Abstract
Background
Impulsive compulsive behaviors (ICBs) can have a deleterious impact on the lives of patients with PD with orally active dopamine agonist treatment recognized as the greatest risk factor. However, the relationship between subcutaneous administration of the dopamine agonist, apomorphine, and impulsive compulsive behaviors is unknown.
Methods
We conducted a retrospective analysis of 28 advanced PD patients treated with subcutaneous waking day apomorphine ambulatory minipumps at the National Hospital for Neurology and Neurosurgery (London, UK).
Results
Twelve of the patients had experienced impulsive compulsive behaviors before starting apomorphine. Reduction of oral dopamine agonist dose before apomorphine had led to complete resolution in 6 cases with no recurrence on long‐term apomorphine maintenance therapy. Six patients still had active impulsive compulsive behaviors when apomorphine was started. Four of these improved, and in the other 2 there was no worsening. Of the 16 patients with no previous history of impulsive compulsive behaviors who started apomorphine, only 1, who was still receiving concurrent levodopa, developed impulsive compulsive behaviors.
Conclusions
These data provide preliminary evidence that continuous apomorphine pump therapy has a lower proclivity to trigger or exacerbate impulsive compulsive behaviors than oral dopamine agonists. This is likely to be attributed to a more tonic stimulation of striatal dopamine receptors leading to desensitisation, but could also be attributed to a different pharmacological profile of apomorphine compared with orally active dopamine agonists. Apomorphine can be considered as a treatment option in patients who have developed disabling impulsive compulsive behaviors on oral agonist therapy whose motor handicap cannot be controlled adequately on l‐dopa alone. Further prospective studies are needed to provide a definitive answer to this question.
Keywords: apomorphine, Parkinson's disease, impulse control disorders, impulsive compulsive behaviors
Introduction
Impulsive compulsive behaviors (ICBs), such as pathological gambling, compulsive sexual behavior, binge eating, and compulsive shopping, can have devastating consequences on the lives of patients with Parkinson's disease (PD) and their families. Initially, it was reported that ICBs occur in around 14% of all treated patients, and approximately one quarter of them had more than one abnormal behavior.1 However, more‐recent studies have shown that ICBs may be more common and affect up to 39% of all PD patients using an oral or transdermal dopamine agonist (DA).2
Risk factors for developing ICBs include younger age at disease onset, pre‐existing novelty‐seeking personality traits, male sex, a personal or family history of addictive behaviors, and a past or family history of depression. However, the greatest risk factor for triggering these addictive behaviors is DA therapy.1, 3 Immediate release orally active agonists appear to carry increased risk for ICBs when compared to transdermal or prolonged release oral agonists.2, 4
The association of ICBs with oral dopamine D2/D3 agonists is well documented, but the potential of apomorphine to induce them is unclear, with conflicting results reported. One study showed that in 5 of 7 patients, pre‐existing ICBs improved after apomorphine pump therapy (5 partially and 2 completely). However, there was onset of de novo ICBs in 6 other cases (excessive eating, compulsive shopping and Internet use, and hypersexuality) using apomorphine pump therapy. Only 2 patients with new‐onset ICBs were using a DA, in both cases rotigotine.5 Other studies, of 15 and 30 individuals, respectively, reported no ICBs or improvement of ICBs after treatment with apomorphine.6, 7
More than 500 patients have been treated with apomorphine pumps at the University College London Hospitals over the last 15 years, and it has been our clinical experience that the emergence of ICBs during long‐term apomorphine therapy in these patients is rare, especially in those who have managed to withdraw all their oral agonist and levodopa therapy (A.J.L., personal experience).
In this study, we have performed a detailed review of clinical notes to assess whether apomorphine can trigger or improve ICBs in PD.
Patients and Methods
We conducted a retrospective audit of PD patients treated with apomorphine pumps at the National Hospital for Neurology and Neurosurgery (London, UK). We included only patients who were registered using the apomorphine pump in the years of 2013 and 2014 to ensure complete data acquisition and, importantly, covering a period when all patients would have been questioned carefully about ICBs.
All files were reviewed by a movement disorders specialist (P.B.) and screened for ICBs and other neuropsychiatric disorders, including depression, anxiety, psychosis, and cognitive impairment. Clinical indications for apomorphine, the daily dosage, side effects, pre‐existing ICBs, and therapies were noted as well as the therapeutic outcome. Data were analyzed using the software, SPSS (version 22; SPSS, Inc., Chicago, IL).
Results
A total of 28 (18 males) patients were included in this study. Demographic characteristics of the patients are summarized in Table 1. All patients started apomorphine because of refractory motor fluctuations with significant OFF periods for at least 2 hours per day. Five patients (17.9%) discontinued the pump for the following reasons: technical problems with the pump (3 patients); hypersomnolence (1 patient); and deteriorating cognition (1 patient). The data of the 5 patients who discontinued apomorphine were included in the analysis.
Table 1.
Demographic characteristics and side effects
| N | Range or % | SD | |
|---|---|---|---|
| No. of patients | 28 | ||
| Male/female | 18/10 | ||
| Mean age at PD onset (years) | 51.03 | 35–76 | ±8.85 |
| Mean disease duration (years) | 18.64 | 8–35 | ±6.28 |
| Mean disease duration when starting apomorphine (years) | 15.21 | 6–28 | ±5.41 |
| Mean duration of apomorphine treatment (months) | 43.32 | 2–120 | ±34.65 |
| Mean hours per day on apomorphine | 13.94 | 12–24 | ±3.05 |
| Mean maximum apomorphine dosage (mg) | 56.34 | 24–96 | ±23.46 |
| Patients who stopped apomorphine (n) | 5 | 17.85% | |
| Patient experiencing side effects | 25 | 89.3% | |
| Skin nodules | 13 | 46.4% | |
| Hypersomnolence | 10 | 35.7% | |
| Cognitive impairment | 8 | 28.5% | |
| Visual hallucinations | 6 | 21.4% | |
| Dizziness | 5 | 17.8% | |
| Postural hypotension | 5 | 17.8% | |
| Bruising injection site | 4 | 14.3% | |
| Nausea | 2 | 7.1% | |
| Excessive sweating | 2 | 7.1% | |
| Dry mouth | 1 | 3.6% | |
| Itching injection site | 1 | 3.6% |
N = total number.
Patients were divided into two groups based on the presence or absence of ICBs before the start of apomorphine and were followed up for a mean period of 43 months. ICBs were diagnosed by a movement disorder specialist. Follow‐up visits were conducted in the same clinical setting. Twelve patients had experienced ICBs before apomorphine use, with more men affected than women. Eight patients had more than one ICB. Furthermore, patients with ICBs (PD+ICB) had a younger onset of PD compared to those without ICBs (PD−ICB). Both groups had similar disease duration and duration of apomorphine treatment. There was no significant difference in apomorphine dosage between the two groups (57.40 mg in PD−ICB and 55.55 mg in PD+ICB patients; P = 0.84), as well as side effects. Four PD−ICB patients and 1 PD+ICB patient needed to stop apomorphine prematurely (P = 0.25; Table 2).
Table 2.
Comparison between PD patients with and without ICBs
| PD Patients With ICBs | PD Patients Without ICBs | P Value | |||||
|---|---|---|---|---|---|---|---|
| N | SD | N | SD | ||||
| No. of patients | 12 | 42.85% | 16 | 57.15% | |||
| Male/female | 11/1 | 7/9 | 0.009a | ||||
| Mean age at PD onset (years) | 46.58 | 35–57 | ±8.09 | 54.37 | 43–76 | ±8.09 | 0.018b |
| Mean disease duration (years) | 19.08 | 8–35 | ±7.84 | 18.31 | 8–28 | ±5.06 | 0.75b |
| Mean disease duration when starting apomorphine (years) | 16.16 | 7–28 | ±6.82 | 14.5 | 6–20 | ±4.17 | 0.43b |
| Mean duration of apomorphine treatment (months) | 39.41 | 3–120 | ±32.57 | 46.25 | 2–120 | ±36.91 | 0.61b |
| Mean maximum apomorphine dosage (mg) | 57.40 | 30–91 | ±19.89 | 55.55 | 24–96 | ±26.45 | 0.84b |
| Patients experiencing side effects (n) | 10 | 83.3% | 15 | 93.75% | 0.37a | ||
| Patients who stopped apomorphine (n) | 1 | 8.3% | 4 | 22.2% | 0.25a | ||
N = total number.
Chi‐square test.
t test for independent samples.
In total, 19 of the 28 patients (67.8%) were treated with a DA before apomorphine. Five PD–ICB patients were still using a DA after initiation of apomorphine pump treatment. Six of 12 PD+ICB patients experienced complete resolution of their ICBs and/or addictive behaviors after reduction (n = 2) or withdrawal (n = 4) of the DA (see Fig. 1), meaning that 2 patients in the PD+ICB group were still using a DA after apomorphine was introduced, albeit in a smaller dose. There was no recurrence of ICBs in this group of individuals. The remaining 6 patients had active ICBs at the time apomorphine was started. After initiation of apomorphine pump treatment, 1 patient with compulsive sexual disorder improved completely, 3 patients showed complete resolution of dopamine dysregulation syndrome (DDS) and partial improvement of impulsivity (1 with DDS and compulsive sexual disorder, 1 with pathological gambling, and 1 with DDS, compulsive sexual disorder, and pathological gambling) and 2 experienced no change in their symptoms (1 with hoarding and 1 with DDS, compulsive sexual disorder, and punding). Details on the ICBs are described in Table 3 and Figure 1.
Figure 1.
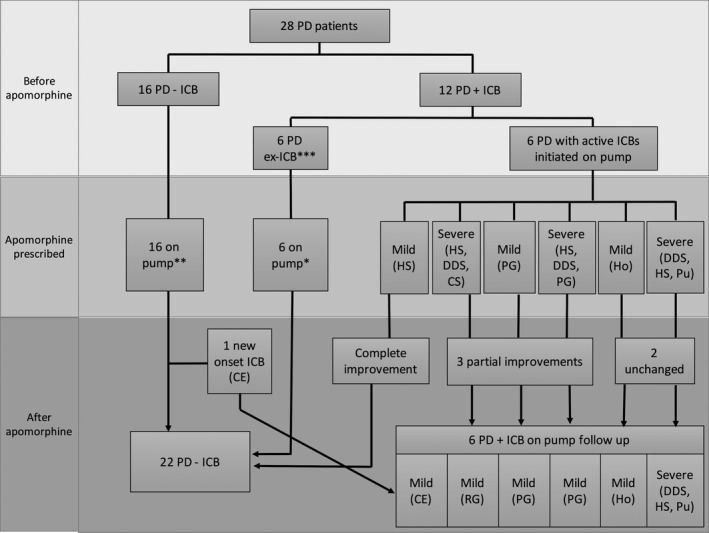
Outcome of patients with ICBs. Six patients improved before apomorphine and 6 had active ICBs. After apomorphine, 3 patients had partial improvement (1 with compulsive sexual disorder, DDS, and compulsive shopping, 1 with pathological gambling, and 1 DDS, compulsive sexual disorder, and pathological gambling), 1 patient with compulsive sexual disorder improved completely, 2 patients remained unchanged (1 with hoarding and 1 with DDS, compulsive sexual disorder, and punding), and 1 patient developed de novo compulsive eating. *One patient was also using the pen formulation. **Two patients were also using pen formulation. *** ICBs resolved after reduction or withdrawal of DAs. CS, compulsive shopping; HS, hypersexuality; PG, pathological gambling; Ho, hoarding; Pu, punding; CE, compulsive eating; RG, reckless generosity.
Table 3.
ICBs outcome
| Case | ICBs Before Apomorphine | Frequency | ICBs After Apomorphine | Frequency | LEDD |
|---|---|---|---|---|---|
| 2 | DDS and compulsive sexual disorder | 2 | ICBs resolved and did not occur | 0 | 1,220 |
| 3 | Compulsive sexual disorder and compulsive shopping | 2 | ICBs resolved and did not occur | 0 | 1,750 |
| 4 | Compulsive sexual disorder, compulsive shopping, and punding | 4 | ICBs resolved and did not occur | 0 | 1,310 |
| 5a | Compulsive sexual disorder, DDS, and compulsive shopping | 4 | Improved partially; reckless generosity after apomorphine, not causing financial strains | 1 | 1,738.5 |
| 10a | Compulsive sexual disorder | 1 | Improved after apomorphine | 0 | 1,020 |
| 11a | Pathological gambling | 2 | Improved partially, not problematic | 1 | 1,330 |
| 18 | Compulsive sexual disorder and pathological gambling | 3 | ICBs resolved and did not occur | 0 | 1,787.5 |
| 20a | Hoarding | 1 | ICB unchanged | 1 | 1,550 |
| 24 | None | 0 | Compulsive eating after starting apomorphine | 2 | 1,500 |
| 27 | Compulsive sexual disorder, punding, and compulsive shopping | 4 | ICBs resolved and did not occur | 0 | 1,738.5 |
| 31a | DDS, compulsive sexual disorder, and pathological gambling | 4 | Improved partially; gambling persisted, not problematic | 2 | 1,587 |
| 35a | DDS, compulsive sexual disorder, and punding | 4 | ICBs unchanged | 4 | 1,510 |
| 41 | Compulsive sexual disorder and pathological gambling | 2 | ICBs resolved and did not occur | 0 | 860 |
ICBs outcome displayed by individual cases. Severity of ICBs: 0 = never; 1 = rarely; 2 = sometimes; 3 = often; 4 = very often.
Patients who had active ICBs after initiation of apomorphine.
One patient who had no previous history of ICBs and no previous exposure to a DA developed new‐onset compulsive eating approximately 15 months after starting treatment with apomorphine pump (see Table 3 and Fig. 1 for details on the ICBs).
Levodopa equivalent daily dose (LEDD) was calculated as previously described.8 Considering all participants (n = 28), total LEDD was lower before apomorphine (before, 1,241.53; after, 1,414.71; P = 0.062; Wilcoxon matched pairs test). We also calculated oral/transdermal dopamine agonists LEDD (DA‐LEDD) for the total cohort, which was significantly lower after apormorphine (159.2; 45.14; P = 0.01). We did a similar analysis in the group of patients with ICBs before apomorphine (n = 12). Although there was no difference in total LEDD before (1,407.19; standard deviation [SD]: 473.17) and after (1,450.12; SD, 304.90) apomorphine treatment (P = 0.75), there was a statistically significant difference in DA‐LEDD before (188.98; SD, 170.19) and after (10.83; SD, 25.39) apomorphine (Wilcoxon matched pairs test; P = 0.008).
The majority of the patients (85%) had l‐dopa‐induced dyskinesias. A total of 25 patients (89.3%), experienced side effects from apomorphine, with 16 of them (57.14%) experiencing two or more side effects (Table 1). The most commons side effects were skin nodules, which occurred in 46.4% of the patients, and hypersomnolence in 35.7%.
Discussion
This study suggests that apomorphine pump therapy has a lower risk of generating ICBs than oral DA treatment. All 12 patients who had ICBs before apomorphine had been treated with an oral DA and 6 of them showed complete remission of their abnormal behavior after reducing or stopping the DA. ICBs did not reoccur on the apomorphine pump treatment in any of these patients.
Six patients still had ongoing ICBs, despite reduction of DA, at the time of starting the apomorphine pump. In 1 of these, damaging impulsivity disappeared, 3 more had considerable improvement so that it no longer represented a problem for the patient and to the family, and 2 did not experience any change in severity of their ICBs. None of these patients were still using a DA after apomorphine was started. Oral DA may induce long‐term pharmacodynamic changes,9 which may explain persistence of some of the pathological behaviors observed after discontinuation of treatment.
It seems probable that the reduction in DA dose was primarily responsible for the improvement noted in the patients. The fact that ICBs did not recur after apomorphine treatment initiation suggests that the drug has a lower tendency to trigger ICBs in PD, because previous studies have shown that attempts to reinstute oral dopamine agonists, even at lower dosage, commonly lead to a recurrence of ICBs.10 There are a number of potential reasons for this. There is evidence from imaging studies that large and rapid increases in extracellular dopamine may be the encoding mechanisms through which dopamine attributes salience to an event in normal conditions. Dopaminergic drugs with fast brain uptake and clearance closely mimic this natural process and tend to be more implicated in the experience of “high” and drug‐induced reinforcement.11 A continuous steady‐state delivery of apomorphine to the dopamine receptor would have the opposite effect and may explain its low proclivity to induce addictive behaviors. In line with this, a recent study has shown that continuous jejunal infusion of l‐dopa also improved DDS as well as ICBs.12 Further evidence to support this notion comes from a 3‐year observational study showing that infusion therapies, apomorphine and intrajejunal l‐dopa, carry a reduced risk for the development of ICBs in individuals with PD.13
Differences in affinity to dopaminergic receptors may also contribute to the occurrence of ICBs. Apomorphine has a receptor profile similar to dopamine with higher affinity for D1 and D2 receptors,14 in contrast to other DAs which have a more marked affinity for D3 receptors. It has been suggested that this dopamine D3 selectivity may be linked to the occurrence of ICBs.15, 16
Apomorphine has also been claimed to possess sedative and antipsychotic effects and its molecular structure includes a piperidine moiety similar to that of the major tranquilizer, thioridazine.17 Historically, repeated hourly injections of apomorphine for 5 days have been used as a treatment for alcohol and other forms of substance dependence.18, 19, 20 Neuropsychiatric symptoms have been reported to improve in some PD patients after the introduction of apomorphine pump therapy,21 although it is unclear whether this improvement can be a consequence of the aforementioned neuroleptic effect.
It has been suggested that ICBs and punding may be the ventral striatal equivalent to l‐dopa‐induced dyskinesias and that both phenomena are attributed to dopaminergic sensitisation.22 Rodriguez‐Oroz and collaborators identified a pattern of oscillatory activity in the STN in both conditions, albeit in a different topography.23 The similarity between l‐dopa‐induced dyskinesias and ICBs, coupled with the evidence that apomorphine infusion has been shown to be effective in reducing PD dyskinesias,24 may explain why none of our patients with pre‐existent ICBs relapsed.
In our study, the mean apomorphine pump dosage was 56.3 mg per day, which is lower than the 69.8 mg reported by Tyne and collaborators and the 98 mg per day reported in an earlier era from our own center by Manson and colleagues.25, 26 The pump treatment was delivered for an average period of 13.94 hours, which is similar to the data from Tyne and collaborators, but significantly less than the 16.5 hours found by Manson and colleagues.25, 26 It is possible that higher doses and longer duration of apomorphine may be an independent risk factor for ICBs27; therefore, the lower doses used in this cohort might be another factor contributing to reduced occurrence of ICBs.
Although apomorphine appears to be safer than other DA, previous studies have shown that de novo cases of ICBs can occasionally occur in patients using apomorphine pump therapy.16, 28, 29 One of our patients who had no previous history of addictive behaviors developed compulsive eating 15 months after treatment with apomorphine pump. This behavior was mild and did not lead to a premature cessation of therapy.
The limitations of this study are that it is descriptive and retrospective and has been carried out on a relatively small number of patients, the variable follow‐up period after initiation of apomorphine treatment, and the relative high rate of discontinuation of the pump. In addition, it is believed that DDS and other types of ICBs may have different pathophysiological origins. By grouping these behavioral abnormalities together, we further limit the generalizability of our results to a wider PD population.
Considering that PD patients with ICBs have a lower insight and therefore a tendency to under‐report their symptoms,30 it is possible that ICBs, despite being present in 46% of the cases, were still under‐reported. So far, only retrospective and open‐label studies have addressed the issue of apomorphine and ICBs,5, 6, 7, 16, 29 and the data linking DA are largely based on descriptive and epidemiological grounds.
Based on the full case notes in our patients and the research interest in dopamine dysregulation going back to 2000, apomorphine pump seems to have lower tendency to worsen or trigger ICBs. Continuous infusion of apomorphine therefore appears to be a valid treatment option for patients with ICBs and DDS when other treatment options have failed. Further prospective studies are needed to provide a definitive answer to this question.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
P.B.: 1A, 1B, 1C, 2A, 2B, 3A, 3B
A.J.L.: 2C, 3B
C.M.: 1B, 1C, 3B
A.D.: 1A, 1B, 2A, 2C, 3B
T.T.W.: 1A, 1B, 2C, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: The Reta Lila Weston Institute of Neurological Studies received financial support from The Reta Lila Weston Trust for Medical Research and Britannia Pharmaceuticals for this research. P.B. received honoraria from Britannia Pharmaceuticals. A.J.L. has a consultancy agreement with Britannia Pharmaceuticals. A.J.L. has a consultancy agreement with Britannia Pharmaceuticals. T.T.W. received honoraria from Britannia Pharmaceuticals. A.D. received honoraria from Britannia Pharmaceuticals.
Financial Disclosures for previous 12 months: P.B. is currently supported by a grant from Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (Brazilian National Council for Scientific and Technological Development). A.J.L. is a consultant for Britannia Pharmaceuticals and BIAL Portela and received honoraria from Britannia, Novartis, Teva, Meda, Boehringer Ingelheim, GlaxoSmithKline, Ipsen, Lundbeck, Allergan, Orion, BIAL, Noscira, Roche, UCB, NordcInfu Care, and Windrose Consulting Group. T.T.W. received honoraria from TEVA, and Lundbeck. AD received honoraria from TEVA and UCB.
Acknowledgments
The authors thank the Reta Lila Weston Institute of Neurological Studies for the support received during this research project.
Relevant disclosures and conflicts of interest are listed at the end of this article.
Contributor Information
Atbin Djamshidian, Email: atbin.djamshidian-Tehrani@i-med.ac.at.
Thomas T. Warner, Email: t.warner@ucl.ac.uk.
References
- 1. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross‐sectional study of 3090 patients. Arch Neurol 2010;67:589–595. [DOI] [PubMed] [Google Scholar]
- 2. Garcia‐Ruiz PJ, Martinez Castrillo JC, Alonso‐Canovas A, et al. Impulse control disorder in patients with Parkinson's disease under dopamine agonist therapy: a multicentre study. J Neurol Neurosurg Psychiatry 2014;85:840–844. [DOI] [PubMed] [Google Scholar]
- 3. Voon V, Thomsen T, Miyasaki JM, et al. Factors associated with dopaminergic drug‐related pathological gambling in Parkinson disease. Arch Neurol 2007;64:212–216. [DOI] [PubMed] [Google Scholar]
- 4. Rizos A, Sauerbier A, Antonini A, et al.; EUROPAR and the IPMDS Non‐Motor‐PD‐Study Group . A European multicentre survey of impulse control behaviours in Parkinson's disease patients treated with short‐ and long‐acting dopamine agonists. Eur J Neurol 2016;23:1255–1261. [DOI] [PubMed] [Google Scholar]
- 5. Todorova A, Martin A, Okai D, Samuel M, Brown R, David A, Ray Chaudhuri K. Assessment of impulse control disorders in Parkinson's patients with infusion therapies: a single center experience [abstract]. Mov Disord 2013;28(suppl 1):366. [Google Scholar]
- 6. Todorova A, Martinez‐Martin P, Martin A, Rizos A, Reddy P, Chaudhuri KR. Daytime apomorphine infusion combined with transdermal Rotigotine patch therapy is tolerated at 2 years: a 24‐h treatment option in Parkinson's disease. Basal Ganglia 2013;3:127–130. [Google Scholar]
- 7. Magennis B, Cashell A, O'Brien D, Lynch T. An audit of apomorphine in the management of complex idiopathic Parkinson's disease in Ireland. Mov Disord 2012;27(suppl 1):144. [Google Scholar]
- 8. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25:2649–2653. [DOI] [PubMed] [Google Scholar]
- 9. van Eimeren T, Pellecchia G, Cilia R, et al. Drug‐induced deactivation of inhibitory networks predicts pathological gambling in PD. Neurology 2010;75:1711–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rabinak CA, Nirenberg MJ. Dopamine agonist withdrawal syndrome in Parkinson disease. Arch Neurol 2010;67:58–63. [DOI] [PubMed] [Google Scholar]
- 11. Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry 2004;9:557–569. [DOI] [PubMed] [Google Scholar]
- 12. Catalan MJ, de Pablo‐Fernandez E, Villanueva C, et al. Levodopa infusion improves impulsivity and dopamine dysregulation syndrome in Parkinson's disease. Mov Disord 2013;28:2007–2010. [DOI] [PubMed] [Google Scholar]
- 13. Todorova A, Samuel M, Brown RG, Chaudhuri KR. Infusion therapies and development of impulse control disorders in advanced Parkinson disease: clinical experience after 3 years' follow‐up. Clin Neuropharmacol 2015;38:132–134. [DOI] [PubMed] [Google Scholar]
- 14. Fahn S, Jankovic J, Hallet M. Principles and Practice of Movement Disorders. 2nd ed Philadelphia, PA: Elsevier Saunders; 2011. [Google Scholar]
- 15. Seeman P. Parkinson's disease treatment may cause impulse‐control disorder via dopamine D3 receptors. Synapse 2015;69:183–189. [DOI] [PubMed] [Google Scholar]
- 16. Samuel M, Rodriguez‐Oroz M, Antonini A, et al. Management of impulse control disorders in Parkinson's disease: controversies and future approaches. Mov Disord 2015;30:150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chase TN, Tamminga CA. Pharmacologic studies of tardive dyskinesia. Adv Biochem Psychopharmacol 1980;24:457–461. [PubMed] [Google Scholar]
- 18. Dent JY. Apomorphine in the treatment of addiction to other drugs. Br J Addict Alcohol Other Drugs 1952;50:43–45. [Google Scholar]
- 19. Scheel‐Kruger J, Golembiowska K, Mogilnicka E. Evidence for increased apomorphine‐sensitive dopaminergic effects after acute treatment with morphine. Psychopharmacology 1977;53:55–63. [DOI] [PubMed] [Google Scholar]
- 20. Beil H, Trojan A. The use of apomorphine in the treatment of alcoholism and other addictions: results of a general practitioner. Br J Addict Alcohol Other Drugs 1977;72:129–134. [DOI] [PubMed] [Google Scholar]
- 21. Ellis C, Lemmens G, Parkes JD, et al. Use of apomorphine in parkinsonian patients with neuropsychiatric complications to oral treatment. Parkinsonism Relat Disord 1997;3:103–107. [DOI] [PubMed] [Google Scholar]
- 22. Voon V, Fernagut PO, Wickens J, et al. Chronic dopaminergic stimulation in Parkinson's disease: from dyskinesias to impulse control disorders. Lancet Neurol 2009;8:1140–1149. [DOI] [PubMed] [Google Scholar]
- 23. Rodriguez‐Oroz MC, Lopez‐Azcarate J, Garcia‐Garcia D, et al. Involvement of the subthalamic nucleus in impulse control disorders associated with Parkinson's disease. Brain 2011;134(Pt 1):36–49. [DOI] [PubMed] [Google Scholar]
- 24. Katzenschlager R, Hughes A, Evans A, et al. Continuous subcutaneous apomorphine therapy improves dyskinesias in Parkinson's disease: a prospective study using single‐dose challenges. Mov Disord 2005;20:151–157. [DOI] [PubMed] [Google Scholar]
- 25. Tyne HL, Parsons J, Sinnott A, Fox SH, Fletcher NA, Steiger MJ. A 10 year retrospective audit of long‐term apomorphine use in Parkinson's disease. J Neurol 2004;251:1370–1374. [DOI] [PubMed] [Google Scholar]
- 26. Manson AJ, Turner K, Lees AJ. Apomorphine monotherapy in the treatment of refractory motor complications of Parkinson's disease: long‐term follow‐up study of 64 patients. Mov Disord 2002;17:1235–1241. [DOI] [PubMed] [Google Scholar]
- 27. Antonini A. Continuous dopaminergic stimulation—from theory to clinical practice. Parkinsonism Relat Disord 2007;13(suppl):S24–S28. [DOI] [PubMed] [Google Scholar]
- 28. Martinez‐Martin P, Reddy P, Katzenschlager R, et al. EuroInf: a multicenter comparative observational study of apomorphine and levodopa infusion in Parkinson's disease. Mov Disord 2015;30:510–516. [DOI] [PubMed] [Google Scholar]
- 29. Garcia Ruiz PJ, Sesar Ignacio A, Ares Pensado B, et al. Efficacy of long‐term continuous subcutaneous apomorphine infusion in advanced Parkinson's disease with motor fluctuations: a multicenter study. Mov Disord 2008;23:1130–1136. [DOI] [PubMed] [Google Scholar]
- 30. Averbeck BB, O'Sullivan SS, Djamshidian A. Impulsive and compulsive behaviors in Parkinson's disease. Annu Rev Clin Psychol 2014;10:553–580. [DOI] [PMC free article] [PubMed] [Google Scholar]


