Abstract
Pacemakers can be directly involved in initiating or sustaining different forms of arrhythmia. These can cause symptoms such as dyspnea, palpitations, and decompensated heart failure. Early detection of these arrhythmias and optimal pacemaker programming is pivotal. The aim of this review article is to summarize the different types of pacemaker‐mediated arrhythmias, their predisposing factors, and mechanisms of prevention or termination.
Keywords: endless loop tachycardia, pacemaker‐mediated arrhythmia, retrograde ventriculo‐atrial conduction
1. INTRODUCTION
The term pacemaker‐mediated arrhythmia includes all abnormal rhythms in which the pacing system plays a major role.1
These arrhythmias can be categorized as:
Reentrant tachycardias in which pacemaker works as the antegrade limb (antidromic)
Reentrant tachycardias in which pacemaker operates as the retrograde limb (orthodromic)
Repetitive nonreentrant ventriculo‐atrial synchrony (AV desynchronization arrhythmia)
Tracking of supraventricular arrhythmias, myopotentials, electromagnetic interference (EMI), or lead noise
Sensor‐driven tachycardia (inappropriate rate response of a pacemaker)
Runaway pacemaker
Pacemaker‐mediated arrhythmias in cardiac resynchronization therapy (CRT) systems
Atrial or ventricular arrhythmias induced by pacing.
1.1. Antidromic pacemaker‐mediated reentrant arrhythmias
This include near‐field endless loop tachycardia and far‐field endless loop tachycardia.
1.1.1. Near‐field endless loop tachycardia (ELT)
Near‐Field Endless Loop Tachycardia is the classic pacemaker‐mediated tachycardia (PMT). This occurs in dual‐chamber tracking modes (DDD or VDD) in patients with intact ventriculo‐atrial (VA) conduction through the atrioventricular (AV) node or an accessory pathway.
Approximately 80% of patients with sick sinus syndrome (SSS) and 35% of patients with AV block have retrograde VA conduction. Hence, this condition is more common in patients with sick sinus syndrome.2 One study revealed that 6% of all pacemaker patients and 20% of patients with retrograde conduction had at least one episode of documented PMT.3
In this arrhythmia, VA conduction works as the retrograde limb and consequent atrial tracking by the pacemaker as the antegrade limb of the macro‐reentrant circuit.1, 4 Therefore, during this arrhythmia, an atrial sensing‐ventricular pacing sequence will be observed.
Any condition which separates the P wave from the QRS complex (paced or spontaneous) in a patient with retrograde VA conduction may initiate PMT.2, 5
The most common triggers are ventricular premature beats (VPBs), atrial premature beats (APBs), loss of atrial capture, loss of atrial sensing, long AV delays, external interference or myopotentials sensed by the atrial channel, return to DDD mode after asynchronous mode pacing and VDD pacing at a higher rate than sinus rate.2, 5, 6
After initiation, the tachycardia will continue until terminated spontaneously due to VA block resulting from the fatigue of the conduction system or another short coupled VPB. It can be actively terminated by carotid sinus massage, with verapamil or beta‐blockers which disrupt VA conduction, placing a magnet directly over the device which can result in asynchronous mode, or pacemaker‐based algorithms as detailed below.2
The rate of arrhythmia depends on the VA conduction time, the programmed maximum tracking rate and the AV interval. It typically occurs at the maximum tracking rate of the device when the sum of VA interval and AV interval is less than the upper rate interval. The device extends the AV interval to follow the upper tracking rate (Figure 1).
Figure 1.
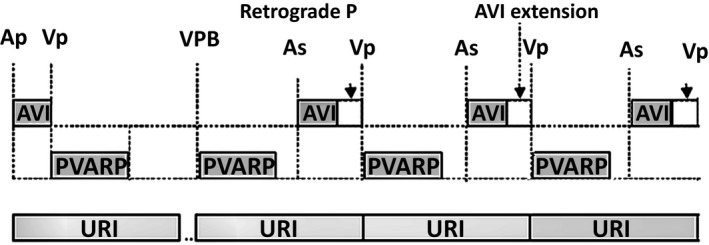
Endless loop tachycardia (ELT) is classically initiated by a ventricular premature beat (VPB). The retrograde P wave is sensed by the atrial channel because the postventricular atrial refractory period (PVARP) is set too short. The tracked P wave initiates an atrioventricular (AV) interval resulting in ventricular pacing. The loop then reoccurs in a cyclical fashion. To not violate upper rate interval, AV interval will be extended
If the sum of the VA interval and AV interval is more than the upper rate interval (due to long VA conduction), arrhythmia occurs at a rate slower than the maximum tracking rate and is called balanced ELT.1, 7
This type of arrhythmia is almost always regular; however, ELT with variable cycle lengths has been reported. This irregularity may be related to the variable conduction time in retrograde or anterograde limbs of the circuit, triggered pacing or a change in the circuit of ELT.8 Furthermore, using algorithms such as IRSplus algorithm (Biotronik) to promote intrinsic atrioventricular conduction and limit right ventricular pacing may cause pacemaker‐mediated tachycardia with several changes in cycle length.9
A useful way to observe the inducibility of this arrhythmia is performing an atrial threshold test to see the effect of loss of atrial capture. However, using an appropriate postventricular atrial refractory period (PVARP) interval is the ultimate way of preventing pacemaker‐mediated tachycardia. Measurement of VA time during implantation is a simple way to guide programming an appropriate duration of PVARP (It should be programmed 50‐75 ms more than VA conduction time). However, it should be emphasized that retrograde VA conduction depends on the autonomic tone and can vary over time in different clinical settings.1
Because a long PVARP can limit the upper tracking rate of the device, different algorithms for PMT prevention, detection, and termination have been used by various pacemaker manufacturers over years. One such algorithm is PVARP extension during VPB detection. When a VPB is sensed (any sensed ventricular event without a preceding atrial event), the device will automatically prolong PVARP (Figure 2). This PVARP extension can be programmed to either a fixed value or a variable value. One of the disadvantages of this system is the occurrence of atrial noncapture because of pacing during the refractory period after a retrograde P which can result in another form of pacemaker‐induced arrhythmia, repetitive non‐reentrant VA synchrony.1
Figure 2.
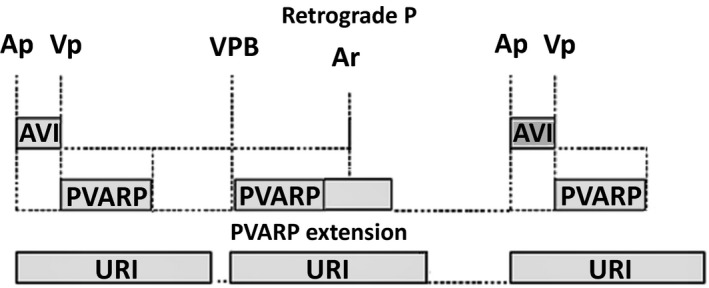
To prevent endless loop tachycardia (ELT), any sensed ventricular event without a preceding atrial event is considered a ventricular premature beat (VPB) by the device and the postventricular atrial refractory period (PVARP) will be prolonged. Hence, the retrograde P wave will not be sensed and hence no pacemaker‐mediated tachycardia (PMT) will occur
Programming a low atrial sensitivity to distinguish high‐amplitude sinus P waves from low‐amplitude retrograde P waves is not recommended as it can cause undersensing of P waves especially at high sinus rates.1
In most devices, if a specific number of P waves are tracked at the maximum tracking rate, it will be labeled as PMT. Since PMT can occur even at a rate lower that the maximum tracking rate, some pacemaker manufacturers use VA interval stability as a marker of PMT.1
Different manufacturers use various methods to terminate PMT. Extension of PVARP, PPM mode switching to DVI for one cycle, delivering an atrial output after a period sufficient to allow for atrial recovery or withholding ventricular pacing for one cycle are some of these methods.1, 5
Observing an increase in the percentage of RV pacing and atrial sensing during interrogation of the device, especially when the heart rate of such episodes is high, can suggest PMT occurrence.5
As a long AV interval permits recovery of AV refractory period and facilitates retrograde VA conduction, programming a long AV interval or using algorithms to avoid ventricular pacing like ventricular intrinsic preference (VIP) [Abbott(St. Jude Medical, Saint Paul, MN, USA)] can lead to PMT. In these patients, setting a long AV detection interval (programmed or via VIP function) should be avoided. Additionally, during automatic threshold testing, loss of atrial capture will happen and the AV interval will be extended, so automatic threshold testing may also need to be turned off.10
The same phenomenon had been reported in the RYTHMIQ AV search algorithm (Boston Scientific, Marlborough, MA, USA). It should be noted that in patients with PMT both the RYTHMIQ algorithm and AV Search+™ algorithm (Boston Scientific) should be turned off.11, 12 Occurrence of PMT due to AAI‐SafeR pacing mode [LivoNova (Sorin, London, UK)] is relatively rare but has been reported previously.13
Furthermore, it should be noted that interaction between different pacemaker algorithms may cause pacemaker‐mediated arrhythmia. There is a report about a patient with a dual chamber pacemaker (Advantio, Boston Scientific) and a PMT caused by interaction between right ventricular automatic capture (RVAC) and rate smoothing down (RSD) algorithms.14
In patients with a CRT device, exercising may cause incorrect diagnosis of PMT and algorithms to stop PMT can cause loss of biventricular stimulation.12 Therefore, in these patients, PMT algorithms may need to be inactive. Moreover, algorithms to enhance biventricular pacing like Tracking Preference (Boston Scientific) which maintains atrial‐tracked biventricular pacing in CRT devices may cause PMT.15
1.1.2. Far‐field endless loop arrhythmia
This can happen even in patients without VA conduction. In this type of arrhythmia, far‐field atrial sensing of ventricular electrical activity (the terminal portion of the QRS or the T wave) can be tracked and trigger an AV interval and ventricular pacing without any relation to P waves which can be sustained (Figure 3). This can lead to pacemaker syndrome due to AV dissociation. Prolonging PVARP can prevent this type of arrhythmia.1
Figure 3.
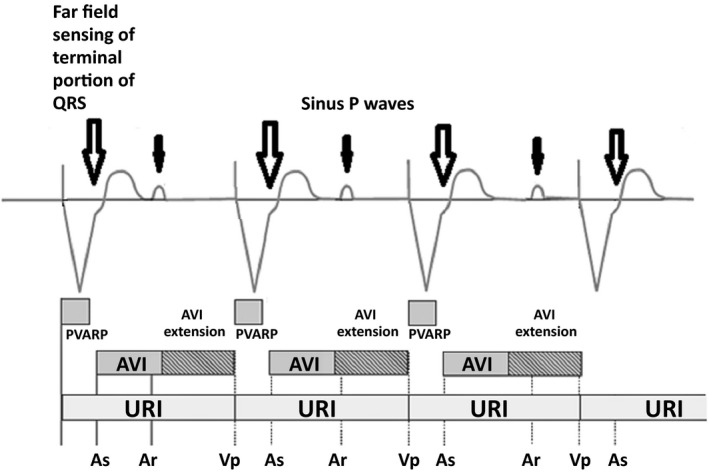
The atrial channel senses the terminal portion of QRS wave (arrows) beyond the postventricular atrial refractory period (PVARP) which triggers a ventricular stimulus after an extended atrioventricular (AV) delay that coincides with the termination of the upper rate interval. There is no correlation between sinus P waves (solid arrows) and QRS complexes
There is a report of a patient with repetitive far‐field endless loop tachycardia caused by oversensing of the RV signal in the atrial channel when the device was programmed to only LV pacing.16
1.2. Orthodromic pacemaker‐mediated tachycardia
This is also called reverse endless‐loop tachycardia. In this rare arrhythmia, intrinsic AV conduction forms the anterograde limb and the pacemaker works as the retrograde limb. It can occur in dual‐chamber pacemakers especially when synchronized atrial stimulation (SAS) is active (SAS, which was present in some older devices, triggered atrial pacing following a VPB) (Figure 4) or in a single chamber pacemaker in AAT mode (because of far‐field sensing of ventricular activation).1
Figure 4.
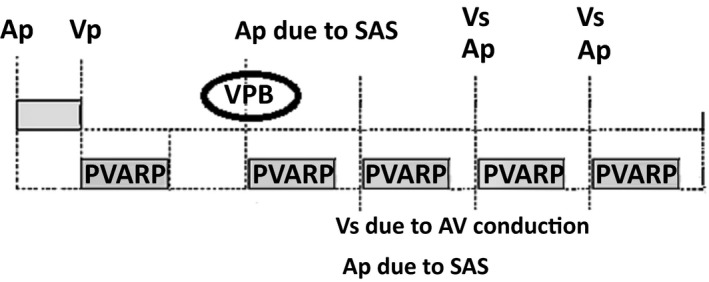
An atrial stimulus delivers on detection of a ventricular premature beat (VPB) because of synchronous atrial stimulation (SAS) algorithm. Therefore, retrograde ventriculo‐atrial (VA) conduction cannot cause atrial depolarization. However, atrial capture following the stimulus may conduct to ventricle with a long PR and give rise to an intrinsic QRS complex that is sensed beyond the ventricular refractory period. The pacemaker interprets this conducted QRS complex as another VPB and delivers SAS. This again leads to a conducted QRS complex and this will continue
Orthodromic PMT has also been reported following inverted connection of the atrial and ventricular leads during generator replacement or the dislodgement of the leads. In these conditions, ventricular activation is sensed by the atrial electrode as an atrial event, which then triggers a stimulus through the ventricular electrode in the atrium, which is conducted to ventricle via the intrinsic conduction system and again sensed by the atrial electrode.17, 18, 19
1.3. Repetitive non‐reentrant VA synchrony (RNRVAS)
Similar to the classic PMT, this occurs in patients with a dual‐chamber device with AV sequential pacing and retrograde VA conduction. Its initiation and termination is very similar to classic PMT.2, 20, 21
This is a repetitive sequence characterized by functional atrial undersensing due to retrograde atrial activation falling within the PVARP (or even undersensing of retrograde P wave after PVARP) with subsequent functional atrial noncapture due to pacing during the absolute refractory period of the recently depolarized atrium.20, 21 The atrial pacing stimulus that cannot capture the atrium will trigger ventricular pacing and the sequence repeats itself (Figure 5).1, 20
Figure 5.
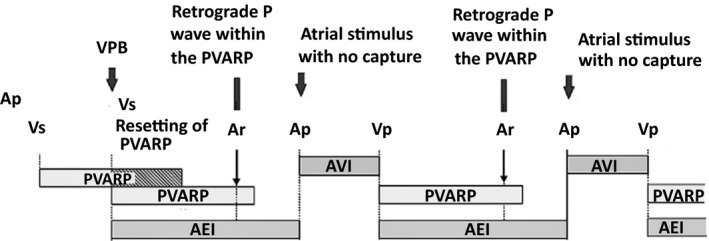
Ventriculo‐atrial (VA) conduction after ventricular pacing results in a P wave that cannot trigger ventricular pacing because of falling within postventricular atrial refractory period (PVARP). Atrial pacing happens within atrial refractory period (shortly after retrograde P). Thus, there is no atrial capture; however, it triggers ventricular pacing after atrioventricular (AV) interval and the cycle repeats
The predisposing factors include a relatively long VA conduction time, a long PVARP, and a relatively short atrial escape interval which commonly occurs during sensor‐driven rate. However, it may happen even at rest according to the programmed parameters and the VA conduction time. Using rate‐responsive modes and algorithms to avoid ventricular pacing can increase the chance of this arrhythmia.20, 21 Moreover, enabling special features like rate‐drop response (Medtronic, Minneapolis, MN, USA) and atrial overdrive pacing algorithm [Abbott (St. Jude Medical)] can also expose the device to this arrhythmia.20 Sometimes, a magnet placed on the pacemaker to terminate classic PMT may initiate RNRVAS.22
Most cases occur during physical activity due to a sensor‐driven increase in the pacing rate. After cessation of physical activity, the sensor‐driven rate will decrease and produce a longer atrial escape interval that will cause atrial pacing after the atrial refractory period and eventually capturing the atrium, terminating the arrhythmia. Therefore, RNRVAS episodes on activity are usually self‐limited.21 Using a rate‐adaptive PVARP with the shortest minimum PVARP can prevent RNRVAS occurring with activity. It should be noted that a short PVARP can predispose the device to ELT. In addition, decreasing the lower rate limit to less than 70 bpm or decreasing sensor threshold, sensor reaction time, or upper sensor rate can be useful.20
In the absence of a substantial increase in the pacing rate, ventricular premature beats are the most common initiating and terminating mechanisms for this type of PMT.2, 23 AF suppression functions can also cause RNRVAS.24
Repetitive non‐reentrant VA synchrony can be terminated by extending the noncompetitive atrial pacing (Medtronic) to allow atrial recovery and capture with atrial pacing; enabling algorithms that allow AAI and backup DDD or VVI pacing modes due to the lack of AV sequential pacing, such as MVP (Medtronic) and AAI‐SafeR [LivaNova (Sorin)], and using dynamic AV delay (Boston Scientific) or rate adaptive AV delay (Medtronic) to shorten the AV delay at higher rates.20
Repetitive non‐reentrant VA synchrony can produce loss of efficient AV synchrony. Therefore, the patient may complain of dyspnea, fatigue, and palpitations; and it may exacerbate heart failure.25 It can also cause inappropriate mode switching.22 RNRVAS is one of the most common causes of false‐positive Atrial High Rate Events (AHREs).1, 20, 26
Unlike classic PMT, RNRVAS does not occur at the programmed upper rate interval and the electrogram during device interrogation shows two atrial signals for every ventricular signal.27
1.4. Tracking of atrial arrhythmias or myopotentials
Tracking of atrial signals in atrial fibrillation, atrial flutter, or atrial tachycardia can cause ventricular pacing at the maximum tracking rate. This particularly occurs if the mode switch option is not active or the pacemaker cannot detect a sufficient number of atrial events to activate mode switching due to the undersensing of atrial signals.1
Myopotential tracking is more common in unipolar leads and is often caused by tracking of electrical activity of muscles.1
1.5. Sensor‐driven tachycardia
This may happen in any sensor‐driven pacing system. Programming a threshold too low or a slope too high in pacemakers with accelerometer‐based sensors can increase the rate for low levels of activity or even nonphysiologic events.1
It has been reported that a generator change with a smaller device programmed unipolar led to pectoral muscle stimulation and then triggered a sensor‐driven tachycardia in certain positions. This complication is not expected to occur with bipolar pacing systems.28
Pacemakers which use minute‐ventilation are prone to increase the rate in conjunction with external currents like electrocautery or RF ablation, or internal currents like measurement of transthoracic impedance.1
1.6. Runaway pacemaker
In contemporary devices, this is extremely rare. It is a malfunction of a pacemaker due to battery depletion, software errors or damage to electrical components. In this condition, the pacemaker will pace with a rate much higher than the programmed rate and hence can be detrimental.1
1.7. Pacemaker‐mediated arrhythmias in biventricular pacing systems
1.7.1. Cross‐ventricular endless‐loop tachycardia (or interventricular PMT)
This occurs due to T‐wave oversensing when a biventricular device has been programmed to trigger left ventricular (LV) pacing following a right ventricular (RV) sensed event. Prevention of T‐wave oversensing, or turning LV triggering off will solve this problem.29
This arrhythmia can also result after the dislodgement of the LV lead into the coronary sinus and LV pacing with resulting left atrial capture which after AV node conduction will trigger LV pacing.1, 30, 31
Interventricular PMT may also occur when a dual chamber pacemaker has been repurposed for use as a biventricular pacemaker in patients with atrial fibrillation, due to T‐wave oversensing by the LV lead which is connected to atrial port (Figure 6). In this condition, programming an appropriate atrial sensing level can prevent oversensing of T waves.1, 32, 33
Figure 6.
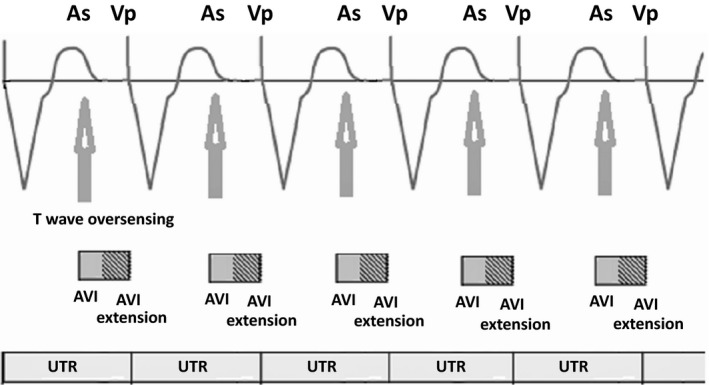
Cross‐ventricular endless loop tachycardia at the programmed upper tracking rate during biventricular pacing with a conventional dual chamber pacemaker. The atrial channel senses the T wave (arrows) that triggers a ventricular stimulus after an extended atrioventricular (AV) delay that coincides with the termination of the upper rate interval. This causes RV only pacing and loss of resynchronization
If a dual chamber pacemaker is used for biventricular pacing in patients with chronic AF, it is better to ablate the AV node to provide permanent biventricular pacing. Ideally in this scenario, the left ventricular lead should be connected to the atrial port and the right ventricular lead to the ventricular port allowing LV pacing to precede RV pacing. LV only pacing and using bipolar LV sensing are other effective ways to prevent this arrhythmia.6
1.7.2. Left ventricular upper rate interval (LVURI) lock‐in
With the widespread usage of devices with LV sensing parameters and an LV T‐wave protection option, another reason for loss of resynchronization and cardiac failure decompensation is LVURI lock‐in. This phenomenon can occur when the LVURI is programmed longer than the right ventricle upper rate interval (RVURI).34, 35
LV T‐wave protection is a parameter whose function is to prevent LV pacing into the vulnerable period of the left ventricular refractory period. Therefore, when it is active, the device can sense LV premature beats and prevent LV pacing into the vulnerable period.35
Cardiac resynchronization therapy (CRT) devices with LV sensing options have an important LV‐based time parameter, called LVURI. LVURI is the period of time that is started by LV events in which LV pacing cannot happen. This interval is programmable and corresponds to the programmed maximum trigger rate. RVURI and LVURI can be programmed individually.34
Left ventricular (LV) pacing always starts an LVURI, regardless of RV pacing offset and LV T‐wave protection programming. LV‐sensed events will start an LVURI only when the left ventricle T‐wave protection function is active. When LVTP is programmed off, the device cannot sense LV events and instead sensed RV events will initiate and reset an LVURI.34, 35
Left ventricular upper rate interval should be usually programmed shorter than the RVURI, and the difference should be sufficient in order to compensate for the interventricular delay. Otherwise, it can lead to a form of desynchronization arrhythmia, which is characterized by RV pacing followed by an LV sensed event and loss of LV pacing.
The arrhythmia can be initiated by a single loss of LV capture or by ventricular premature beats (VPBs) (Figure 7), and is then perpetuated (LVURI lock‐in) until a pause from a VPB or a decrease in the RV pacing rate can restore biventricular pacing.34, 35, 36
Figure 7.
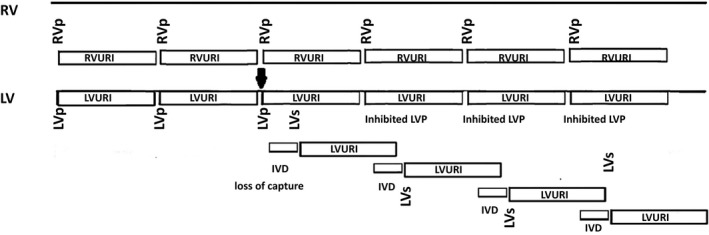
Initiation of left ventricular upper rate interval (LVURI) lock‐in after loss of capture in the left ventricular (LV) (arrow). LV pacing (LVp) failed to capture the LV, but it started a LVURI. RV pacing (RVp) captured the RV and after interventricular delay (IVD) and conduction to the left ventricle, sensing of the LV signal (LVs) restarted LVURI. The RVp was delivered without an accompanying LVp because the LVp would have fallen into an ongoing LVURI. The inhibited LVp restarted another LVURI and, again after RVp and conduction to the LV, the LVs resets the LVURI, and the cycle continues
1.8. Atrial or ventricular arrhythmias induced by pacing
The occurrence of these arrhythmias is rare, especially with a normal functioning pacemaker with no secondary causes such as acute myocardial ischemia or metabolic disturbance. There are reports of the induction of ventricular tachycardia with an abnormal circuit close to the right ventricular apex or by the fusion of pacemaker impulses with multiple ventricular premature beats.37, 38
Although it is very rare, PMT protection algorithms may cause ventricular arrhythmia in patient with structural heart disease.39
2. CONCLUSION
Pacemakers can be directly involved in initiating or sustaining multiple different forms of arrhythmia. These can result in deterioration in the patient's condition. Being familiar with these arrhythmias will allow early detection and appropriate modification of pacemaker settings during device interrogation is necessary to cure and prevent arrhythmias. Figure 8 illustrates a simple diagram, which is helpful for different diagnosis of various pacemaker‐mediated arrhythmias.
Figure 8.
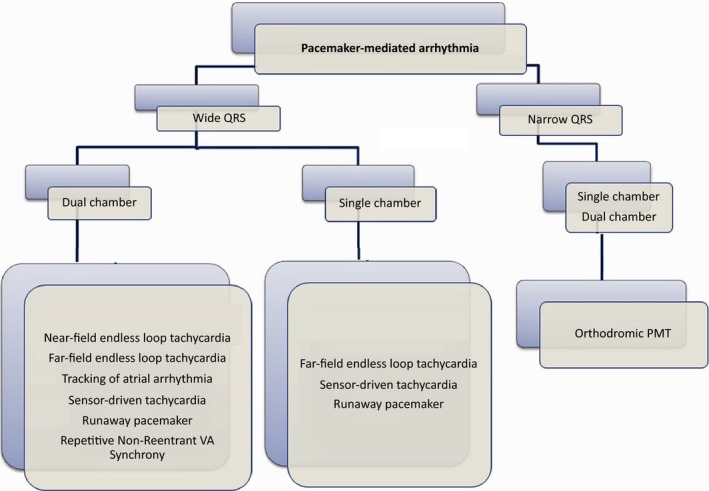
The diagram shows the differential diagnosis of pacemaker‐mediated arrhythmia types in patients with dual chamber and single chamber pacemakers
CONFLICT OF INTEREST
Authors declare no conflict of interests for this article.
Alasti M, Machado C, Rangasamy K, et al. Pacemaker‐mediated arrhythmias. J Arrhythmia. 2018;34:485–492. 10.1002/joa3.12098
REFERENCES
- 1. Doppalapudi H. Timing Cycles of Implantable Devices Clinical Cardiac Pacing, Defibrillation, and Resynchronization Therapy In: Clinical cardiac pacing, defibrillation, and resynchronization therapy, 5th edn Ellenbogen KA, Wilkoff BL, Kay GN, Lau CP, Auricchio A. (eds). Philadelphia, PA: Elsevier; 2017: pp. 961–1030. [Google Scholar]
- 2. Barold SS. Repetitive reentrant and non‐reentrant ventriculoatrial synchrony in dual chamber pacing. Clin Cardiol. 1991;14:754–63. [DOI] [PubMed] [Google Scholar]
- 3. Richter S, Muessigbrodt A, Salmas J, et al. Ventriculoatrial conduction and related pacemaker‐mediated arrhythmias in patients implanted for atrioventricular block: an old problem revisited. Int J Cardiol 2013;168:3300–3308. [DOI] [PubMed] [Google Scholar]
- 4. Roblendo‐Nolasco R, Ortiz‐Avalos M, Rodroguez‐Diez G, Castro‐Villacorta H. Catheter ablation of accessory pathway in the treatment of pacemaker‐mediated tachycardia. Pacing Clin Electrophysiol 2012;35:e84–6. [DOI] [PubMed] [Google Scholar]
- 5. Monteil B, Ploux S, Eschalier R, et al. Pacemaker‐mediated tachycardia: manufacturer specifics and spectrum of cases. Pacing Clin Electrophysiol 2015;38:1489–1498. [DOI] [PubMed] [Google Scholar]
- 6. Blanc JJ, Fatemi M. A new cause of pacemaker‐mediated tachycardia in patients implanted with a biventricular device. Pacing Clin Electrophysiol. 2001;24(12):1711–2. [DOI] [PubMed] [Google Scholar]
- 7. Sakamoto A, Takeuchi R, Hosoya N, et al. Endless loop tachycardia below the upper tracking rate of a pacemaker: a case report. J Arrhythm. 2012;28:356–9. [Google Scholar]
- 8. Gilmore M, Lim S. Intermittent irregular pacemaker‐mediated tachycardia. Pacing Clin Electrophysiol. 2015;38:1117–20. [DOI] [PubMed] [Google Scholar]
- 9. Verrijcken A, Huybrechts W, Willems R. Pacemaker‐mediated tachycardia with varying cycle length: What is the mechanism? Europcae. 2009;11(10):1400–2. [DOI] [PubMed] [Google Scholar]
- 10. Barold SS, Stroobandt RX. Pacemaker‐mediated tachycardia initiated by an atrioventricular search algorithm to minimize right ventricular pacing. J Electrocardiol. 2012;45(3):336–9. [DOI] [PubMed] [Google Scholar]
- 11. Bhattacharya S, Wang NC. Atypical initiation of endless‐loop. Pacemaker‐mediated tachycardia. Pacing Clin Electrophysiol. 2011;34:617–20. [DOI] [PubMed] [Google Scholar]
- 12. Strik M, Frontera A, Eschalier R, et al. Accuracy of the pacemaker‐mediated tachycardia algorithm in Boston Scientific devices. J Electrocardiol. 2016;49(4):522–9. [DOI] [PubMed] [Google Scholar]
- 13. Crea P, Crea T, Picciolo G, Luzza F. SafeR and escape junctional rhythm: a singular trigger for pacemaker‐mediated tachycardia. J Electrocardiol. 2017;50(5):504–6. [DOI] [PubMed] [Google Scholar]
- 14. Sharma D, Narayanan K, Wang X, Shehata M. An unusual form of pacemaker mediated tachycardia. Heart Rhythm. 2014;11:328–9. [DOI] [PubMed] [Google Scholar]
- 15. Crea P, Picciolo G, Luzza F. Tracking preference as unusual trigger of pacemaker‐mediated tachycardia in a resynchronization device. J Electrocardiol. 2016;49:509–11. [DOI] [PubMed] [Google Scholar]
- 16. Jastrzebski M. Repetitive pacemaker‐mediated tachycardia occurring only during left ventricular pacing: what is the mechanism? Heart Rhythm. 2008;5:1482–4. [DOI] [PubMed] [Google Scholar]
- 17. Ozeke O, Cay S, Ozcan F, Topaloglu S, Aras D. Pin‐port misconnection induced endless loop tachycardia. J Arrhythm. 2018;34:213–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alcalde Ó, Fuertes M, Villuendas R, Pereferrer D. Atypical pacemaker‐mediated tachycardia from the atrial channel: what is the mechanism? Heart Rhythm. 2011;8:636–8. [DOI] [PubMed] [Google Scholar]
- 19. Yoo HS, Albino E, Fernandez P, et al. Pacemaker‐mediated tachycardia with narrow QRS complexes. Clin Case Rep. 2018;14:1040–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sharma PS, Kaszala K, Tan AY, et al. Repetitive non‐reentrant ventriculoatrial synchrony: an underrecognized cause of pacemaker‐related arrhythmia. Heart Rhythm. 2016;13:1739–47. [DOI] [PubMed] [Google Scholar]
- 21. Barold SS, Levine PA. Pacemaker repetitive nonreentrant ventriculoatrial synchronous rhythm: a review. J Interv Card Electrophysiol. 2001;5:45–58. [DOI] [PubMed] [Google Scholar]
- 22. Barold SS, Falkoff MD, Ong LS, Heinle RA. Magnet unresponsive pacemaker endless loop tachycardia. Am Heart J. 1988;116:726–32. [DOI] [PubMed] [Google Scholar]
- 23. Barold SS, Stroobandt RX, Van Heuverswyn F. Pacemaker repetitive nonreentrant ventriculoatrial synchrony. Why did automatic mode switching occur? J Electrocardiol. 2012;45:420–5. [DOI] [PubMed] [Google Scholar]
- 24. Kohno R, Abe H, Nagatomo T, Otsuji Y. Repetitive non‐reentrant VA Synchrony and pacemaker‐mediated tachycardia induced by the af suppression algorithm. Pacing Clin Electrophysiol. 2009;32:1333–5. [DOI] [PubMed] [Google Scholar]
- 25. Toyoshima Y, Inoue K, Kimura R, et al. A case of repetitive non‐reentrant ventriculo‐atrial synchrony exacerbating heart failure in dilated cardiomyopathy. J Arrhythm. 2012;28:297–9. [Google Scholar]
- 26. Tzeis S, Pastromas S, Andrikopoulos G. Repetitive non‐reentrant ventriculoatrial synchrony: A rare cause of overestimating atrial fibrillation burden. EP Europace. 2014;16(7):1091. [DOI] [PubMed] [Google Scholar]
- 27. Francis J. Repetitive nonreentrant ventriculoatrial synchrony. Indian Pacing Electrophysiol J. 2010;10(5):203–4. [PMC free article] [PubMed] [Google Scholar]
- 28. Bohm A, Kiss R, Dorian P, Pinter A. Single‐chamber, rate responsive pacemaker‐mediated tachycardia. Can J Cardiol. 2010;26(9):e340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barold SS, Delnoy PP, Kucher A. Interventricular pacemaker‐mediated tachycardia during biventricular pacing. Pacing Clin Electrophysiol. 2015;38:645–50. [DOI] [PubMed] [Google Scholar]
- 30. Berruezo A, Mont L, Scalise A, Brugada J. Orthodromic pacemaker‐mediated tachycardia in a biventricular system without an atrial electrode. J Cardiovasc Electrophysiol. 2004;15:1100–2. [DOI] [PubMed] [Google Scholar]
- 31. Herczku C, Clemens M, Edes I, Csanadi Z. Pacemaker‐mediated tachycardia over the upper rate limit in a biventricular pacemaker system: what is the mechanism? Pacing Clin Electrophysiol. 2010;33:1421–4. [DOI] [PubMed] [Google Scholar]
- 32. Van Gelder BM, Bracke FA, Meijer A. Pacemaker‐mediated tachycardia in a biventricular pacing system. Pacing Clin Electrophysiol. 2001;24:1819–20. [DOI] [PubMed] [Google Scholar]
- 33. Barold SS, Byrd CL. Cross‐ventricular endless loop tachycardia during biventricular pacing. Pacing Clin Electrophysiol. 2001;24:1821–3. [DOI] [PubMed] [Google Scholar]
- 34. Barold SS, Kucher A. Understanding the timing cycles of a cardiac resynchronization device designed with left ventricular sensing. Pacing Clin Electrophysiol. 2014;37:1324–37. [DOI] [PubMed] [Google Scholar]
- 35. Barold SS, Porto FM, Kucher A. Pacemaker rhythm recorded by a cardiac resynchronization device capable of left ventricular sensing. Pacing Clin Electrophysiol. 2014;37:904–8. [DOI] [PubMed] [Google Scholar]
- 36. Alasti M, Rangasamy K, Healy S, Adam D, Kotschet E. Loss of cardiac resynchronization therapy in a patient with a biventricular implantable cardioverter‐defibrillator. J Arrhythm. 2017;33:652–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morin DP. An uncommon cause of pacemaker‐mediated ventricular tachycardia. J Cardiovasc Electrophysiol. 2014;25:107–9. [DOI] [PubMed] [Google Scholar]
- 38. Lefroy DC, Crake T, Davies DW. Ventricular tachycardia: an unusual pacemaker‐mediated tachycardia. Br Heart J. 1994;71:481–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Galand V, Behar N, Martins RP. Ventricular fibrillation triggered by a pacemaker‐mediated tachycardia protection algorithm. EP Europace. 2017;19(8):1342. [DOI] [PubMed] [Google Scholar]


