Abstract
Background
Orthostatic Tremor (OT) is characterized by the presence of a sensation of instability while standing, associated with high frequency (13–18 Hz) lower extremity tremor. Diagnosis is confirmed with surface electromyography (EMG). An accurate screening tool that could be used in the routine clinical setting, without any specialized equipment, would be useful in earlier detection of OT and judicial use of additional testing.
Objective
The objective of this study was to evaluate OT diagnostic test characteristics at bedside using iPhone's built‐in accelerometer and available applications for tremor recordings.
Methods
We obtained recordings using iPhones (Model 5, 5s, and 6) and free Applications (“LiftPulse” by LiftLabs [App1] and “iSeismometer” by ObjectGraph LLC [App2]) at default settings.
Results
24 EMG‐confirmed OT subjects (mostly females, 22/24) and 15 age‐matched controls (mostly males, 11/15) were evaluated. App1 detected OT range tremor in 22/24 patients and none of the controls. (Sensitivity = 92%, Specificity = 100%, NPV = 88%). App2 detected OT range tremor in 21/24 patients and in 1/13 controls (Sensitivity = 88%, Specificity = 92%, NPV = 80%). When combined, 24/24 patients and 1/13 controls had OT range tremor (Sensitivity = 100%, Specificity = 92%, NPV = 100%).
Conclusions
Smartphone apps that use the built‐in accelerometer provide a simple, accurate and inexpensive bedside screening diagnostic tool for patients with OT.
Keywords: orthostatic tremor, accelerometer, smartphone, application, tremor frequency
Orthostatic tremor (OT) is characterized by the presence of a sensation of instability while standing that disappears on sitting and at least improves by leaning and walking. This is associated with a very high frequency tremor in the legs, usually with a range of 13–18 Hz. The presence of a high frequency leg tremor has been considered the key feature in differentiating unsteadiness of OT from other causes of instability. The currently accepted diagnostic criterion for OT requires determination of a tremor in the legs with a frequency in the OT range.1 OT patients usually suffer many years before they get a diagnosis,2, 3 and therefore a screening tool would be very helpful to minimize this delay in diagnosis.
The determination of tremor frequency traditionally requires the use of a multichannel surface electromyography (EMG) with a specifically written application for power spectral analysis of the recorded data.4 However, these tools are typically only available in motor unit labs at tertiary care or research centers, have to be custom‐written (fast Fourier transformation for tremor peak using MATLAB), are expensive to buy, and require training before use. The reliability of visual analysis of EMG recordings for determination of tremor frequency has not been studied. There have been other efforts at testing devices for identifying tremor frequency that would bypass the cumbersome surface EMG process. There are currently free applications on the market for accelerometers, gyroscopes, and digitizing tablets that have shown good accuracy, though these devices may not be as readily available as a smart phone to the average practitioner.5 Other small studies have reported the use of an accelerometer device attached to the knee for evaluation of OT tremor, but most find a tremor lower than the OT range.6, 7 Though all of these devices have shown poor accuracy for amplitude, diagnosis of OT is based on tremor frequency, and we feel there could be utility of these types of devices for OT. This has led to a continued search for readily available alternatives to EMG for detection of tremors in OT.
In our study, we aimed at recording OT tremors by using a smartphone with a built‐in accelerometer and easily available applications that run algorithms for tremor frequency. This could drastically improve the diagnosis of OT by confirming clinical suspicion with bedside frequency determination.
Methods
Study Design
This was a prospective study comparing the gold standard surface EMG tremor frequency in OT subjects to the frequency recorded on two separate iPhone apps in a single visit. This study was reviewed and approved by our Institutional Review Board.
Participants
After obtaining informed consent, we prospectively enrolled subjects with EMG‐confirmed OT as well as controls. In addition to review of EMG record, clinical diagnosis of OT was reconfirmed with detailed history. Clinically evident leg tremor was documented if present but not required. Exclusion criteria included: 1) Previous history of vestibular disease, 2) high risk for falling, and 3) other causes of instability or ataxia (beyond OT) in both groups. Cases of secondary OT were also excluded. Potentially eligible participants were identified prior to September 2014 and were part of the University of Nebraska Medical Center OT study, a large prospective study that includes patients from the USA, Canada, Europe, and Australia. These participants formed a consecutive sampling as all meeting the eligibility criteria were invited to participate.
Test Methods
We obtained tremor recordings using iPhones (models 5, 5s, and 6) with their built‐in accelerometer. We used free, publicly available applications, including Liftpulse by LiftLabs (App1) and iSeismometer by ObjectGraph LLC (App2) at their default settings. These two apps have built in frequency detection and do not require any interpretation on the part of the user. App1 is now only available on Android devices and is specifically written for tremor detection. App2 is written to measure the frequency of seismographic activity in any direction. The phone was placed just above patella on the quadriceps tendon while sitting and it was held in place with a tourniquet. Recording started after the participants stood up (Fig. 1). App1 displays frequency power spectrum with a visible peak on the graph along with a software detected peak frequency as a number (Fig. 2). In App1 we recorded both the “predominant peak” (provided by the app), and any visible second peak (see results). If any of those two measures showed a tremor within the OT range, this was counted as a positive test. App2 reports data as a continuous tremor graph like an oscilloscope in three standard axes in relation to the ground (Fig. 2). The graph usually shows a clear tremor pattern, and when paused, the software gives the mean frequency for each of the three axes for the last 10 seconds of recording. The peak frequency in all three axes was individually recorded for App2. If any of these revealed a tremor in the OT range, this was counted as a positive test. The tremor frequencies on the two apps were compared to record of tremor frequency on surface EMG report, which is the current gold standard for diagnosis of OT. Surface EMG was not performed for every subject at our facility. Records of surface EMGs performed outside our facility were reviewed as part of subject eligibility. Controls did not have surface EMG. The investigators did know the diagnostic status of the participants prior to using the two apps. Physicians performing the EMGs were presumably aware of a suspected diagnosis of OT.
Figure 1.
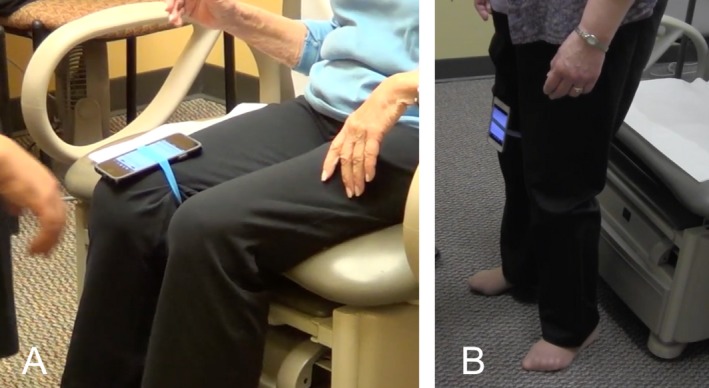
Example of using the iPhone apps. Panel A shows the iPhone tied to the subjects leg and the app is open on the screen. Panel B shows the subjects standing and the app running.
Figure 2.
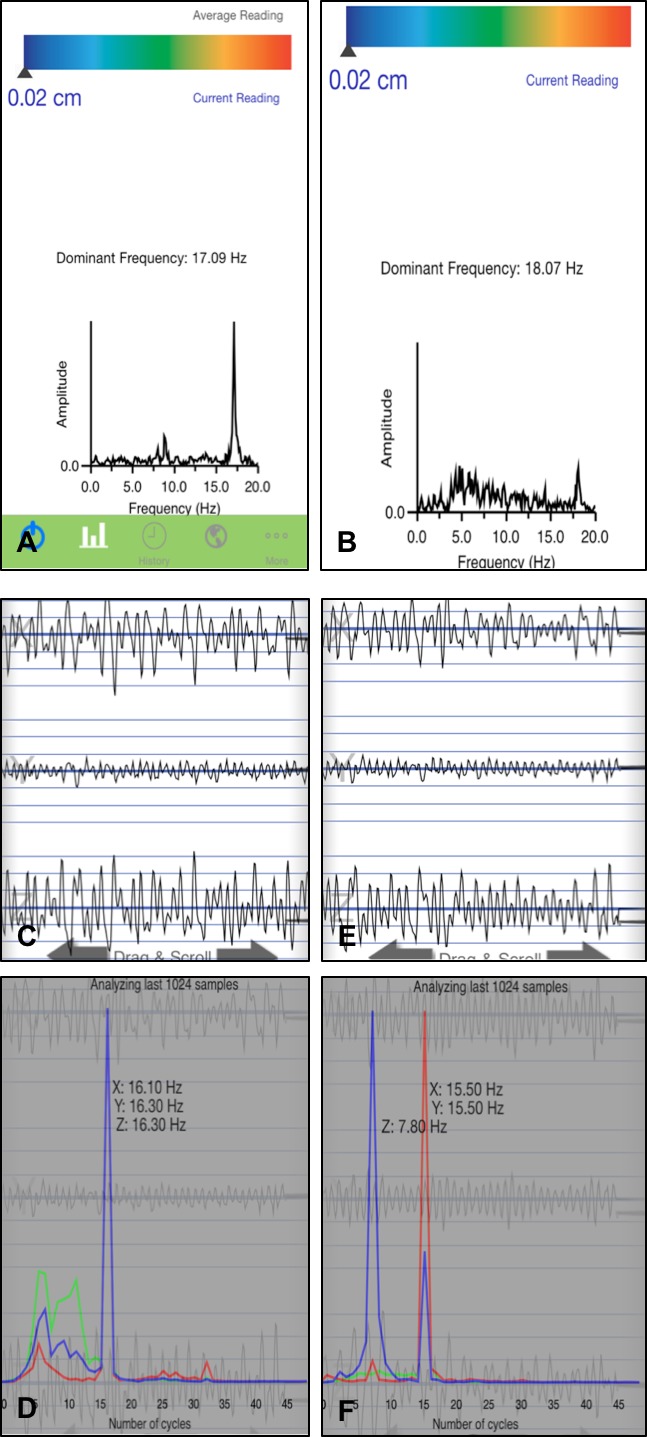
Screen shots from App1 and App2. Panels A and B show screen shots of recordings from App1 with a single dominant frequency given. Panel C‐F show screen shots from App2. Panel C shows a recording from a subject with corresponding analysis giving three peak frequencies (1 for each direction of leg tremor X, Y, and Z) below in panel D. The subject recording in panel E corresponds to the analysis below in panel F.
Analysis
Statistical analysis followed the typical calculations for test characteristics, including sensitivity, specificity, predictive value (PV), and likelihood ratios (LR). We analyzed the data for predictive values of presence and absence of OT range tremor in comparison with the gold standard provided by the EMG‐based diagnosis. We also compared the frequency provided by the app to that of the EMG using a Bland‐Altman plot. Subjects with missing data for one of the apps were not included in analysis for that app.
Results
Participants
Twenty‐four EMG confirmed OT subjects and 15 spousal controls were evaluated. OT subjects were mostly female (21/24) and controls predominantly males (11/15). The predominance of female OT patients is similar to the trend that has been identified in previous retrospective studies and literature review.2, 3, 8, 9, 10 The average age for OT patients was 68.6 years (range 54–87 years), and 72.7 years (range 56–86 years) for controls. Average duration of disease was 16.5 years (range 4–44 years). App1 was used on all 24 OT subjects and 15 controls. App2 was used on all 24 OT subjects and only 13 controls due to unintentional omission of that task by one of the investigators as this testing was taking place during a broader comprehensive evaluation of subjects. The reference standard surface EMG was performed prior to study visit for app testing (range 16 years–1 year prior). Participants were not limited from starting any medications for OT between time of EMG and app testing. Specific clinical interventions in this time period were not recorded.
Test Results
All of the EMG confirmed subjects had an OT range tremor on at least one of the applications (22/24 LiftPulse; 21/24 iSeismometer; see confusion matrixes Table 1). App1 detected OT range tremor (>12 and <19 Hz) in 22/24 OT subjects and none of the controls (Sensitivity = 92%, Specificity = 100%, Negative Predictive Value [NPV] = 88%, Negative Likelihood Ratio [NLR] <0.1). App2 detected OT range tremor in 21/24 OT subjects and 1/13 controls (Sensitivity = 88%, Specificity = 92%, NPV = 80%, NLR = 0.14). When combined 24/24 OT subjects and 1/13 controls had OT range tremor on at least one of the two apps (Sensitivity = 100%, Specificity = 92%, NPV = 100%, NLR = 0).
Table 1.
Confusion matrix for App1, App2, and both apps together
| OT subjects App1 | Controls App1 | OT subjects App2 | Controls App2 | OT subjects both apps | Controls both apps | |
|---|---|---|---|---|---|---|
| 13–18 Hz tremor present | 22 | 0 | 21 | 1 | 24 | 1 |
| 13–18 Hz tremor absent | 2 | 15 | 3 | 12 | 0 | 12 |
| Totals | 24 | 15 | 24 | 13 | 24 | 13 |
Two of the controls did not have an evaluation with App2 due to investigator error. All of the OT subjects had a 13–18 Hz tremor on at least one of the apps. There was one false positive using App2 and none using App1.
The comparison between the frequencies measured by the apps versus those reported by EMG showed no significant difference. See Bland‐Altman plot (Fig. 3 and 4). Tremor frequency measured by previously obtained EMG was available in 16/24 subjects and showed an average frequency of 15.4 Hz (range 13–17.5 Hz, mode 17 Hz). Fifteen of the 16 subjects had an OT range tremor detectable on App1. The average difference between the frequency measured by App1 and the frequency measured by EMG was 0.72 Hz (range 0–3.8, mode 0.5 Hz), which was not statistically significant (P = 0.18). App2 measured OT range tremor in 14/16 subjects with an average difference from EMG‐measured frequency of 0.92 Hz (range 0.1–2.3), which was also not statistically significant (P = 0.19). When taking the tremor frequency from either app that was closest to EMG frequency in all 16 subjects, the average difference was further reduced to 0.56 Hz (range 0–2.7; P = 0.79).
Figure 3.
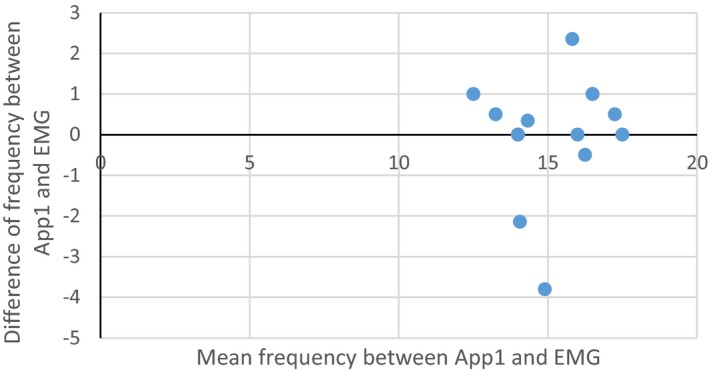
Bland‐Altman plot showing average frequency between App1 result and EMG report on x‐axis verses the difference between the frequency on App1 and EMG report on y‐axis for each subject with all the available data.
Figure 4.
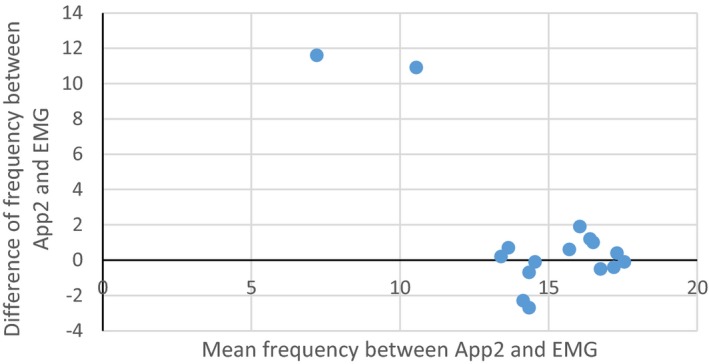
Bland‐Altman plot showing average frequency between App2 and EMG report on x‐axis verses the difference between the frequency on App2 and EMG report on y‐axis for each subject with all the available information. We used the highest reported frequency on App2 of the three axes. The two outliers are both subjects with EMG results in the OT tremor frequency range but tremor frequencies lower than OT range in all axes using App2.
Two subjects had an easily appreciable visible peak above 12 Hz on the power spectrum of App1, which was not detected by the software. App1 tremor frequency logarithm provides the “predominant frequency” based significantly on amplitude of the movement. Since OT tremor amplitude is small, the reported predominant frequency peak was occasionally incongruent with the visible second peak in the OT range.
App2 provides tremor frequencies in all three axes individually without amplitude (Fig. 2). It was interesting to note that OT range tremor was seen in all three axes in only 2/24 subjects, in two axes in 11/24 subjects, and in one axis in 11/24 subjects. Y‐axis seemed to be the most likely to detect OT range tremor (14/24), followed closely by X‐axis (12/24). Z‐axis showed tremor in 7/24 subjects.
Discussion
Surface Electromyography (EMG) evaluation is the gold standard for the diagnosis of OT. The standard finding is a fast (13–18 Hz) tremor that is usually synchronous between the two sides, alternating between agonist and antagonist muscle groups and resolving with sitting, leaning, or walking (in the swinging leg), as first noted by Dr Lugarasi in 1970.11 Currently, there does not seem to be a standard for EMG analysis in OT and the impact of differences in recording is not clear. Studies have reported EMG results using various electrode placements, ranges of band‐pass filter (0.5–5000 Hz), and gain of signal (1000 to 20,000), as well as recording different muscles (calf vs. thighs), and digitizing the signal with variable sampling rates (from 64–5000 Hz).12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 A sub‐harmonic tremor is sometimes noted with dominant tremor, with the frequency of either the dominant tremor or the sub‐harmonic tremor in OT range.18, 20, 23
Most studies report a much lower frequency of tremor on accelerometer devices, which might be a more visible sub‐harmonic of the peak frequency.6, 7, 22, 23 Our study also showed that App1 recorded subharmonic tremors and misread the tremor peak as below OT range in 2/24 subjects with a clear visible peak on the power spectrum within OT range (Fig. 2). No systematic study has been done to determine the ideal location of device placement on the leg, but patella and lower quadriceps are the most common reported sites. Only one previous study reported a fast tremor of OT range (16–17 Hz tremor) consistent with EMG in two subjects with an accelerometer placed over the distal aspect of the vastus medialis just above the knee.24
Our study had some limitations. We enrolled a relatively small number of patients. However, due to the low prevalence of the disease, this was still one of the largest prospective studies in OT. Importantly, we also relied on historical EMG data, as we did not repeat the EMG at the time of measuring the tremor with the apps, making frequency comparison less reliable. Another limitation was that our sample had a very large prevalence of OT, and although this did not affect the test's screening ability, it can artificially amplify the positive predictive value (PPV). Therefore, we have chosen not to make assumptions about the PPV of the apps.
However, our study has robust results, providing data to prove that the use of apps in an iPhone could serve as a screening tool for OT. In fact, a NPV of 100% would signify that by using these apps together, the clinician would never miss a single OT case to be referred for confirmation. The significant improvement in accuracy compared with previously well‐done accelerometer studies could be due to advancements in hardware and better understanding of data analysis when recording tremor as supported by another recent study.24
Availability of an easy to use bedside tool for diagnosis of OT can be very useful in patients with a clinical presentation consistent with OT and in other unexplained cases of unsteadiness and/or leg tremors. In a review of Mayo Clinic database from 1976 to 1990, 256 cases of unusual tremor were found and seven would fulfill diagnostic criteria for OT.4 Lang et al. reported searching medical records of movement disorders clinics at two teaching hospitals of Canada and Portugal from 1988–2011, finding 35 cases of suspected OT.25 Easy availability of a screening tool could also help to understand if tremor is present in a patient suspected of having OT. It will also help us consider other conditions of instability and easily evaluate the presence of an OT‐like tremor.
In conclusion, smartphones with their built‐in accelerometer and available applications have tremendous potential to make bedside detection of OT tremors easy. Further studies are needed to evaluate accuracy and reliability of these devices using the intended population and with direct comparison with EMG.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
AH: 3B
CP: 1B, 1C, 3B
DB: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
DTR: 1B, 1C, 3B
JB: 3B
RT: 1B, 1C, 3B
Disclosures
Ethical Compliance Statement: Institutional review board (IRB) approval was obtained from the University of Nebraska Medical Center IRB for this study. We confirm that we have read the journal's position on issues involved in ethical publication and confirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: Funding by the University of Nebraska Medical Center, Department of Neurological Sciences internal funds. No conflicts of interest reported.
Full Financial Disclosures for last 12 months: Danish Bhatti had a speaking engagement with Teva Neurosciences and Acadia pharmaceuticals; research participation with Abbvie pharmaceutical funded trial and internal grants within University of Nebraska Medical Center. Diego Torres‐Russotto has been a consultant or speaker for AbbVie, Allergan, Ipsen, Lundbek and Teva.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Mov Disord 1998;13(Suppl 3):2–23. [DOI] [PubMed] [Google Scholar]
- 2. Gerschlager W, Munchau A, Katzenschlager R, et al. Natural history and syndromic associations of orthostatic tremor: a review of 41 patients. Mov Disord 2004;19:788–795. [DOI] [PubMed] [Google Scholar]
- 3. Hassan A, Ahlskog JE, Matsumoto JY, Milber JM, Bower JH, Wilkinson JR. Orthostatic tremor: clinical, electrophysiologic, and treatment findings in 184 patients. Neurology 2016;86:458–464. [DOI] [PubMed] [Google Scholar]
- 4. Thompson PD, Rothwell JC, Day BL, et al. The physiology of orthostatic tremor. Arch Neurol 1986;43:584–587. [DOI] [PubMed] [Google Scholar]
- 5. Elble RJ, McNames J. Using portable transducers to measure tremor severity. Tremor Other Hyperkinet Mov (N Y) 2016;6:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cleeves L, Cowan J, Findley LJ. Orthostatic tremor: diagnostic entity or variant of essential tremor? J Neurol Neurosurg Psychiatry 1989;52:130–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McManis PG, Sharbrough FW. Orthostatic tremor: clinical and electrophysiologic characteristics. Muscle Nerve 1993;16:1254–1260. [DOI] [PubMed] [Google Scholar]
- 8. Ganos C, Maugest L, Apartis E, et al. The long‐term outcome of orthostatic tremor. J Neurol Neurosurg Psychiatry 2016;87:167–172. [DOI] [PubMed] [Google Scholar]
- 9. Piboolnurak P, Yu QP, Pullman SL. Clinical and neurophysiologic spectrum of orthostatic tremor: case series of 26 subjects. Mov Disord 2005;20:1455–1461. [DOI] [PubMed] [Google Scholar]
- 10. Benito‐Leon J, Domingo‐Santos A, Hassan A, Ahlskog JE, Matsumoto JY, Bower JH. Orthostatic tremor: clinical, electrophysiologic, and treatment findings in 184 patients. Neurology 2016;87:341. [DOI] [PubMed] [Google Scholar]
- 11. Pazzaglia P, Sabattini L, Lugaresi E. On an unusual disorder of erect standing position (observation of three cases). Riv Sper Freniatr Med Leg Alien Ment 1970;94(2):450–457. [PubMed] [Google Scholar]
- 12. Onofrj M, Thomas A, Paci C, D'Andreamatteo G. Gabapentin in orthostatic tremor: results of a double‐blind crossover with placebo in four patients. Neurology 1998;51:880–882. [DOI] [PubMed] [Google Scholar]
- 13. Tsai CH, Semmler JG, Kimber TE, et al. Modulation of primary orthostatic tremor by magnetic stimulation over the motor cortex. J Neurol Neurosurg Psychiatry 1998;64:33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manto MU, Setta F, Legros B, Jacquy J, Godaux E. Resetting of orthostatic tremor associated with cerebellar cortical atrophy by transcranial magnetic stimulation. Arch Neurol 1999;56:1497–1500. [DOI] [PubMed] [Google Scholar]
- 15. Wu YR, Ashby P, Lang AE. Orthostatic tremor arises from an oscillator in the posterior fossa. Mov Disord 2001;16:272–279. [DOI] [PubMed] [Google Scholar]
- 16. Spiegel J, Fuss G, Krick C, Dillmann U. Impact of different stimulation types on orthostatic tremor. Clin Neurophysiol 2004;115:569–575. [DOI] [PubMed] [Google Scholar]
- 17. Bacsi AM, Fung VSC, Colebatch JG. Sway patterns in orthostatic tremor: impairment of postural control mechanisms. Mov Disord 2005;20:1469–1475. [DOI] [PubMed] [Google Scholar]
- 18. Thomas A, Bonanni L, Antonini A, Barone P, Onofrj M. Dopa‐responsive pseudo‐orthostatic tremor in parkinsonism. Mov Disord 2007;22:1652–1656. [DOI] [PubMed] [Google Scholar]
- 19. van Gerpen JA. A retrospective study of the clinical and electrophysiological characteristics of 32 patients with orthostatic myoclonus. Parkinsonism Relat Disord 2014;20(8):889–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wills AJ, Brusa L, Wang HC, Brown P, Marsden CD. Levodopa may improve orthostatic tremor: case report and trial of treatment. J Neurol Neurosurg Psychiatry 1999;66:681–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wee AS, Subramony SH, Currier RD. ‘Orthostatic tremor’ in familial‐essential tremor. Neurology 1986;36:1241–1245. [DOI] [PubMed] [Google Scholar]
- 22. McAuley JH, Britton TC, Rothwell JC, Findley LJ, Marsden CD. The timing of primary orthostatic tremor bursts has a task‐specific plasticity. Brain J Neurol 2000;123(Pt 2):254–266. [DOI] [PubMed] [Google Scholar]
- 23. Baker M, Fisher K, Lai M, Duddy M, Baker S. Slow orthostatic tremor in multiple sclerosis. Mov Disord 2009;24:1550–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sander HW, Masdeu JC, Tavoulareas G, Walters A, Zimmerman T, Chokroverty S. Orthostatic tremor: an electrophysiological analysis. Mov Disord 1998;13:735–738. [DOI] [PubMed] [Google Scholar]
- 25. Mestre TA, Lang AE, Ferreira JJ, et al. Associated movement disorders in orthostatic tremor. J Neurol Neurosurg Psychiatry 2012;83:725–729. [DOI] [PubMed] [Google Scholar]


