Abstract
Catalytic hydrogenation of various azides in the presence of functionalized mixed carbonates afforded the corresponding urethanes in high yields.
The syntheses of functionalized urethanes have received a growing interest because of their important applications in the synthesis of biologically active polyfunctional molecules.1 Although several methods are now available for their synthesis,2 the importance of this functionality continues to provide the impetus to develop more efficient and convenient procedures. Recently, we have described3 convenient and mild procedures for the synthesis of variously functionalized mixed active carbonates utilizing readily available disuccinimidyl and di(2-pyridyl) carbonates. These active carbonates readily react with various amines to provide the corresponding urethanes in high yields. Since the azides have been utilized extensively as incipient amines in the context of amino sugar and amino acid syntheses,4 the conversion of various azides into the corresponding urethane derivatives would be of considerable value, particularly in medicinal chemistry. In this communication, we describe a facile synthetic protocol to transform various azides into the corresponding functionalized urethanes in high yields.
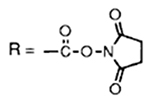
In general, mixed carbonates of variously protected alcohols were prepared by reaction of excess disuccinimidyl carbonate (DSC) or di(2-pyridyl) carbonate (DPC) as described previously.3 For example, reaction of (+)-alcohol 1 with 1.5 equiv. of DSC and 1.5 equiv. of triethylamine in dry acetonitrile at 23 °C for 4 h provided the mixed carbonate 2 after work-up with aqueous sodium hydrogen carbonate solution (Scheme 1). The mixed carbonates so obtained are fairly stable and can be chromatographed and stored in the refrigerator for several months. Exposure of carbonate 2 to catalytic hydrogenation condition with azide 35 in the presence of 10% palladium on charcoal (Pd/C) in tetrahydrofuran (THF)† furnished the corresponding urethane 6 in 60% yield after 12 h. Interestingly, the use of triethylamine as a promoter has a notable effect on the yield and the rate of the alkoxycarbonylation process. Thus, when the above hydrogenation was carried out in the presence of triethylamine, the reaction time (2 h) and the yield (76%) of the isolated urethane 6 were significantly improved. Furthermore, hydrogenation of azide 56 with the mixed carbonate 2 under the above mentioned conditions provided the urethane 7 in 80% isolated yield. In view of further synthetic applications, it is worthy to note that the reaction of N-benzyloxycarbonyl (Z) protected amine 4 with the mixed carbonate 2 under these hydrogenation conditions resulted in urethane 6 in 75% yield after silica gel chromatography. In a typical experiment, commercially available 10% Pd/C was suspended in a mixture of active carbonate, azide or Z-protected amine (1.2 equiv.) and triethylamine (2 equiv.) in tetrahydrofuran (THF). The resulting mixture was stirred under hydrogen filled balloon until no starting azide or Z-derivative remained by TLC (2–4 h). The catalyst was removed by filtration through Celite and the solvents were removed under reduced pressure to give a residue which was chromatographed (25% ethyl acetate–hexane) over a short silica gel column to provide the corresponding urethane.
Scheme 1,
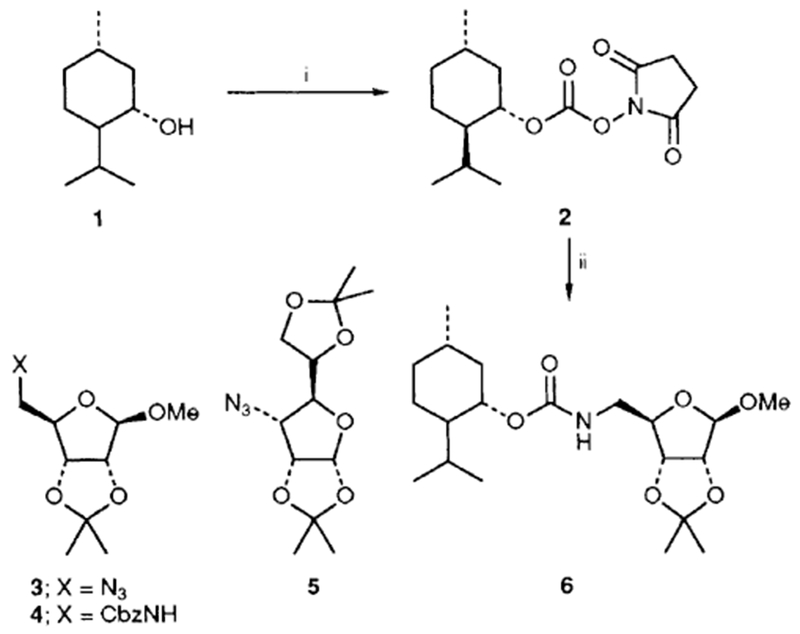
Reagents and conditions: i, disuccinimidyl carbonate, Et3N, MeCN, 23 °C; ii, azide 3, H2, 10% Pd/C, Et3N, THF
The above alkoxycarbonylation protocol was then employed to a series of three different types of alcohols‡ and the effectiveness of this procedure is summarized in Table 1. As is evident, the corresponding urethanes were obtained in very good yields from the mixed carbonates derived from DSC or DPC. It is interesting to note that the presence of sulfur (entry 5) does not limit the scope of this methodology. Since Z-protected amines are available frequently, the exchange of urethanes under this conditions will add a useful dimension to synthetic and medicinal chemistry.
Table 1.
Synthesis of urethanes with azidesa
| Entry | Carbonate | Azide | Carbamates | Yield (%)b |
|---|---|---|---|---|
| 1 | 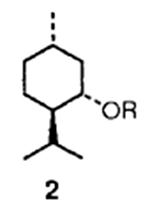 |
3 | 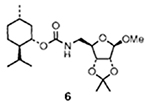 |
76 |
| 2 | 2 | 5 | 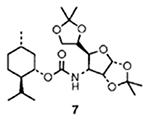 |
80 |
| 3 | 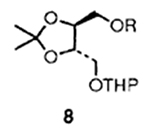 |
3 | 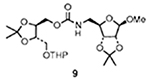 |
74 |
| 4 | 8 | 5 | 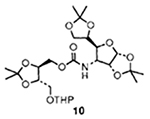 |
69 |
| 5 | 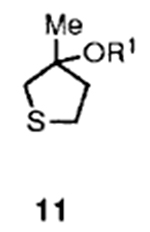 |
3 | 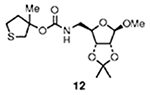 |
80 |
 |
||||
All new compounds have been fully characterized by 1H NMR, IR and mass spectral analysis.
Yield of pure products after silica gel chromatography (0.4–2.0 mmol scale).
In conclusion, we have demonstrated that various azides or Z-protected amines can be utilized efficiently for the preparation of structurally diverse urethanes derived from a variety of alcohols (yield 69–80%).§ Application of this methodology in the synthesis of biologically active molecules is currently in progress.
Acknowledgments
The authors acknowledge the encouragement and support of Drs Joel R. Huff and Paul S. Anderson.
Footnotes
No change in reaction rate or yield was observed when ethyl acetate was used as solvent for this reaction.
Alcohol of carbonate 11 was obtained by reaction of methylmagnesium bromide and tetrahydrothiophen-3-one in dry THF at 0 to 23 °C for 12 h (56% isolated yield).
All new compounds gave satisfactory spectroscopic and analytical results. For example, carbamate 6: IR (neat) v/cm−1 1702; 1H NMR (CDCl3) δ 5.2 (1 H, br s), 5.0 (1 H, s), 4.6 (3 H, m), 4.3 (1 H, t, J 6.1 Hz), 3.35 (3 H, s), 3.31 (2 H, m), 1.85–2.1 (2 H, m), 1.0–1.65 (7 H, m), 1.48 (3 H, s), 1.32 (3 H, s), 0.9 (6 H, d, J 7.0 Hz) and 0.82 (3 H, d, J 7.0 Hz); mass (FAB): 386 (M+ + H)+, 354 (M+ − OMe).
References
- 1.Bourne GT, Horwell DC and Pritchard MC, Tetrahedron, 1991, 47, 4763; [Google Scholar]; Matassa VG, Maduskuie TP, Shapiro HS, Hesp B, Snyder DW, Aharony D, Krell RD and Keith RA, J. Med. Chem, 1990, 33,1781; [DOI] [PubMed] [Google Scholar]; Firestone RA, Pisano JM, Falck JR, McPhaul MM and Krieger M, J. Med. Chem, 1984, 27, 1037; [DOI] [PubMed] [Google Scholar]; Khur RJ and Dorough HW, Carbamate Insecticides: Chemistry, Biochemistry and Toxicology, CRC Press, Cleveland, Ohio, 1976. [Google Scholar]
- 2.Eckert H and Forster B, Angew. Chem., Int. Ed. Engl, 1987, 26, 894; [Google Scholar]; Denarie M, Grenouillat D, Malfroot T, Senet J-P, Sennyey G and Wolf P, Tetrahedron Lett, 1987, 28, 5823; [Google Scholar]; Bruncan C and Dixneuf PH, Tetrahedron Lett, 1987, 28, 2005; [Google Scholar]; Takeda K, Tsuboyama K, Yamaguchi K and Ogura H, J. Org. Chem, 1985, 50, 273; [Google Scholar]; Shute RE and Rich DH, Synthesis, 1987, 346. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh AK, Duong TT and McKee SP, Tetrahedron Lett, 1991, 32, 4251; [DOI] [PMC free article] [PubMed] [Google Scholar]; Ghosh AK, Duong TT, McKee SP and Thompson WJ, Tetrahedron Lett, 1992, 33, 2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams RM, Synthesis of Optically Active α-Amino Acids, Pergamon, Oxford, 1989; [Google Scholar]; Evans DA, Britton TC, Ellman JA and Dorow RL, J. Am. Chem. Soc, 1990, 112, 4011 and references cited therein. [Google Scholar]
- 5.Levene PA and Marker RE, J. Biol. Chem, 1934, 299; [Google Scholar]; Kinoshita M, Hagiwara A and Aburaki S Bull Chem. Soc. Jpn, 1975, 48, 570. [Google Scholar]
- 6.Netscher T, Tetrahedron Lett, 1988, 29, 455; [Google Scholar]; Whistler RL and Doner LW, J. Org. Chem, 1970, 35, 3562. [Google Scholar]


