Abstract
Background
Impaired dexterity is a common symptom in Parkinson's disease (PD) and has been related to limb kinetic apraxia (LKA). LKA negatively influenced activities of daily living (ADL) in PD. However, the impact on quality of life (QoL) remains to be clarified, which was the aim of the current study.
Methods
Eighty nondemented patients with PD and 60 age‐matched, sex‐matched healthy individuals participated in this study. The 39‐item Parkinson's Disease Questionnaire was used to measure QoL. Dexterity was assessed by the coin rotation (CR) task and the ADL‐related Dexterity Questionnaire 24. Nonmotor symptoms were monitored with part I of the Movement Disorder Society‐Unified Parkinson's Disease Rating Scale, and motor symptoms were measured with parts II and III of the modified Movement Disorder Society‐Unified Parkinson's Disease Rating Scale.
Results
Regression analysis revealed that dexterity scores controlled for parkinsonian motor symptoms were a strong and independent predictor of QoL in patients with PD.
Conclusion
The study demonstrated that the strong association of impaired dexterity and QoL is independent of bradykinesia, thereby underscoring the clinical relevance of LKA in PD.
Keywords: dexterity, limb‐kinetic apraxia, Parkinson's disease, quality of life
Patients with Parkinson's disease (PD) often suffer from dexterous deficits,1, 2, 3 which affect different activities of daily living (ADL), such as dressing, tying shoe laces, and using computer keyboards.1 These impairments add to the burden of PD4, 5 and thus are expected to reduce quality of life (QoL). Interestingly, the dexterous deficit may remain even when patients are in good on state; that is, when motor functioning is rather good.1 Therefore, it has been suggested that limb kinetic apraxia (LKA), a disorder of fine motor control not explained by elementary sensorimotor deficits being assessed by a coin rotation (CR) task, may explain impaired dexterity in PD.1, 2, 3, 5 LKA has been described as an higher order (i.e., apraxic) motor disorder characterized by impaired control of selective and coordinated hand and finger movements not explained by motor or sensory deficits.1, 2, 3, 5 Recently, it was demonstrated that LKA was predictive for buttoning/unbuttoning a cardigan.5 However, QoL, as measured by an ADL‐related subscore on the Parkinson Disease Questionnaire 39 (PDQ‐39), did not influence this dexterity‐specific ADL task. Therefore, the role of impaired dexterity for QoL in PD remains to be explored. Specifically, the impact of defective fine motor skill on QoL, which is independent of bradykinesia, was of main interest.
The aim of the present study was to elucidate in the impact of LKA on the QoL of patients with PD, as measured using the PDQ‐396 and controlled for parkinsonian motor symptoms using the modified Movement Disorder Society‐Unified Parkinson's Disease Rating Scale (MDS‐UPDRS). Based on its everyday impact, we hypothesized that LKA is an independent and significant predictor for QoL.
Patients and Methods
Participants
Eighty patients with PD (Hoehn & Yahr stages I–IV) and 60 age‐matched and sex‐matched healthy individuals participated in this study. Patients were recruited at the Neurocenter of the General Hospital of Lucerne. The healthy individuals were relatives and people working at the Neurocenter. They were all naive to the purpose of the study. The study was performed in accordance with the Declaration of Helsinki and was approved by the local ethics committee. All participants provided written informed consent. Patients were included if they had an established diagnosis of PD, as determined by the UK Brain Bank diagnostic criteria,7 and had been under stable medical treatment for at least 1 month to ensure task performance in good on state. Exclusion criteria for all participants were significant psychiatric or medical comorbidity, including severe cognitive disorders (Montreal Cognitive Assessment [MoCA] scores <21).8 Hand dominance was assessed with the Edinburgh Handedness Inventory.9 Detailed clinical characteristics are presented in Table 1.
Table 1.
Characteristics of Patients and Healthy Participants
| Characteristic | Mean ± SD (range) | |
|---|---|---|
| PD patients, n = 80 | Healthy participants, n = 60 | |
| Age, y | 68.5 ± 7.5 (49–81) | 66.9 ± 9.9 (52–83) |
| Sex: no. of men/women | 40/40 | 30/30 |
| Handedness: no. right/left | 78/2 | 56/4 |
| MoCA score | 26.4 ± 2.1 (21–30) | |
| Disease duration, y | 5.8 ± 3.3 (0–14) | |
| Hoehn & Yahr stage on | 2.0 ± 0.7 (1–4) | |
| Levodopa equivalent, mg/d | 624.3 ± 392 (60–1855) | |
| PDQ‐39 score | 39.93 ± 22.2 (5.0–91.0) | |
| CR task: Mean score | 8.6 ± 3.0 (2.5–15.5) | 14.2 ± 3.0 (6.5–22.5) |
| Right | 8.9 ± 3.4 (1.0–17.5) | 14.9 ± 3.1 (6.5–22.5) |
| Left | 8.2 ± 3.6 (2.5–16.0) | 13.8 ± 3.2 (5.4–22.0) |
| DextQ‐24 score | 38.5 ± 10.1 (24–73) | |
| MDS‐UPDRS scoresa | ||
| Part I | 10.1 ± 5.2 (1–23) | |
| Part II | 4.9 ± 2.6 (0–11) | |
| Part III | 14.5 ± 5.5 (4–29) | |
MDS‐UPDRS part II contains Items 2.4 through 2.7, and part III contains Items 3.3 through 3.6 and Items 3.15 through 3.18.
Abbreviations: SD, standard deviation: PD, Parkinson's disease; MoCA, Montreal Cognitive Assessment; on, on medication; PDQ, Parkinson's Disease Questionnaire 39; CR, coin rotation; DextQ‐24, Dexterity questionnaire 24; MDS‐UPDRS, Movement Disorder Society‐Unified Parkinson's Disease Rating Scale.
Behavioral Assessments
QoL was measured by the widely used and fully validated PDQ‐39,6 which comprises 39 questions, each with 5 different answer options (never, occasionally, sometimes, often, or always). A total index can be calculated according to the scoring algorithm as defined by Peto and colleagues.6 The maximum score is 100, indicating worst level of problem.
LKA was measured by the CR task, which is a valid and sensitive screening for dexterity and has been used in several studies to assess LKA in PD.1, 2, 3, 5 During the CR task, the participants were instructed to flip a 20‐Rappen coin between thumb, index finger, and middle finger as fast as possible. The score reflects the number of half turns adjusted for coin drops (CR score = half turns − [coin drops × 0.1 × half turns]).1 The CR task was performed 3 times with each hand separately, and each trial lasted 10 seconds. To assure correct CR execution, the first trial was excluded. The CR score represents the mean of the resulting 2 trials. Dexterity‐related ADL difficulties were measured by the DextQ‐24, which is a recently validated patient‐recorded outcome measure that contains 24 questions, which are divided into 5 subgroups (“washing/grooming,” “dressing,” “meals and kitchen,” “everyday tasks,” and “TV/CD/DVD”).10 For each question, patients had to state whether they had no problems (1 points), minor problems (2 points), major problems (3 points), or needed aid (4 point) to perform the task. Total scores ranged from a minimum of 24 points (no problems) to a maximum of 96 points (needed aid).
Nonmotor symptoms were measured by using part I of the MDS‐UPDRS.11 For motor‐related ADL, a modified version of the MDS‐UPDRS, part II (Items 2.4–2.7) was used. For motor symptoms, upper limb items from part III (Items 3.3–3.6 and 3.15–3.18) were used.
Statistical Analysis
We explored which behavioral measures would most strongly predict QoL. For this purpose, we applied a stepwise, hierarchical regression analysis with the PDQ‐39 total score as the dependent variable and the clinical and behavioral measures as the independent variables. In a first step, clinical scores, such as disease severity (Hoehn & Yahr stage), disease duration, and cognitive scores (MoCA), were integrated within the hierarchical regression analysis. In a second step, PD‐related scores, including MDS‐UPDRS part I as well as modified part II and part III scores, followed. Finally, dexterity scores (composite mean score of right and left CR and total DextQ‐24) were hierarchically modeled. The association between dexterity and QoL, controlling for parkinsonian motor symptoms, was further analyzed by partial correlation analyses. Between‐group difference analyses (PD vs. controls) were done by using independent t tests. For all statistical analyses, the level of significance was set at P < 0.05 (2‐tailed). Statistical analyses were performed using PASW for Windows (version 24.0; SPSS, Inc., Chicago, IL).
Results
The dexterity measures CR and DextQ‐24 were significantly reduced in patients with PD (both P < 0.0001) compared with healthy participants (age matched: t138 = 1.06 [P = 0.29]; sex matched: χ2 = 0.0 [P = 1.0]).
The hierarchical regression analysis revealed that the predictive model including Hoehn & Yahr stage, disease duration, cognition (MoCA), MDS‐UPDRS scores from part I and from modified versions of parts II and III, and dexterity measures (CR and DextQ‐24) explained 60% of the variance of QoL (R2 = 0.60; F = 13.41; P < 0.001). The clinical scores for disease stage, duration, and cognition accounted for 22% of the variation in QoL. The MDS‐UPDRS scores for parts I, II, and III accounted for 25% of the variance, and dexterity accounted for 13%. However, only the dexterity measures CR and DextQ‐24 and MDS‐UPDRS scores on parts I and II were significant independent predictors of QoL (CR: β = −0.21; P < 0.05; DextQ‐24: β = 0.40; P < 0.01; MDS‐UPDRS part I: β = −0.16; P < 0.05; MDS‐UPDRS part II: β = 0.28; P < 0.05).
The independence of the amount of variance in QoL explained by dexterity also was underlined by partial correlation analyses controlling for parkinsonian motor symptoms (UPDRS part III). The findings revealed a strong association of residuals between both CR scores and QoL (rp = −0.47; P < 0.001) and between DextQ‐24 scores and QoL (rp = 0.61; P < 0.001) (see Fig. 1A,B).
Figure 1.
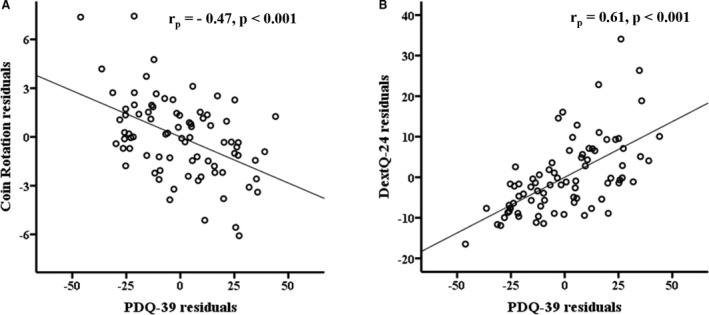
A,B: Scatter plots present significant partial correlations between dexterity measures (the coin rotation task and the activities of daily living‐related Dexterity Questionnaire 24) and the Parkinson Disease Questionnaire 39 (PDQ‐39) controlled for motor parkinsonian symptoms (Movement Disorder Society‐Unified Parkinson's Disease Rating Scale part III).
Discussion
The current study demonstrates for the first time that, in PD, impaired dexterity not explained by parkinsonian motor symptoms is an independent and strong predictor of QoL. The close association was observed for both the objective measure (CR) and the subjective patient's perspective (DextQ‐24). The finding is in line with earlier studies demonstrating that impaired dexterity in PD significantly adds to the burden of the disease.4, 5 Furthermore, as expected from the literature, nonmotor symptoms and motor‐related ADL function play an important independent role for QoL in patients with PD.12, 13, 14
We believe impaired dexterity in PD that is not explained by bradykinesia is best described as LKA.1, 2, 3, 5 The tight relationship between CR (a measure for LKA) and QoL observed herein, controlled for elemental parkinsonian symptoms, adds further evidence to this concept. Our findings extend on previous reports demonstrating associations of impaired dexterity with apraxia3 and ADL5 beyond bradykinesia.
Neuroanatomical support in favor of the LKA nature of dexterity deficits in PD comes from a very recently published functional magnetic resonance imaging (fMRI) study.15 The findings in PD demonstrated increased fMRI activity in the left inferior parietal lobe and the left ventral premotor area associated with the CR task.15 These areas are considered core regions of the limb praxis network in the left hemisphere16 that are activated independent of the hand involved.
The reason why LKA affects QoL in PD is that dexterous interaction with objects (turning keys, opening a jar, writing notes, and using newer technologies, such as smart phones and tablets) is highly prevalent in everyday life. Efficient manipulation of these tools depends on the control of independent, coordinated finger movements, which typically are affected in LKA.1, 2, 3, 5 Even in mild‐to‐moderate stages of the disease, patients with PD may complain about this type of manipulation difficulties, despite being in a good on state, because more time is needed to achieve their personal goals.5 The impact of LKA on QoL in PD may be explained by hindering the patient's participation in leisure activities,17 which play an important role in subjective well‐being. Furthermore, leisure activities provide the opportunity to acquire additional motor skills and ultimately to build social relationships.18
In conclusion, LKA is an independent predictor for QoL in PD and contributes significantly to the burden of the disease. Therefore, LKA may put a significant strain on patients, requiring targeted treatment. Our findings clearly favor the concept that LKA accounts for dexterous impairment beyond parkinsonian symptoms, a controversial debate that started a decade ago.19, 20 Because dopaminergic treatment only minimally improves LKA,1 nonpharmacologic treatment options, such as noninvasive, repetitive brain‐stimulation techniques21, 22 or specific home‐based dexterity training,23 may offer an improvement in dexterous functions for patients with PD.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
T.V.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
D.H.: 1C; 2B, 2C, 3A, 3B
S.K.: 2B, 2C, 3B
S.B.: 1A, 1B, 2A, 2C, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest: This study was supported by the Swiss National Foundation (32003B_155954) and the Jacques and Gloria Gossweiler Foundation. The authors report no conflicts of interest.
Financial Disclosures for the previous 12 months: The authors report no sources of funding and no conflicts of interest.
Acknowledgments
We are grateful to all participants for their commitment to this research.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Gebhardt A, Vanbellingen T, Baronti F, Kersten B, Bohlhalter S. Poor dopaminergic response of impaired dexterity in Parkinson's disease: bradykinesia or limb kinetic apraxia? Mov Disord 2008;23:1701–1706. [DOI] [PubMed] [Google Scholar]
- 2. Quencer K, Okun MS, Crucian G, Fernandez HH, Skidmore F, Heilman KM. Limb‐kinetic apraxia in Parkinson disease. Neurology 2007;68:150–151. [DOI] [PubMed] [Google Scholar]
- 3. Vanbellingen T, Kersten B, Bellion M, Temperli P, Baronti F, Müri R, Bohlhalter S. Impaired finger dexterity in Parkinson's disease is associated with praxis function. Brain Cogn 2011;77:48–52. [DOI] [PubMed] [Google Scholar]
- 4. Pohar SL, Allyson Jones C. The burden of Parkinson disease (PD) and concomitant comorbidities. Arch Gerontol Geriatr 2009;49:317–321. [DOI] [PubMed] [Google Scholar]
- 5. Foki T, Vanbellingen T, Lungu C, et al. Limb‐kinetic apraxia affects activities of daily living in Parkinson's disease: a multi‐center study. Eur J Neurol 2016;23:1301–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peto V, Jenkinson C, Fitzpatrick R. Determining minimally important differences for the PDQ‐39 Parkinson's disease questionnaire. Age Ageing 2001;30:299–302. [DOI] [PubMed] [Google Scholar]
- 7. Hughes AJ, Ben‐Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology 1992;42:1142–1146. [DOI] [PubMed] [Google Scholar]
- 8. Gill DJ, Freshman A, Blender J, Ravina B. The Montreal Cognitive Assessment as a screening tool for cognitive impairment in Parkinson's disease. Mov Disord 2008;23:1043–1046. [DOI] [PubMed] [Google Scholar]
- 9. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- 10. Vanbellingen T, Nyffeler T, Nef T, Kwakkel G, Bohlhalter S, van Wegen EEH. Reliability and validity of a new dexterity questionnaire (DextQ‐24) in Parkinson's disease. Parkinsonism Relat Disord 2016;33:78–83. [DOI] [PubMed] [Google Scholar]
- 11. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–2170. [DOI] [PubMed] [Google Scholar]
- 12. Schrag A, Jahanshahi M, Quinn N. How does Parkinson's disease affect quality of life? A comparison with quality of life in the general population. Mov Disord 2000;15:1112–1118. [DOI] [PubMed] [Google Scholar]
- 13. Findley L, Eichhorn T, Janca A. Factors impacting on quality of life in Parkinson's disease: results from an international survey. Mov Disord 2002;17:60–67. [DOI] [PubMed] [Google Scholar]
- 14. Müller B, Assmus J, Herlofson K, Larsen JP, Tysnes OB. Importance of motor vs. non‐motor symptoms for health‐related quality of life in early Parkinson's disease. Parkinsonism Relat Disord 2013;19:1027–1032. [DOI] [PubMed] [Google Scholar]
- 15. Kübel S, Stegmayer K, Vanbellingen T, et al. Altered praxis network underlying limb kinetic apraxia in Parkinson's disease–an fMRI study. Neuroimage Clin 2017;16:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goldenberg G. Apraxia–the cognitive side of motor control. Cortex 2014;57:270–274. [DOI] [PubMed] [Google Scholar]
- 17. Wressle E, Engstrand C, Granerus A. Living with Parkinson's disease: elderly patients’ and relatives’ perspective on daily living. Aust Occup Ther J 2007;54:131–139. [Google Scholar]
- 18. Brajsa‐Zganec A, Merkas M, Sverko I. Quality of life and leisure activities: how do leisure activities contribute to subjective well‐being? Soc Indic Res 2011;102:81–91. [Google Scholar]
- 19. Landau WM, Mink JW. Is decreased dexterity in Parkinson disease due to apraxia? Neurology 2007;68:90–91. [DOI] [PubMed] [Google Scholar]
- 20. Heilman K, Quencer K, Okun MS, Crucian G, Fernandez HH, Skidmore F. Is decreased dexterity in Parkinson disease due to apraxia [letter]? Neurology 2007;68:2044. [DOI] [PubMed] [Google Scholar]
- 21. Vanbellingen T, Wapp M, Stegmayer K, et al. Theta burst stimulation over premotor cortex in Parkinson's disease: an explorative study on manual dexterity. J Neural Transm (Vienna) 2016;123:1387–1393. [DOI] [PubMed] [Google Scholar]
- 22. Benninger DH, Hallett M. Non‐invasive brain stimulation for Parkinson's disease: current concepts and outlook 2015. NeuroRehabilitation 2015;37:11–24. [DOI] [PubMed] [Google Scholar]
- 23. Vanbellingen T, Nyffeler T, Nigg J, et al. Home based training for dexterity in Parkinson's disease: a randomized controlled trial. Parkinsonism Relat Disord 2017;41:92–98. [DOI] [PubMed] [Google Scholar]


