Abstract
Background
Huntington's disease (HD) is a genetic neurodegenerative condition that involves impairments in movement, cognition, and mood. Research is lacking in HD with regard to the prevalence of pain and the relationships between psychological factors and pain. The aim of this research was to investigate the prevalence of pain and identify the psychological factors associated with pain severity in people with HD.
Methods
This data‐mining study used data from 1474 people who participated in the European Huntington's Disease Network (EHDN) REGISTRY study. Pain severity was measured using the Medical Outcome Study 36‐item short‐form health survey. Separate ordinal regression analyses were conducted with participant‐rated and interviewer‐rated psychological measures (the Hospital Anxiety and Depression Scale–Snaith Irritability Scale and the Unified Huntington's Disease Rating Scale). The psychological factors considered were anxiety, depression, irritability, aggression, low self‐esteem, and apathy.
Results
The prevalence of pain in the total sample was 41% (stage I, 42%; stage II, 44%; stage III, 39%; stages IV and V, 50%). After controlling for confounding variables, pain severity was significantly associated with participant‐rated anxiety and depression. Interviewer‐rated anxiety, depression, and irritability also were significantly associated with severity of pain after controlling for confounding variables.
Conclusions
This research confirmed that pain is indeed an issue for people with HD, particularly during the later stages of the disease. Caregivers and health professionals should consider the possibility that people with HD might be experiencing pain, particularly if they are showing signs of anxiety, depression, or irritability.
Keywords: Huntington's disease, pain, anxiety, depression, irritability
Huntington's disease (HD) is a rare genetic neurodegenerative condition involving impairments in movement, cognition, and mood.1 Psychological changes in HD can include depression, anxiety, irritability, and apathy.2, 3 Chronic pain is common in many neurological diseases; however, the prevalence of pain in HD is uncertain,4 and only 4 small to moderately sized studies have reported a prevalence ranging between 11% and 62%.5, 6, 7, 8
Only 2 studies have been found that considered psychological factors and pain in HD.9, 10 Albin and Young described 2 patients with HD and depression who experienced painful somatosensory symptoms.9 In a sample of 87 individuals with HD, Arran and colleagues observed that pain correlated significantly with both anxiety and depression, with higher levels of pain associated with higher levels of anxiety and depression.10 There is substantial evidence to suggest a reciprocal relationship between depression and pain in the wider literature, with the conditions frequently coexisting and sharing common neurobiological mechanisms.11, 12, 13, 14 There is also evidence for relationships between other psychological factors and pain, including anxiety,15, 16, 17, 18 aggression,19, 20, 21, 22, 23 irritability,21 self‐esteem,24 and apathy.25 In the current study, our objective was to gain an understanding of the prevalence of pain in individuals with HD at different stages of the disease and to identify which psychological factors are associated with the severity of their pain.
Materials and Methods
Design
Data from the European Huntington's Disease Network (EHDN) REGISTRY versions 2 and 3 were used. REGISTRY is a multicentre, multinational observational study. Participants were recruited through annual clinical interviews at their local EHDN study site and provided written, informed consent. Ethical approval was gained locally from ethics committees for all study sites that contributed to REGISTRY.
The dependent variable for this study was pain severity, with 6 levels: none, very mild, mild, moderate, severe, and very severe. The independent variables were patient‐rated (PR) measures of anxiety, depression, and irritability and interviewer‐rated (IR) measures of anxiety, depression, irritability, aggression, self‐esteem, and apathy. The covariates and demographic variables considered were age, gender, disease stage, motor ability, functional capacity, dementia, comorbidity with pain‐related conditions, and analgesic medication.
Disease stage was separated into 5 categories: premanifest, stage I, stage II, stage III, and a final category combining stages IV and V. Confidence in a diagnosis of HD had been assessed by the interviewer using scores from 0 to 4 according to the presence of motor abnormalities observed during the visit. Premanifest participants were HD gene carriers with a diagnostic confidence between 0 (no motor abnormalities) and 2 (nonspecific motor abnormalities or motor abnormalities that may be signs of HD, suggesting less than 90% confidence). HD gene carriers with a diagnostic confidence of 3 or 4 (motor abnormalities that are likely or unequivocal signs of HD, suggesting confidence of 90% or greater) were separated into the remaining stages according to their total functional capacity score on the Unified Huntington's Disease Rating Scale (UHDRS).26
Based on previous studies, the following ongoing conditions were considered potentially related to pain: diabetes, peripheral neuropathy, osteoporosis, rheumatic conditions (including arthritis, spondylosis, fibromyalgia, gout, rheumatism, Raynaud's syndrome, back pain, and sciatica), stroke, cancer, fractures, headache, and migraine.27, 28, 29, 30, 31 Data regarding ongoing use of medication registered to relieve pain also were included.
Measures
The following measures were used, including translated versions of assessment forms where appropriate:
Medical Outcome Study 36‐Item Short Form Health Survey
The Medical Outcome Study 36‐item short form health survey (SF‐36) is a generic health outcome measure that is completed by the participant with 36 questions, including: “how much bodily pain have you had during the last 4 weeks?”32 Pain severity is measured on a scale from 0 to 5, with higher numbers representing greater severity.
UHDRS
The UHDRS33 assesses different characteristics of HD, including behavioral and motor domains. The behavioral assessment of the UHDRS includes severity and frequency information for 11 symptoms, of which the following were included in the study: depressed mood, anxiety, aggressive behavior, irritable behavior, low self‐esteem, and apathy. Severity and frequency are both measured on scales from 0 to 4, with higher numbers representing greater frequency or severity. The motor assessment considers 15 motor symptoms to produce an overall motor score ranging between 0 and 124, with greater scores representing reduced abilities. In addition, the UHDRS includes a question regarding whether the interviewer believes the participant has dementia.
Hospital Anxiety and Depression Scale
The Hospital Anxiety and Depression Scale‐Snaith Irritability Scale (HADS‐SIS) is a self‐report measure comprised of 3 subscales: anxiety (7 items), depression (7 items), and irritability (8 items).34, 35 Each of the 22 items is rated on a scale from 0 to 3. Higher scores represent greater levels of anxiety, depression, or irritability. The HADS‐SIS is an extended version of the Hospital Anxiety and Depression Scale (HADS),35 which includes items from the Irritability Depression Anxiety (IDA) scale to assess irritability.34
Participants
All available data were obtained for participants who had completed the relevant pain question on the SF‐36. For participants who had provided pain information at multiple appointments, data were selected only from their most recent visit. Participants were excluded if they did not have the HD genetic expansion (i.e., with less than 36 CAG repeats). Participants in the area of reduced penetrance (with between 36 and 39 CAG repeats) were only included if diagnostic confidence was 99% or greater that they had unequivocal motor signs of HD. Our sample totaled 1474 participants who were HD gene carriers.
Participants visited the EHDN study sites between June 2011 and February 2014. Participants ranged in age from 14 to 88 years (mean ± standard deviation, 49 ± 13.8 years). Six participants were younger than 20 years. There were 787 female participants (53.4%). The majority of participants (71.0%) for whom information was available relating to disease stage were between the first and third stages of HD. Data were collected from 15 different European countries, and 45% of the data were collected in France and Germany. In total, 299 participants (21.6%) were considered to have a diagnosis of dementia. Of the participants who had dementia, 81.1% were at stage III or above. A total of 97 participants (6.6%) reportedly had been taking analgesic medication, and 213 participants (14.5%) reportedly had a comorbid disorder related to pain.
Statistical Analysis
The statistical software package IBM SPSS Statistics version 20 (IBM Corporation, Armonk, NY) was used to analyze the data. Two‐sided 95% confidence intervals (CIs) were used, and P values ≤ 0.05 were considered statistically significant. A frequency analysis was performed to identify the prevalence of pain in HD. A cross‐tabulation analysis was then conducted to identify the prevalence of pain according to disease stage. IR measures that included both severity and frequency ratings were combined into a single score by multiplying the severity score by the frequency score, consistent with other research.2, 36, 37, 38 To identify potential indicators of pain in individuals with HD for both PR and IR measures, proportional odds ordinal regression analyses were conducted including covariates that correlated with pain. Best‐fit models were investigated, and further analyses were conducted adjusting for comorbid conditions and medication.
Results
Prevalence of Pain in HD
Of a total of 1474 participants, 40.8% reported experiencing some pain during the previous 4 weeks. The prevalence of pain was slightly higher at 42.6% for participants who were motor symptomatic. The prevalence of moderate to very severe pain was 18.7%. In considering the prevalence of pain across the stages of HD, there were 90 patients of missing data, providing a sample of 1384 participants. The prevalence of pain was higher in the later stages of the disease (premanifest, 32%; stage I, 42%; stage II, 44%; stage III, 39%; stages IV and V, 50%). Figure 1 illustrates prevalence according to pain severity across stages.
Figure 1.
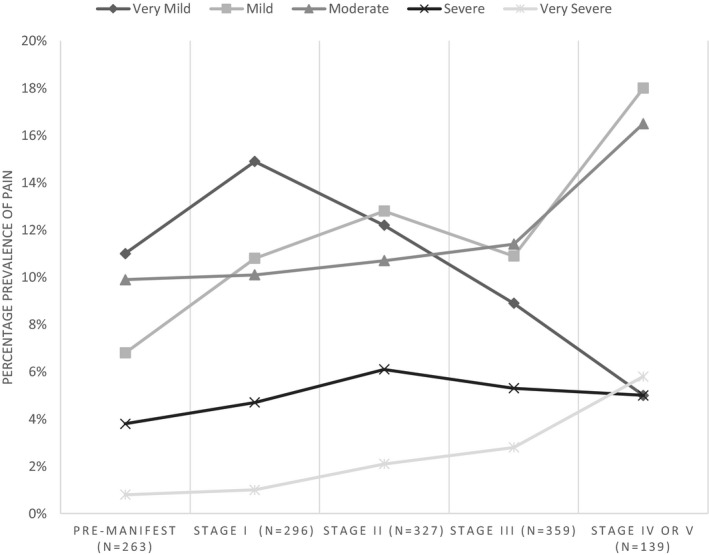
Percentage prevalence of pain severity according to disease stage.
Indicators of Pain in HD
There were significant associations between the severity of pain and age, gender, disease stage, motor function, comorbid conditions related to pain, analgesic use, and dementia. Therefore, these were considered as confounding variables in the ordinal regression analyses. All PR and IR measures were highly correlated with the severity of pain. PR measures were more highly correlated than IR measures, and PR anxiety was the most strongly correlated of all the measures (rs = 0.307; P < 0.001).
PR Ordinal Regression Analysis
A proportional odds ordinal regression was run to determine the effect of PR depression, PR anxiety, and PR irritability on the severity of pain in people with HD controlling for gender, age, disease stage, motor function, and dementia. A higher depression score was associated with an increase in the odds of greater pain severity, with an odds ratio of 1.056 (95% CI, 1.022–1.090; Wald χ2 with 1 degree of freedom [1 df] = 11.044; P = 0.001). A higher anxiety score was associated with an increase in the odds of greater pain severity, with an odds ratio of 1.095 (95% CI, 1.054–1.137; Wald χ2 [1 df] = 21.913; P < 0.001). A higher irritability score was not associated with a significant increase in the odds of greater pain severity, with an odds ratio of 1.031 (95% CI, 0.999–1.065; Wald χ2 [1 df] = 3.648; P = 0.056).
PR irritability was removed from the analysis for the best‐fit model (Table 1). A higher depression score was associated with an increase in the odds of greater pain severity, with an odds ratio of 1.061 (95% CI, 1.028–1.094; Wald χ2 [1 df] = 13.542; P < 0.001). A higher anxiety score was associated with an increase in the odds of greater pain severity, with an odds ratio of 1.119 (95% CI, 1.084–1.155; Wald χ2 [1 df] = 47.743; P < 0.001).
Table 1.
Ordinal Regression Analysis: Patient‐Rated Best‐fit Model
| Parameter | Hypothesis Test | OR | 95% Wald CI for OR | |||
|---|---|---|---|---|---|---|
| Wald | df | P Value | Lower | Upper | ||
| Age | 10.713 | 1 | 0.001 | 1.016 | 1.006 | 1.025 |
| Female vs. male | 8.808 | 1 | 0.003 | 1.415 | 1.125 | 1.780 |
| Dementia vs. no dementia | 1.699 | 1 | 0.192 | 0.802 | 0.576 | 1.118 |
| Premanifest vs. stage IV or V | 1.834 | 1 | 0.176 | 0.601 | 0.287 | 1.256 |
| Stage I vs. stage IV or V | 0.675 | 1 | 0.411 | 0.779 | 0.429 | 1.414 |
| Stage II vs. stage IV or V | 0.811 | 1 | 0.368 | 0.790 | 0.472 | 1.320 |
| Stage III vs. stage IV or V | 2.500 | 1 | 0.114 | 0.697 | 0.446 | 1.090 |
| Motor score | 1.361 | 1 | 0.243 | 0.995 | 0.986 | 1.004 |
| PR anxiety | 47.743 | 1 | 0.000 | 1.119 | 1.084 | 1.155 |
| PR depression | 13.542 | 1 | 0.000 | 1.061 | 1.028 | 1.094 |
CI, confidence interval, OR, odds ratio; df, degrees of freedom; PR, patient‐rated.
The PR best‐fit model ordinal regression was rerun adjusting for comorbidity and analgesic use (Table 2). A higher depression score was associated with a slightly larger increase in the odds of greater pain severity, with an odds ratio of 1.064 (95% CI, 1.030–1.098; Wald χ2 [1 df] = 14.582; P < 0.001). A higher anxiety score was associated with a similar increase in the odds of greater pain severity, with an odds ratio of 1.118 (95% CI, 1.083–1.155; Wald χ2 [1 df] = 46.191; P < 0.001). Taking analgesic medication was associated with an increase in the odds of greater pain severity, with an odds ratio of 3.667 (95% CI, 2.364–5.688; Wald χ2 [1 df] = 33.671; P < 0.001). Having comorbidities associated with pain was associated with an increase in the odds of greater pain severity, with an odds ratio of 1.789 (95% CI, 1.300–2.461; Wald χ2 [1 df] = 12.748; P < 0.001).
Table 2.
Ordinal Regression Analysis: Patient‐rated Adjusted Best‐fit Model
| Parameter | Hypothesis Test | OR | 95% Wald CI for OR | |||
|---|---|---|---|---|---|---|
| Wald | df | P Value | Lower | Upper | ||
| Analgesics vs. no analgesics | 33.671 | 1 | 0.000 | 3.667 | 2.364 | 5.688 |
| Comorbidities vs. no comorbidities | 12.748 | 1 | 0.000 | 1.789 | 1.300 | 2.461 |
| Age | 3.638 | 1 | 0.056 | 1.009 | 1.000 | 1.019 |
| Female vs. male | 6.097 | 1 | 0.014 | 1.339 | 1.062 | 1.687 |
| Dementia vs. no dementia | 1.140 | 1 | 0.286 | 0.833 | 0.597 | 1.165 |
| Premanifest vs. stage IV or V | 1.058 | 1 | 0.304 | 0.677 | 0.322 | 1.424 |
| Stage I vs. stage IV or V | 0.136 | 1 | 0.713 | 0.893 | 0.489 | 1.630 |
| Stage II vs. stage IV or V | 0.176 | 1 | 0.675 | 0.895 | 0.531 | 1.506 |
| Stage III vs. stage IV or V | 0.981 | 1 | 0.322 | 0.796 | 0.506 | 1.251 |
| Motor score | 0.777 | 1 | 0.378 | 0.996 | 0.987 | 1.005 |
| PR anxiety | 46.191 | 1 | 0.000 | 1.118 | 1.083 | 1.155 |
| PR depression | 14.582 | 1 | 0.000 | 1.064 | 1.030 | 1.098 |
CI, confidence interval, OR, odds ratio; df, degrees of freedom; PR, patient‐rated.
IR Ordinal Regression Analysis
A proportional odds ordinal regression was run to determine the effect of IR measures of depression, anxiety, irritability, aggression, apathy, and low self‐esteem on the severity of pain in people with HD controlling for gender, age, disease stage, motor function, and dementia. A higher anxiety score was associated with an increase in the odds of greater pain severity, with an odds ratio of 1.061 (95% CI, 1.024–1.100; Wald χ2 [1 df] = 10.806; P = 0.001). Higher depression, irritability, aggression, apathy, or low self‐esteem scores were not associated with a significant increase in the odds of greater pain severity.
For the best‐fit model, IR measures that were least significant were removed from the analysis 1 by 1, and the ordinal regression analysis was repeated until all remaining IR measures were significant (Table 3). A higher depression score was associated with a greater increase in the odds of greater pain severity, with an odds ratio of 1.047 (95% CI, 1.013–1.083; Wald χ2 [1 df] = 7.270; P = 0.007). A higher anxiety score was also associated with a slightly greater increase in the odds of greater pain severity, with an odds ratio of 1.064 (95% CI, 1.029–1.102; Wald χ2 [1 df] = 12.707; P < 0.001). A higher irritability score was associated with a significant increase in the odds of greater pain severity, with an odds ratio of 1.051 (95% CI, 1.017–1.087; Wald χ2 [1 df] = 8.804; P = 0.003).
Table 3.
Ordinal Regression Analysis: Interviewer‐rated Best‐fit Model
| Parameter | Hypothesis Test | OR | 95% Wald CI for OR | |||
|---|---|---|---|---|---|---|
| Wald | df | P Value | Lower | Upper | ||
| Age | 10.882 | 1 | 0.001 | 1.015 | 1.006 | 1.023 |
| Premanifest vs. stage IV or V | 11.125 | 1 | 0.001 | 0.313 | 0.159 | 0.620 |
| Stage I vs. stage IV or V | 11.261 | 1 | 0.001 | 0.390 | 0.225 | 0.676 |
| Stage II vs. stage IV or V | 8.567 | 1 | 0.003 | 0.493 | 0.307 | 0.792 |
| Stage III vs. stage IV or V | 10.650 | 1 | 0.001 | 0.510 | 0.341 | 0.764 |
| Female vs. male | 8.130 | 1 | 0.004 | 1.366 | 1.103 | 1.693 |
| Dementia vs. no dementia | 4.437 | 1 | 0.035 | 0.716 | 0.525 | 0.977 |
| Motor score | 2.614 | 1 | 0.106 | 0.993 | 0.985 | 1.001 |
| IR anxiety | 12.707 | 1 | 0.000 | 1.064 | 1.029 | 1.102 |
| IR depression | 7.270 | 1 | 0.007 | 1.047 | 1.013 | 1.083 |
| IR irritability | 8.804 | 1 | 0.003 | 1.051 | 1.017 | 1.087 |
CI, confidence interval, IR, interviewer‐rated; OR, odds ratio; df, degrees of freedom.
The IR ordinal regression best‐fit model was rerun adjusting for comorbidity and analgesics (Table 4). After controlling for comorbidities and analgesic medication, a higher depression score was associated with a smaller increase in the odds of greater pain severity than the unadjusted best‐fit model, with a lower odds ratio of 1.040 (95% CI, 1.005–1.076; Wald χ2 [1 df] = 5.091; P = 0.024). A higher anxiety score was also associated with an increase in the odds of greater pain severity, with a slightly greater odds ratio of 1.069 (95% CI, 1.032–1.106; Wald χ2 [1 df] = 14.080; P < 0.001). A higher irritability score was associated with an increase in the odds of greater pain severity, with a slightly greater odds ratio than the unadjusted model of 1.053 (95% CI, 1.018–1.088; Wald χ2 [1 df] = 9.167; P = 0.002).
Table 4.
Ordinal Regression Analysis: Interviewer‐rated Adjusted Best‐fit Model
| Parameter | Hypothesis Test | OR | 95% Wald CI for OR | |||
|---|---|---|---|---|---|---|
| Wald | df | P Value | Lower | Upper | ||
| Analgesics vs. no analgesics | 34.707 | 1 | 0.000 | 3.456 | 2.288 | 5.221 |
| Comorbidities vs. no comorbidities | 12.548 | 1 | 0.000 | 1.719 | 1.274 | 2.319 |
| Age | 4.099 | 1 | 0.043 | 1.009 | 1.000 | 1.018 |
| Premanifest vs. stage IV or V | 9.504 | 1 | 0.002 | 0.340 | 0.171 | 0.675 |
| Stage I vs. stage IV or V | 8.546 | 1 | 0.003 | 0.438 | 0.252 | 0.762 |
| Stage II vs. stage IV or V | 5.958 | 1 | 0.015 | 0.552 | 0.342 | 0.889 |
| Stage III vs. stage IV or V | 7.391 | 1 | 0.007 | 0.568 | 0.378 | 0.854 |
| Female vs. male | 6.306 | 1 | 0.012 | 1.319 | 1.063 | 1.638 |
| Dementia vs. no dementia | 3.253 | 1 | 0.071 | 0.750 | 0.549 | 1.025 |
| Motor score | 1.841 | 1 | 0.175 | 0.994 | 0.986 | 1.003 |
| IR anxiety | 14.080 | 1 | 0.000 | 1.069 | 1.032 | 1.106 |
| IR depression | 5.091 | 1 | 0.024 | 1.040 | 1.005 | 1.076 |
| IR irritability | 9.167 | 1 | 0.002 | 1.053 | 1.018 | 1.088 |
CI, confidence interval, IR, interviewer‐rated; OR, odds ratio; df, degrees of freedom.
Discussion
The prevalence of pain in this sample of 1474 people with genetically confirmed HD was 41%, and the prevalence increased slightly to 43% when considering only those who were motor symptomatic. This was consistent with other research on smaller sample sizes in which the prevalence of pain in HD ranged from 11% to 62% across studies.5, 6, 7, 8 A review by Borsook showed a similar prevalence of pain in other neurodegenerative conditions (40–60% in Parkinson's disease; 57% in Alzheimer's disease).4 Borsook suggested this may be due to alterations in brain circuitry, such as atrophy of the basal ganglia in HD, that are involved in pain processing. In the normal population, for comparison, Breivik and colleagues estimated a prevalence of 19% for chronic pain.39 The current research yielded a higher prevalence of pain overall together with severe pain in the later stages of the disease. The prevalence of pain was only 39% in the third stage, however, which is when occupational and domestic activity is reduced, perhaps also leading to a reduction in pain as responsibilities move away from the patient and towards the carer. The higher prevalence of severe pain in the later stages of the disease (11%) is of particular concern given the debilitating effects of severe pain.
Regression analyses controlled for confounding variables showed that greater anxiety and depression were significantly associated with greater severity of pain using either PR or IR measures. After also accounting for analgesic medication and pain‐related comorbidities, the results confirmed that PR anxiety followed by PR depression were the best indicators of the severity of pain. It is possible that PR measures were more highly correlated to the severity of pain than IR measures because patients were more aware of their own state than interviewers. Moreover, the PR measures were more in‐depth, whereas the IR measures only considered the overall state. The more prominent role of anxiety was consistent with the study by Arran and colleagues10 and the general literature highlighting the relationship between anxiety and pain,15, 16, 17, 18 which perhaps could be understood better in terms of the threat matrix of Visser and Davies, with anxiety considered a response to a perceived threat that increases pain.40
IR irritability was significantly associated with severity of pain only in the best‐fit model but remained significant after adjusting for analgesic medication and pain‐related comorbidities. An increase in PR irritability was associated with an increase in the severity of pain, although this relationship did not quite reach significance. However, PR irritability did become significant when nonsignificant control variables were removed from the model. Aggression, self‐esteem, and apathy were not significantly associated with the severity of pain.
The treatment of pain is particularly challenging in people with HD, who might find it difficult to express their suffering due to communication difficulties, cognitive difficulties, apathy, or merely not wanting to complain. The findings suggest that caregivers and health professionals should consider the possibility that a patient with HD might be experiencing pain, particularly if they are showing signs of anxiety, depression, or irritability. The use of PR measures should be considered, because patients' assessments of their depression and anxiety were stronger indicators of their pain than interviewers' assessments. The role of anxiety highlighted the relevance of treatment approaches, such as relaxation in addition to medication. Pain, anxiety, and depression should be monitored in patients with HD so that treatment can be provided, improving quality of life and reducing the risk of suicide.
This study had a number of limitations. First, it was cross‐sectional; thus, causality could not be determined. Second, standardized PR measures of aggression, apathy, or self‐esteem were not used. Third, the outcome measure representing pain was limited to a single question, and it was not possible to distinguish between non‐HD–related pain and HD‐related pain. This limitation was mitigated by controlling for pain‐related comorbid conditions; however, other causes of non‐HD–related pain were not addressed. In addition, normative data were not available for the individual SF‐36 pain item used in this study, so it was not possible to compare the results directly with a healthy population. Finally, participants' weight was not considered in this study, and its inclusion is recommended for further research.
In summary, the prevalence of pain in this sample of patients with HD ranged from 32% in the premanifest stage to 50% in the later stages. Greater severity of pain was significantly associated with greater levels of anxiety, depression, and IR irritability. PR measures of anxiety and depression were more strongly associated with pain severity than IR measures. These findings suggest that caregivers and health professionals should consider the possibility that people with HD might be experiencing pain, particularly if they are showing signs of anxiety, depression, or irritability. Further research should attempt to distinguish between HD‐related pain and non‐HD–related pain, with longitudinal research providing more information regarding how the relationship between psychological variables and pain changes over time. Finally, investigation into the best treatments for people with HD who are experiencing pain and psychological distress would greatly help to improve their quality of life.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
M.U.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
S.B.: 1A, 2A, 2C, 3B
M.D.: 1A, 2A, 2C, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest: The authors report no sources of funding and no conflicts of interest.
Financial Disclosures for the previous 12 months: The authors report no sources of funding and no conflicts of interest.
Supporting information
Appendix S1. Investigators of the European Huntington's Disease Network
Acknowledgments
We thank Dr. Eric Gardner for his statistical advice and Dr. Reza Kiani for his assistance in the areas of medication and comorbid conditions. Additionally, we thank all European Huntington's Disease Network REGISTRY Study Group investigators for collecting the data and all participating REGISTRY patients for their time and efforts. More details can be found in Appendix S1.
Supporting information may be found in the online version of this article.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Novak MJ, Tabrizi SJ. Huntington's disease [serial online]. BMJ 2010;340:c3109. [DOI] [PubMed] [Google Scholar]
- 2. Craufurd D, Thompson JC, Snowden JS. Behavioral changes in Huntington disease. Cogn Behav Neurol 2001;14:219–226. [PubMed] [Google Scholar]
- 3. Van Duijn E, Kingma E, Van der Mast R. Psychopathology in verified Huntington's disease gene carriers. J Neuropsychiatry Clin Neurosci 2007;19:441–448. [DOI] [PubMed] [Google Scholar]
- 4. Borsook D. Neurological diseases and pain. Brain 2012;135(pt 2):320–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Calvert M, Pall H, Hoppitt T, Eaton B, Savill E, Sackley C. Health‐related quality of life and supportive care in patients with rare long‐term neurological conditions. Qual Life Res 2013;22:1231–1238. [DOI] [PubMed] [Google Scholar]
- 6. Dorey J, Cohen J, Mraidi M, Urbinati D, Toumi M. Burden of Huntington's disease in the USA [abstract]. Neurotherapeutics 2012;9:232. [Google Scholar]
- 7. Scherder E, Statema M. Huntington's disease [letter]. Lancet 2010;376:1464. [DOI] [PubMed] [Google Scholar]
- 8. De Tommaso M, Serpino C, Difruscolo O, et al. Nociceptive inputs transmission in Huntington's disease: study by laser evoked potentials. Acta Neurol Belg 2011;111:33–40. [PubMed] [Google Scholar]
- 9. Albin RL, Young AB. Somatosensory phenomena in Huntington's disease. Mov Disord 1988;3:343–346. [DOI] [PubMed] [Google Scholar]
- 10. Arran N, Craufurd D, Simpson J. Illness perceptions, coping styles and psychological distress in adults with Huntington's disease. Psychol Health Med 2014;19:169–179. [DOI] [PubMed] [Google Scholar]
- 11. Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med 2003;163:2433–2445. [DOI] [PubMed] [Google Scholar]
- 12. Chopra K, Arora V. An intricate relationship between pain and depression: clinical correlates, coactivation factors and therapeutic targets. Expert Opin Ther Targets 2014;18:159–176. [DOI] [PubMed] [Google Scholar]
- 13. Goesling J, Clauw DJ, Hassett AL. Pain and depression: an integrative review of neurobiological and psychological factors. Curr Psychiatry Rep 2013;15:1–8. [DOI] [PubMed] [Google Scholar]
- 14. Kroenke K, Wu J, Bair MJ, Krebs EE, Damush TM, Tu W. Reciprocal relationship between pain and depression: a 12‐month longitudinal analysis in primary care. J Pain 2011;12:964–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beesdo K, Hoyer J, Jacobi F, Low NC, Hofler M, Wittchen H. Association between generalized anxiety levels and pain in a community sample: evidence for diagnostic specificity. J Anxiety Disord 2009;23:684–693. [DOI] [PubMed] [Google Scholar]
- 16. Gureje O. Comorbidity of pain and anxiety disorders. Curr Psychiatry Rep 2008;10:318–322. [DOI] [PubMed] [Google Scholar]
- 17. Means‐Christensen AJ, Roy‐Byrne PP, Sherbourne CD, Craske MG, Stein MB. Relationships among pain, anxiety, and depression in primary care. Depress Anxiety 2008;25:593–600. [DOI] [PubMed] [Google Scholar]
- 18. Tanasale B, Kits J, Kluin PM, Trip A, Kluin‐Nelemans HC. Pain and anxiety during bone marrow biopsy. Pain Manag Nurs 2013;14:310–317. [DOI] [PubMed] [Google Scholar]
- 19. Anderson CA, Bushman BJ. Human aggression. Psychology 2002;53:27–51. [DOI] [PubMed] [Google Scholar]
- 20. Anderson KB, Anderson CA, Dill KE, Deuser WE. The interactive relations between trait hostility, pain, and aggressive thoughts. Aggress Behav 1998;24:161–171. [Google Scholar]
- 21. Berkowitz L. Pain and aggression: some findings and implications. Motiv Emot 1993;17:277–293. [Google Scholar]
- 22. Corbett A, Husebo BS, Achterberg WP, Aarsland D, Erdal A, Flo E. The importance of pain management in older people with dementia. Br Med Bull 2014;111:139–148. [DOI] [PubMed] [Google Scholar]
- 23. MacDonald G, Leary MR. Why does social exclusion hurt? The relationship between social and physical pain [serial online]. Psychol Bull 2005;131:202. [DOI] [PubMed] [Google Scholar]
- 24. Soares JJ, Grossi G. The relationship between levels of self‐esteem, clinical variables, anxiety/depression and coping among patients with musculoskeletal pain. Scand J Occup Ther 2000;7:87–95. [Google Scholar]
- 25. Benito‐Leon J, Cubo E, Coronell C. Impact of apathy on health‐related quality of life in recently diagnosed Parkinson's disease: the ANIMO study. Mov Disord 2012;27:211–218. [DOI] [PubMed] [Google Scholar]
- 26. Shoulson I, Fahn S. Huntington disease: clinical care and evaluation. Neurology 1979;29:1–3. [DOI] [PubMed] [Google Scholar]
- 27. Defazio G, Gigante A, Mancino P, Tinazzi M. The epidemiology of pain in Parkinson's disease. J Neural Transm 2013;120:583–586. [DOI] [PubMed] [Google Scholar]
- 28. Gruber‐Baldini A, Zimmerman S, Boustani M, Watson LC, Williams CS, Reed PS. Characteristics associated with depression in long‐term care residents with dementia. Gerontologist 2005;45:50–55. [DOI] [PubMed] [Google Scholar]
- 29. Tinazzi M, Del Vesco C, Fincati E, et al. Pain and motor complications in Parkinson's disease. J Neurol Neurosurg Psychiatry 2006;77:822–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Torvik K, Kaasa S, Kirkevold O, Rustoen T. Pain and quality of life among residents of Norwegian nursing homes. Pain Manag Nurs 2010;11:35–44. [DOI] [PubMed] [Google Scholar]
- 31. Zambito Marsala S, Tinazzi M, Vitaliani R, et al. Spontaneous pain, pain threshold, and pain tolerance in Parkinson's disease. J Neurol 2011;258:627–633. [DOI] [PubMed] [Google Scholar]
- 32. Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36): I. Conceptual framework and item selection. Med Care 1992;30:473–483. [PubMed] [Google Scholar]
- 33. Unified Huntington's Disease Rating Scale: reliability and consistency. Huntington Study Group. Mov Disord 1996;11:136–142. [DOI] [PubMed] [Google Scholar]
- 34. Snaith RP, Constantopoulos AA, Jardine MY, McGuffin P. A clinical scale for the self‐assessment of irritability. Br J Psychiatry 1978;132:164–171. [DOI] [PubMed] [Google Scholar]
- 35. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–370. [DOI] [PubMed] [Google Scholar]
- 36. Paulsen JS, Nehl C, Hoth KF, et al. Depression and stages of Huntington's disease. J Neuropsychiatry Clin Neurosci 2005;17:496–502. [DOI] [PubMed] [Google Scholar]
- 37. Ready RE, Mathews M, Leserman A, Paulsen JS. Patient and caregiver quality of life in Huntington's disease. Mov Disord 2008;23:721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thompson JC, Harris J, Sollom AC, Stopford CL, Howard E, Snowden JS, Craufurd D. Longitudinal evaluation of neuropsychiatric symptoms in Huntington's disease. J Neuropsychiatry Clin Neurosci 2012;24:53–60. [DOI] [PubMed] [Google Scholar]
- 39. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006;10:287–333. [DOI] [PubMed] [Google Scholar]
- 40. Visser EJ, Davies S. Expanding Melzack's pain neuromatrix. The Threat Matrix: a super‐system for managing polymodal threats [serial online]. Pain Pract 2010;10:163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Investigators of the European Huntington's Disease Network


