studies125, 138, 139 have evaluated skin biopsies as potential diagnostic markers for PD. Sample sizes in those studies ranged from 16 patients127 to 67 patients,134 and biopsies were performed especially from the trunk and lower limbs. Phosphorylated SNCA (pSNCA) was evaluated in 10 of those studies. The sensitivity of pSNCA examinations was low, because the staining rate ranged from 0% to 100%. The specificity of pSNCA evaluation was satisfactory, because staining was absent in healthy controls. Three studies evaluated whether the presence of pSNCA could differentiate PD from multiple system atrophy and atypical parkinsonism.129, 132, 135 pSNCA‐positive staining was found in 5.3%,131 62%,134 and 100%137 of patients with PD and in 7%, 0%, and 0% of patients with multiple system atrophy or atypical parkinsonism. Studies of related SNCA immunoreactivity found positive staining both in patients with PD and in controls. The frequency of pSNCA deposits seems to decrease from proximal (trunk, chest, thigh) to distal (distal leg, finger) sites.129, 130, 132 Variable sweat gland density may also contribute to this proximodistal gradient of pSNCA distribution.137
Eight studies have evaluated the morphology and distribution of different types of cutaneous nerves.128, 129, 130, 133, 134, 135, 136, 137 Loss of dermal nerve fibers and reduced intraepidermal nerve fiber density were consistent findings. Some studies propose a length‐independent loss of intraepidermal nerve fibers associated with the appearance of pSNCA at proximal sites129 and sweat gland denervation correlating with pSNCA distribution.130 Adjacent to neurodegenerative alterations, Nolano et al.133 reported increased nerve regeneration evidenced by abnormal nerve fiber sprouting and altered neurochemical coding. Rodriguez‐Leyva et al.134 demonstrated significantly decreased intraepidermal nerve fiber density in patients who had PD compared with those who had multiple system atrophy. Autonomic denervation is in line with several studies that demonstrated autonomic skin dysfunction in PD. These may include abnormal vasomotor reflex (which is evaluable using thermography with a cold stress test),140 abnormal skin wrinkling in response to immersion of the hands in hot water,141 abnormal sympathetic skin response and R‐R interval variation tests,29 abnormal skin resistance levels, and skin resistance response.142
Skin fibroblasts may play a significant role in future PD diagnosis and therapy. Hoepken et al.143 reported increased SNCA expression in skin fibroblasts. Mitochondrial dysfunction and decreased energetic metabolism are consistently observed in skin fibroblasts from patients with PD.144 This has been demonstrated in cultures from patients with parkin mutations145, 146 and SNCA triplication.147 Enhanced vulnerability of skin fibroblasts in PARK6 to apoptosis induced by proteosomal stress has been reported.148 Also, decreased cholesterol biosynthesis in skin fibroblasts has been observed.149 Skin fibroblasts may have a significant role in future cell‐replacement therapies through the development of induced pluripotent stem cells.150 However, this topic was beyond the scope of the current review.
Conclusions
To our knowledge, this is the first comprehensive review on skin affections associated with PD. Skin disorders in PD are common and may result in decreased quality of life23, 28; however they are frequently overlooked and underrated. We have identified several areas in which more systematic research will be needed:
Although it is a well‐recognized manifestation of PD, the prevalence of SD was studied mostly in small samples of patients with SD and used varying inclusion criteria. Therefore, more systematic studies on the prevalence of SD in PD would be needed. Also, the etiology of SD has not been fully elucidated. Autonomic dysfunction, sebaceous gland excretion, the presence of Malassezia yeast, and the host‐immune response seem to contribute to the occurrence of SD; however. This needs to be confirmed.
The prevalence of BP is significantly increased in several neurological disorders, including PD. It is believed that the underlying cause of this association is the cross‐reactivity of the endothelial and neuronal isoforms of the hemidesmosomal BPAg1 proteins. This needs further validation.
The prevalence of malignant melanoma is significantly increased in PD, and this relationship is not fully understood. It is important that physicians are aware of this increased risk of melanoma, explain the risk to their patients with PD, and recommend sun protection, self‐surveillance, and periodic skin check‐ups.151 An interesting recent finding is the correlation between substantia nigra hyperechogenicity and light skin pigmentation phenotype; the underlying cause of this relationship is not well understood and would need further research.
Diagnostic utilization of peripheral tissue biopsies, including the skin, is an exciting area of current PD research. However, multiple caveats and pending issues are present in this regard, including optimal tissue selection, site selection, technique and sample processing, optimal immunohistochemistry marker, sensitivity and specificity of these examinations compared with healthy controls, and patients with other synucleinopathies.
Skin disorders may be a common side effect of medication and are often associated with a specific mode of drug delivery, such as skin patches, subcutaneous drug application, PEG delivery in LCIG therapy, and hardware implantation in DBS. These iatrogenic skin disorders often may be effectively prevented or managed using the above‐mentioned procedures. Many of these recommendations, however, are based on expert opinion only and would need to be proven in formal clinical studies.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
M.S.: 1A, 1B, 1C, 3A
K.P.B.: 1A, 1C, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: This work was supported by the Slovak Research and Development Agency under contract no. APVV‐14‐0415 and by the Slovak Scientific Grant Agency under contract no. VEGA 1/0024/14. The authors report no conflicts of interest.
Financial Disclosures for the previous 12 months: “MS received grants from the Slovak Research and Development Agency, the Slovak Scientific Grant Agency, speaker honoraria and compensations for consultations from Abbvie, Actavis, Egis, Krka, Lundbeck, Medtronic, TEVA and UCB. Otherwise the authors declare no conflict of interest.”
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Krestin D. The seborrheic facies as a manifestation of post‐encephalitic parkinsonism and allied disorders. Q J Med 1927;21:177–186. [Google Scholar]
- 2. Pont‐Sunyer C, Hotter A, Gaig C, et al. The onset of nonmotor symptoms in Parkinson's disease (the ONSET PD study). Mov Disord 2015;30:229–237. [DOI] [PubMed] [Google Scholar]
- 3. Chen YJ, Wu CY, Lin MW, et al. Comorbidity profiles among patients with bullous pemphigoid a nationwide population‐based study. Br J Dermatol 2011;165:593–599. [DOI] [PubMed] [Google Scholar]
- 4. Huang P, Yang XD, Chen SD, et al. The association between Parkinson's disease and melanoma: a systematic review and meta‐analysis [serial online]. Transl Neurodegener 2015;4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fedorow H, Tribl F, Halliday G, Gerlach M, Reiderer P, Double KL. Neuromelanin in human dopamine neurons: comparison with peripheral melanins and relevance to Parkinson's disease. Prog Neurobiol 2005;75:109–124. [DOI] [PubMed] [Google Scholar]
- 6. Naldi L, Rebora A. Clinical practice: seborrheic dermatitis. N Engl J Med 2009;360:387–396. [DOI] [PubMed] [Google Scholar]
- 7. Peyri J, Lleonart M; Gruppo Españoldel Estudio SEBDERM . [Clinical and therapeutic profile and quality of life of patients with seborrheic dermatitis]. Actas Dermosifiliogr 2007;98:476–482. [PubMed] [Google Scholar]
- 8. Appenzeller O, Harville D. Effect of L‐dopa on seborrhea of parkinsonism. Lancet 1970;7667:311–312. [PubMed] [Google Scholar]
- 9. Burton JL, Shuster S. Effect of L‐dopa on seborrhea of parkinsonism. Lancet 1970;7662:19–20. [DOI] [PubMed] [Google Scholar]
- 10. Kohn SR, Pochi PE, Strauss JS, et al. Sebaceous gland excretion in Parkinson's disease during L‐dopa treatment. J Invest Dermatol 1973;60:134–136. [DOI] [PubMed] [Google Scholar]
- 11. Martignoni E, Godi L, Pacchetti C, et al. Is seborrhea a sign of autonomic impairment in Parkinson's disease? J Neural Transm 1997;104:1295–1304. [DOI] [PubMed] [Google Scholar]
- 12. Fischer M, Gemende I, Marsch WC, et al. Skin function and skin disorders in Parkinson's disease. J Neural Transm 2001;108:205–213. [DOI] [PubMed] [Google Scholar]
- 13. Tanner CM, Albers K, Goldman S, et al. Seborrheic dermatitis and risk of future Parkinson's disease (PD) [meeting abstract]. Neurology 2012;78:S42.001. [Google Scholar]
- 14. Johnson MT, Roberts J. Skin conditions and related need for medical care among persons 1–74 years. United States, 1971–1974. Vital Health Stat 11 1978;212:i–v, 1–72. [PubMed] [Google Scholar]
- 15. Mastrolonardo M, Diaferio A, Logroscino G. Seborrheic dermatitis, increased sebum excretion, and Parkinson's disease: a survey of (im)possible links. Med Hypotheses 2003;60:907–911. [DOI] [PubMed] [Google Scholar]
- 16. Noda K, Kobayashi T, Matsuoka S, et al. [A 65‐year‐old man with rigid‐bradykinetic parkinsonism, vertical gaze palsy, difficulty of eye‐lid opening, and marked pseudo‐bulbar palsy]. No To Shinkei 2005;57:73–86. [PubMed] [Google Scholar]
- 17. Sariahmetoglu H, Soysal A, Sen A, et al. Forehead sympathetic skin responses in determining autonomic involvement in Parkinson's disease. Clin Neurophysiol 2014;125:2436–2440. [DOI] [PubMed] [Google Scholar]
- 18. Shuster S, Thody AJ, Goolamali SK, et al. Melanocyte‐stimulating hormone and parkinsonism. Lancet 1973;3:463–464. [DOI] [PubMed] [Google Scholar]
- 19. Del Rosso JQ. Adult seborrheic dermatitis. A status report on practical topical management. J Clin Aesthet Dermatol 2011;4:32–38. [PMC free article] [PubMed] [Google Scholar]
- 20. Arsenijevic VSA, Milobratovic D, Barac AM, et al. A laboratory‐based study on patients with Parkinson's disease and seborrheic dermatitis: the presence and density of Malassezia yeasts, their different species and enzymes production [serial online]. BMC Dermatol 2014;14:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goldenberg G. Optimizing treatment approaches in seborrheic dermatitis. J Clin Aesthet Dermatol 2013;6:44–49. [PMC free article] [PubMed] [Google Scholar]
- 22. Pranteda G, Mari E, Feliziani G, et al. Transient acantholytic dermatitis and Parkinson's disease. J Eur Acad Dermatol Venereol 2009;23:455–457. [DOI] [PubMed] [Google Scholar]
- 23. Bloem B, Kalf E, van de Kerkhof E, et al. Debilitating consequences of drooling. J Neurol 2009;256:1382–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aminoff MJ, Wilcox CS. Assessment of autonomic function in patients with a Parkinsonian syndrome. Br Med J 1971;4:80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rajput AH, Rozdilsky B. Dysautonomia in parkinsonism: a clinic‐pathological study. J Neurol Neurosurg Psychiatry 1976;39:1092–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saito H, Kogure K. Thermal sudomotor deficits in Parkinson's disease. Rinsho Shinkeigaku 1989;29:734–740. [PubMed] [Google Scholar]
- 27. De Marinis M, Stocchi F, Gregori B, et al. Sympathetic skin response and cardiovascular autonomic function tests in Parkinson's disease and multiple system atrophy with autonomic failure. Mov Disord 2000;15:1215–1220. [DOI] [PubMed] [Google Scholar]
- 28. Swinn L, Schrag A, Viswanathan R, et al. Sweating dysfunction in Parkinson's disease. Mov Disord 2003;18:1459–1463. [DOI] [PubMed] [Google Scholar]
- 29. Zakrewska‐Pniewska B, Jamrozik Z. Are electrophysiological autonomic tests useful in the assessment of dysautonomia in Parkinson's disease? Parkinsonism Relat Disord 2003;9:179–183. [DOI] [PubMed] [Google Scholar]
- 30. Trachani E, Constantoyannis C, Sirrou V, et al. Effects of subthalamic nucleus deep brain stimulation on sweating function in Parkinson's disease. Clin Neurol Neurosurg 2010;112:213–217. [DOI] [PubMed] [Google Scholar]
- 31. Hirayama M. Hyperhidrosis in Parkinson's disease In: Yamamoto M, ed. Parkinson's Disease: Clinical Issues. 1st ed Tokyo, Japan: Chu‐gai‐Igakusha; 2006:234–242. [Google Scholar]
- 32. Sage JI, Mark MH. Drenching sweats as an off phenomenon in Parkinson's disease: treatment and relation to plasma levodopa profile. Ann Neurol 1995;37:120–122. [DOI] [PubMed] [Google Scholar]
- 33. Sanghera MK, Ward C, Stewart RM, et al. Alleviation of drenching sweats following subthalamic deep brain stimulation in a patient with Parkinson's disease—a case report. J Neurol Sci 2009;285:246–249. [DOI] [PubMed] [Google Scholar]
- 34. Appenzeller O, Goss JE. Autonomic deficits in Parkinson's syndrome. Arch Neurol 1971;24:50–57. [DOI] [PubMed] [Google Scholar]
- 35. Schestatsky P, Valls‐Sole J, Ehlers JA, et al. Hyperhidrosis in Parkinson's disease. Mov Disord 2006;21:1744–1748. [DOI] [PubMed] [Google Scholar]
- 36. Spica V, Pekmezovic T, Svetel M, et al. Prevalence of non‐motor symptoms in young‐onset versus late‐onset Parkinson's disease. J Neurol 2013;260:131–137. [DOI] [PubMed] [Google Scholar]
- 37. Ou R, Guo X, Song W, et al. Characteristics of non‐motor symptoms in patients with Parkinson's disease exhibiting camptocormia. Gait Posture 2014;40:447–450. [DOI] [PubMed] [Google Scholar]
- 38. Antonini A, Barone P, Marconi R, et al. The progression of non‐motor symptoms in Parkinson's disease and their contribution to motor disability and quality of life. J Neurol 2012;259:2621–2631. [DOI] [PubMed] [Google Scholar]
- 39. Olanow CW, Koller WC. An algorithm (decision tree) for the management of Parkinson's disease: treatment guidelines. Neurology 1998;50:1–57. [DOI] [PubMed] [Google Scholar]
- 40. Cheon SM, Park MJ, Kim WJ, et al. Non‐motor symptoms in Parkinson's disease. J Korean Med Sci 2009;24:311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Storch A, Schneider CB, Wolz M, et al. Nonmotor fluctuations in Parkinson's disease. Neurology 2013;80:800–809. [DOI] [PubMed] [Google Scholar]
- 42. Naumann M, Dressler D, Hallett M. Evidence‐based review and assessment of botulinum neurotoxin for the treatment of secretory disorders. Toxicon 2013;67:141–152. [DOI] [PubMed] [Google Scholar]
- 43. Martinez‐Martin P, Reddy P, Antonini A, et al. Chronic subcutaneous infusion therapy with apomorphine in advanced Parkinson's disease compared to conventional therapy: a real life study of non motor effect. J Parkinsons Dis 2011;1:197–203. [DOI] [PubMed] [Google Scholar]
- 44. Pursiainen V, Lyytinen J, Pekkonen E. Effect of duodenal levodopa infusion on blood pressure and sweating. Acta Neurol Scand 2012;126:e20–e24. [DOI] [PubMed] [Google Scholar]
- 45. Halim A, Baumgartner L, Binder DK. Effect of deep brain stimulation on autonomic dysfunction in patients with Parkinson's disease. J Clin Neurosci 2011;18:804–806. [DOI] [PubMed] [Google Scholar]
- 46. Schmidt E, Zillikens D. Pemphygoid diseases. Lancet 2013;381:320–332. [DOI] [PubMed] [Google Scholar]
- 47. Bastuji‐Garin S, Joly P, Lemordant P, et al. Risk factors for bullous pemphigoid in the elderly a prospective case‐control study. J Invest Dermatol 2011;131:637–643. [DOI] [PubMed] [Google Scholar]
- 48. Brick KE, Weaver CH, Savica R, et al. A population‐based study of the association between bullous pemphigoid and neurological disorders. J Am Acad Dermatol 2014;71:1191–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cai SC, Allen JC, Lim YL, et al. Mortality of bullous pemphigoid in Singapore risk factors and causes of death in 359 patients seen at the National Skin Centre. Br J Dermatol 2014;170:1319–1326. [DOI] [PubMed] [Google Scholar]
- 50. Casas‐de‐la‐Asuncion E, Ruano‐Ruiz J, Rodriguez‐Martin AM, Vélez García‐Nieto A, Moreno Giménez JC. Association between bullous pemphigoid and neurologic diseases: a case‐control study. Actas Dermosifiliogr 2014;105:860–865. [DOI] [PubMed] [Google Scholar]
- 51. Cordel N, Chosidow O, Hellot MF, et al. Neurological disorders in patients with bullous pemphigoid. Dermatology 2007;215:187–191. [DOI] [PubMed] [Google Scholar]
- 52. Foureur N, Descamps V, Lebrun‐Vignes B, et al. Bullous pemphigoid in a leg affected with hemiparesia: a possible relation of neurological diseases with bullous pemphigoid? Eur J Dermatol 2001;11:230–233. [PubMed] [Google Scholar]
- 53. Gamblicher T, Segert H, Hoxtermann S, et al. Neurological disorders in patients with bullous pemphigoid clinical and experimental investigations. J Eur Acad Dermatol Venereol 2015;29:1758–1762. [DOI] [PubMed] [Google Scholar]
- 54. Kwan Z, Lai YN, Ch'ng CC, et al. The association between bullous pemphigoid and neurological disorders in a selected Malaysian population. Med J Malaysia 2015;70:81–85. [PubMed] [Google Scholar]
- 55. Langan SM, Groves RW, West J. The relationship between neurological disease and bullous pemphigoid: a population‐based case‐control study. J Invest Dermatol 2011;131:631–636. [DOI] [PubMed] [Google Scholar]
- 56. Stinco G, Codutti R, Scarbolo M, et al. A retrospective epidemiological study on the association of bullous pemphigoid and neurological diseases. Acta Derm Venereol 2005;85:136–139. [DOI] [PubMed] [Google Scholar]
- 57. Taghipour K, Ching‐Chi C, Vincent A, et al. The association of bullous pemphigoid with cerebrovascular disease and dementia. Arch Dermatol 2010;146:1251–1254. [DOI] [PubMed] [Google Scholar]
- 58. Teixeira VB, Cabral R, Brites MM, et al. Bullous pemphigoid and comorbidities a case‐control study in Portuguese patients. An Bras Dermatol 2014;89:274–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Culton DA, Liu Z, Diaz LA. Bullous pemphigoid In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, eds. Fitzpatrick's Dermatology in General Medicine. 8th ed New York: McGraw‐Hill Professional; 2012:608–616. [Google Scholar]
- 60. Behlim T, Sharma YK, Chaudhuri ND, Dash K. Dyshidrosiform pemphigoid with parkinsonism in a nonagenarian Maharashtrian female. Indian Dermatol Online J 2014;5:482–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li L, Chen J, Wang B, et al. Sera from patients with bullous pemphigoid (BP) associated with neurological diseases recognized BP antigen 1 in the skin and brain. Br J Dermatol 2009;160:1343–1345. [DOI] [PubMed] [Google Scholar]
- 62. Bertoni JM, Arlette JP, Fernandez HH, et al. Increased melanoma risk in Parkinson's disease: a prospective clinicopathological study. Arch Neurol 2010;67:347–352. [DOI] [PubMed] [Google Scholar]
- 63. Ong EL, Goldacre R, Goldacre M. Differential risks of cancer types in people with Parkinson's disease: a national record‐linkage study. Eur J Cancer 2014;50:2456–2462. [DOI] [PubMed] [Google Scholar]
- 64. Wirdefeldt K, Weibull CE, Chen H, et al. Parkinson's disease and cancer: a register‐based family study. Am J Epidemiol 2014;179:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Walter U, Heilman E, Voss J, et al. Frequency and profile of Parkinson's disease prodromi in patients with malignant melanoma. J Neurol Neurosurg Psychiatry 2016;87:302–310. [DOI] [PubMed] [Google Scholar]
- 66. Rumpf JJ, Schirmer M, Fricke C, et al. Light pigmentation phenotype is correlated with increased substantia nigra hyperechogenicity. Mov Disord 2015;30:1848–1852. [DOI] [PubMed] [Google Scholar]
- 67. Fiala KH, Whetteckey J, Manyam BV. Malignant melanoma and levodopa in Parkinson's disease: causality or coincidence? Parkinsonism Relat Disord 2003;9:321–327. [DOI] [PubMed] [Google Scholar]
- 68. Skibba JL, Pinckley J, Gilbert EF, et al. Multiple primary melanoma following administration of levodopa. Arch Pathol 1972;93:556–561. [PubMed] [Google Scholar]
- 69. Olsen JH, Tangerud K, Wermuth L, et al. Treatment with levodopa and risk for malignant melanoma. Mov Disord 2007;22:1252–1257. [DOI] [PubMed] [Google Scholar]
- 70. Zanetti R, Loria D, Rosso S. Melanoma, Parkinson's disease and levodopa: causal or spurious link? A review of the literature. Melanoma Res 2006;16:201–206. [DOI] [PubMed] [Google Scholar]
- 71. Bajaj A, Driver JA, Schernhammer ES. Parkinson's disease and cancer risk: a systematic review and meta‐analysis. Cancer Causes Control 2010;21:697–707. [DOI] [PubMed] [Google Scholar]
- 72. Berwick M. Counterpoint: sunscreen use is a safe and effective approach to skin cancer prevention. Cancer Epidemiol Biomarkers Prev 2007;16:1923–1924. [DOI] [PubMed] [Google Scholar]
- 73. Weiner WJ, Singer C, Sanchez‐Ramos JR, Goldenberg JN. Levodopa, melanoma and Parkinson's disease. Neurology 1993;43:674–677. [DOI] [PubMed] [Google Scholar]
- 74. DataPharm . Electronic Medicines Compendium (eMC). Available at: https://www.medicines.org.uk/emc/medicine/9650. Accessed November 15, 2015.
- 75. Pan T, Li X, Jankovic J. The association between Parkinson's disease and melanoma. In J Cancer 2011;128:2251–2260. [DOI] [PubMed] [Google Scholar]
- 76. Dong J, Gao J, Nalls M, et al. Susceptibility loci for pigmentation and melanoma in relation to Parkinson's disease. Neurobiol Aging 2014;35:1512.e5–1512.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Puig‐Butille JA, Escamez MJ, Garcia‐Garcia F, et al. Capturing the biological impact of CDKN2A and MC1R genes as an early predisposing event in melanoma and non‐melanoma skin cancer. Oncotarget 2014;5:1439–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tell‐Marti G, Puig‐Butille JA, Potrony M, et al. The MC1R melanoma risk variant p. R160W is associated with Parkinson's disease. Ann Neurol 2015;77:889–894. [DOI] [PubMed] [Google Scholar]
- 79. Elincx‐Benizri S, Inzelberg R, Greenbaum L, et al. The melanocortin 1 receptor (McR1) variants do not account for the co‐occurrence of Parkinson's disease and malignant melanoma. J Mol Neurosci 2014;54:820–825. [DOI] [PubMed] [Google Scholar]
- 80. Dong J, Gao J, Nalls M, et al. Susceptibility loci for pigmentation and melanoma in relation to Parkinson's disease. Neurobiol Aging 2014; 35: 1512e5–1512e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Inzelberg R, Samuels Y, Azizi E, et al. Parkinson disease (PARK) genes are somatically mutated in cutaneous melanoma. Neurol Genet 2016;2:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Quaresma MV, Gomes AC, Serruya A, Vendramini DL, Braga L, Buçard AM. Amantadine‐induced livedo reticularis—case report. An Bras Dermatol 2015;90:745–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rana AQ, Masroor MS. Patient perception of livedo reticularis due to amantadine. Int J Neurosci 2012;122:363–366. [DOI] [PubMed] [Google Scholar]
- 84. Singer C, Papapetropoulos S, Gonzales MA, et al. Rimantadine in Parkinson's disease patients experiencing peripheral adverse effects from amantadine: report of a case series. Mov Disord 2005;20:873–877. [DOI] [PubMed] [Google Scholar]
- 85. Tietjen GE, Gottwald L, Al‐Quasmi MM, et al. Migraine is associated with livedo reticularis: a prospective study. Headache 2002;42:263–267. [DOI] [PubMed] [Google Scholar]
- 86. Ono S, Tanizaki H, Miyachi Y, et al. Late onset of livedo reticularis induced by amantadine treatment concomitant with erythrocytosis. Eur J Dermatol 2012;22:273–274. [DOI] [PubMed] [Google Scholar]
- 87. Sladden MJ, Nicolaou N, Johnston GA, et al. Livedo reticularis induced by amantadine. Br J Dermatol 2003;149:656–658. [DOI] [PubMed] [Google Scholar]
- 88. Gibbs MB. Livedo reticularis: an update. J Am Acad Dermatol 2005;52:1009–1019. [DOI] [PubMed] [Google Scholar]
- 89. Hayes BB, Cook‐Norris RH, Miller JL, et al. Amantadine‐induced livedo reticularis: a report of two cases. J Drugs Dermatol 2006;5:288–289. [PubMed] [Google Scholar]
- 90. Elshoff JP, Braun M, Andreas JO, et al. Steady‐state plasma concentrations profile of transdermal rotigotine: an integrated analysis of three, open‐label, randomized, phase I multiple doses studies. Clin Ther 2012;34:966–978. [DOI] [PubMed] [Google Scholar]
- 91. Sprenger FS, Seppi K, Poewe W. Drug safety evaluation of rotigotine. Expert Opin Drug Saf 2012;11:503–512. [DOI] [PubMed] [Google Scholar]
- 92. Benitez A, Edens H, Fishman J, et al. Rotigotine transdermal system: developing continuous dopaminergic delivery to treat Parkinson's disease and restless leg syndrome. Ann N Y Acad Sci 2014;1329:45–66. [DOI] [PubMed] [Google Scholar]
- 93. Sanford M, Scott LJ. Rotigotine transdermal patch: a review of its use in the treatment of Parkinson's disease. CNS Drugs 2011;25:699–719. [DOI] [PubMed] [Google Scholar]
- 94. UCB Pharma . Neupro (rotigotine transdermal system) (US prescribing information). Brussels, Belgium: UCB Pharma; 2013. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/.../021829s002lbl.pdf. Accessed 14 June 2016. [Google Scholar]
- 95. Gregory R, Miller S. Parkinson's disease and the skin. Pract Neurol 2015;15:246–249. [DOI] [PubMed] [Google Scholar]
- 96. Eisler T, Hall RP, Kalavar KA, Calne DB. Erythromelalgia‐like eruption in parkinsonian patients treated with bromocriptine. Neurology 1981;31:1368–1370. [DOI] [PubMed] [Google Scholar]
- 97. Monk BE, Parkes JD, Du Vivier A. Erythromelalgia following pergolide administration. Br J Dermatol 1984;111:97–99. [DOI] [PubMed] [Google Scholar]
- 98. Foti C, Cassano N, De Mari M, et al. Bullous skin eruption associated with entacapone. Int J Dermatol 2004;43:471–472. [DOI] [PubMed] [Google Scholar]
- 99. Trenkwalder C, Chaudhuri KR, Garcia‐Ruiz PJ, et al. Expert consensus group report on the use of apomorphine in the treatment of Parkinson's disease—clinical practice recommendations. Parkinsonism Relat Disord 2015;21:1023–1030. [DOI] [PubMed] [Google Scholar]
- 100. Katzenschlager R, Hughes A, Evans A, et al. Continuous subcutaneous apomorphine therapy improves dyskinesias in Parkinson's disease: a prospective study using single‐dose challenges. Mov Disord 2005;20:151–157. [DOI] [PubMed] [Google Scholar]
- 101. Wojtecki L, Südmeyer M, Schnitzel A. Multiple subcutaneous abscesses and necroses due to apomorphine pump treatment [letter]. Parkinsonism Relat Disord 2012;18:1002. [DOI] [PubMed] [Google Scholar]
- 102. Acland KM, Churchyard A, Fletcher CL, et al. Panniculitis in association with apomorphine pump. Br J Dermatol 1998;138:480–482. [DOI] [PubMed] [Google Scholar]
- 103. Poltawski L, Edwards H, Todd A, et al. Ultrasound treatment of cutaneous side‐effects of infused apomorphine: a randomized controlled pilot study. Mov Disord 2009;24:115–118. [DOI] [PubMed] [Google Scholar]
- 104. Buongiorno M, Antonelli F, Camara A, et al. Long‐term response to continuous duodenal infusion of levodopa/carbidopa gel in patients with advanced Parkinson disease: the Barcelona registry. Parkinsonism Relat Disord 2015;21:871–876. [DOI] [PubMed] [Google Scholar]
- 105. Antonini A, Tolosa E. Apomorphine and levodopa infusion therapies for advanced Parkinson's disease: selection criteria and patient management. Expert Rev Neurother 2009;9:859–867. [DOI] [PubMed] [Google Scholar]
- 106. Löser C, Schl G, Hebuterne X, et al. ESPEN guidelines on artificial enteral nutrition—percutaneous endoscopic gastrostomy (PEG). Clin Nutr 2005;24:848–861. [DOI] [PubMed] [Google Scholar]
- 107. Devos D; French DUODOPA Study Group . Patient profile, indications, efficacy and safety of duodenal levodopa infusion in advanced Parkinson's disease. Mov Disord 2009;24:993–1000. [DOI] [PubMed] [Google Scholar]
- 108. Lucendo AJ, Friginal‐Ruiz AB. Percutaneous endoscopic gastrostomy: an update on its indications, management, complications and care. Rev Esp Enferm Dig 2014;106:529–539. [PubMed] [Google Scholar]
- 109. AbbVie Inc . PEG information for users. Available at: http://www.sukl.sk/sk/databazy-a-servis/databazy/vyhladavanie-zdravotnickych-pomocok?page_id=1725&submited=1&zp_nazov=PEG&fir_nazov=AbbVie&kat_kod=0&kod_zp=&fir_nazov2=. Accessed 14 June 2016.
- 110. Sixel‐Döring F, Trenkwalder C, Kappus C, Hellwig D. Skin complications in deep brain stimulation for Parkinson's disease: frequency, time course, and risk factors. Acta Neurochir 2010;152:195–200. [DOI] [PubMed] [Google Scholar]
- 111. Falowski SM, Ooi YC, Bakay RA. Long‐term evaluation of changes in operation technique and hardware‐related complications with deep brain stimulation. Neuromodulation 2015;18:670–677. [DOI] [PubMed] [Google Scholar]
- 112. Solmaz B, Tatarli N, Ceylan D, et al. A sine‐wave‐shaped skin incision for inserting deep brain stimulators. Acta Neurochir 2014;156:1523–1525. [DOI] [PubMed] [Google Scholar]
- 113. Park YS, Kang JH, Kim HY, et al. A combination of procedure with double C‐shaped skin incision and dual‐floor burr hole method to prevent skin erosion on the scalp and reduce postoperative skin complications in deep brain stimulation. Stereotact Funct Neurosurg 2011;89:178–184. [DOI] [PubMed] [Google Scholar]
- 114. Gomez R, Hontanilla B. The reconstructive management of hardware‐related scalp erosion in deep brain stimulation for Parkinson's disease. Ann Plast Surg 2014;73:291–294. [DOI] [PubMed] [Google Scholar]
- 115. Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha‐synuclein gene identified in families with Parkinson's disease. Science 1997;276:2045–2047. [DOI] [PubMed] [Google Scholar]
- 116. Spillantini MG, Schmidt ML, Lee VM, et al. α‐Synuclein in Lewy bodies. Nature 1997;388:839–840. [DOI] [PubMed] [Google Scholar]
- 117. Kordower JH, Chu Y, Hauser RA, et al. Lewy‐body like pathology in long‐term embryonic nigral transplants in Parkinson's disease. Nat Med 2008;5:504–506. [DOI] [PubMed] [Google Scholar]
- 118. Irwin DJ, Lee VM, Trojanowski JQ. Parkinson's disease dementia—convergence of α‐synuclein, tau and amyloid‐beta pathologies. Nat Rev Neurosci 2013;14:626–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Hansen C, Angot E, Bergstrom AL, et al. α‐Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest 2011;2:715–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Luk KC, Kehm V, Carroll J, et al. Pathological a‐synuclein transmission initiates Parkinson‐like neurodegeneration in nontransgenic mice. Science 2012;338:949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ulusoy A, Rusconi R, Perez‐Revuelta BI, et al. Caudo‐rostral brain spreading of a‐synuclein through vagal connections. EMBO Mol Med 2013;5:1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Pan‐Montojo F, Anichtchik O, Dening Y, et al. Progression of Parkinson's disease pathology is reproduced by intragastric administration of rotenone in mice [serial online]. PLoS One 2010;5:e8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Pan‐Montojo F, Schwartz M, Winkler C, et al. Environmental toxins trigger PD‐like progression via increased alpha‐synuclein release from enteric neurons in mice [serial online]. Sci Rep 2012;2:898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bloch A, Probst A, Bissig H, et al. Alpha‐synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol 2006;32:284–295. [DOI] [PubMed] [Google Scholar]
- 125. Beach TG, Adler CH, Sue LI, et al. Multi‐organ distribution of phosphorylated alpha‐synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 2010;119:689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Shannon KM, Keshavarzian A, Dodiya HB, et al. Is alpha‐synuclein in the colon a biomarker for premotor Parkinson's disease? Evidence from 3 cases. Mov Disord 2012;27:716–719. [DOI] [PubMed] [Google Scholar]
- 127. Michell AW, Luheshi LM, Barker RA. Skin and platelet alpha‐synuclein as peripheral biomarkers of Parkinson's disease. Neurosci Lett 2005;381:294–298. [DOI] [PubMed] [Google Scholar]
- 128. Dabby R, Djaldetti R, Shahmurov M, et al. Skin biopsy for assessment of autonomic denervation in Parkinson's disease. J Neural Transm 2006;113:1169–1176. [DOI] [PubMed] [Google Scholar]
- 129. Doppler K, Ebert S, Uceyler N, et al. Cutaneous neuropathy in Parkinson's disease: a window into brain pathology. Acta Neuropathol 2014;128:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Donadio V, Incensi A, Leta V, et al. Skin nerve alpha‐synuclein deposits: a biomarker for idiopathic Parkinson's disease. Neurology 2014;82:1362–1369. [DOI] [PubMed] [Google Scholar]
- 131. Haga R, Sugimoto K, Nishijima H, et al. Clinical utility of skin biopsy in differentiating between Parkinson's disease and multiple system atrophy [serial online]. Parkinsons Dis 2015;2015:167038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Miki Y, Tomiyama M, Ueno T, et al. Clinical availability of skin biopsy in the diagnosis of Parkinson's disease. Neurosci Lett 2010;469:357–359. [DOI] [PubMed] [Google Scholar]
- 133. Nolano M, Provitera V, Estraneo A, et al. Sensory deficit in Parkinson's disease: evidence of a cutaneous denervation. Brain 2008;131:1903–1911. [DOI] [PubMed] [Google Scholar]
- 134. Rodriguez‐Leyva I, Calderon‐Garciduenas AL, Jimenez‐Capdeville ME, et al. α‐Synuclein inclusions in the skin of Parkinson's disease and parkinsonism. Ann Clin Transl Neurol 2014;1:471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Rossi A, Gionvenali P, Benvenuti M, et al. Skin biopsy: a new diagnostic tool for autonomic dysfunctions in Parkinson's disease? Lancet Neurol 2007;6:848–849. [DOI] [PubMed] [Google Scholar]
- 136. Wang N, Gibbons CH, Lafo J, et al. Alpha‐synuclein in cutaneous autonomic nerves. Neurology 2013;81:1604–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zange L, Noack C, Hahn K, et al. Phosphorylated α‐synuclein in skin nerve fibers differentiates Parkinson's disease form multiple system atrophy. Brain 2015;138:2310–2321. [DOI] [PubMed] [Google Scholar]
- 138. Gelpi E, Navarro‐Otano J, Tolosa E, et al. Multiple organ involvement by alpha‐synuclein pathology in Lewy body disorders. Mov Disord 2014;29:1010–1018. [DOI] [PubMed] [Google Scholar]
- 139. Ikemura M, Saito Y, Sengoku R, et al. Lewy body pathology involves cutaneous nerves. J Neuropathol Exp Neurol 2008;67:945–953. [DOI] [PubMed] [Google Scholar]
- 140. Antonio‐Rubio I, Madrid‐Navarro CJ, Salazar‐Lopez E, et al. Abnormal thermography in Parkinson's disease. Parkinsonism Relat Disord 2015;21:852–857. [DOI] [PubMed] [Google Scholar]
- 141. Djaldetti R, Melamed E, Gadoth N. Abnormal skin wrinkling in the less affected side in hemiparkinsonism—a possible test for sympathetic dysfunction in Parkinson's disease. Biomed Pharmacother 2001;55:475–478. [DOI] [PubMed] [Google Scholar]
- 142. Esen F, Celebi G, Ertekin C, Colakoglu Z. Electrodermal activity in patients with Parkinson's disease. Clin Auton Res 1997;7:35–40. [DOI] [PubMed] [Google Scholar]
- 143. Hoepken HH, Gispert Z, Azizov M, et al. Parkinson patients fibroblasts show increased alpha‐synuclein expression. Exp Neurol 2008;212:307–313. [DOI] [PubMed] [Google Scholar]
- 144. Del Hoyo P, Garcia‐Redondo A, de Bustos F, et al. Oxidative stress in skin fibroblasts cultures from patients with Parkinson's disease [serial online]. BMC Neurol 2010;10:12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Van der Merwe C, Loos B, Swart C, et al. Mitochondrial impairment observed in fibroblasts from South African Parkinson's disease patients with parkin mutations. Biochem Biophys Res Commun 2014;447:334–340. [DOI] [PubMed] [Google Scholar]
- 146. Zanelatti MC, Monti V, Barzaghi C, et al. Mitochondrial dysfunction in Parkinson's disease: evidence in mutant PARK2 fibroblasts [serial online]. Front Genet 2015;6:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Mak SK, Tewari D, Tetrud JW, et al. Mitochondrial dysfunction in skin fibroblasts from a Parkinson's disease patient with an alpha‐synuclein triplication. J Parkinson Dis 2011;1:175–183. [DOI] [PubMed] [Google Scholar]
- 148. Klinkenberg M, Thurow N, Gispert S, et al. Enhanced vulnerability of PARK6 patient skin fibroblasts to apoptosis induced by proteosomal stress. Neuroscience 2010;166:422–434. [DOI] [PubMed] [Google Scholar]
- 149. Musanti R, Parati E, Lamperti E, Ghiselli G. Decreased cholesterol biosynthesis in fibroblasts from patients with Parkinson's disease. Biochem Med Metab Biol 1993;49:133–142. [DOI] [PubMed] [Google Scholar]
- 150. Jacobs BM. Stemming the hype: what can we learn from iPSC models of Parkinson's disease and how can we learn it? J Parkinson Dis 2014;4:15–27. [DOI] [PubMed] [Google Scholar]
- 151. Disse M, Reich H, Lee PK, et al. A review of the association between Parkinson disease and malignant melanoma. Dermatol Surg 2016;42:141–146. [DOI] [PubMed] [Google Scholar]


