Abstract
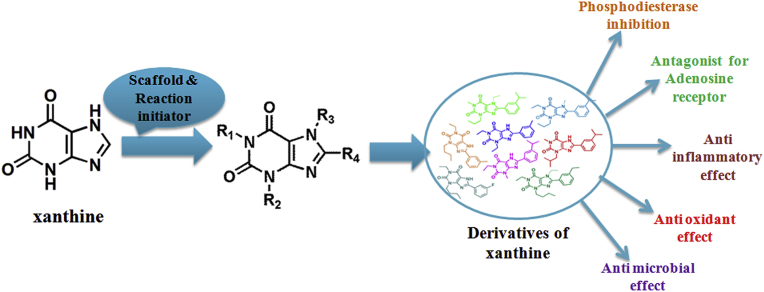
Medicinal plants have been the basis for discovery of various important marketed drugs. Xanthine is one such lead molecule. Xanthines in various forms (caffeine, theophylline, theobromine, etc) are abode in tea, coffee, cocoa, chocolate etc. giving them popular recognition. These compounds are best known for their diverse pharmaceutical applications as cyclic nucleotide phosphodiesterase inhibition, antagonization of adenosine receptor, anti-inflammatory, anti-microbial, anti-oxidant and anti-tumor activities. These properties incentivize to use xanthine as scaffold to develop new derivatives. Chemical synthesis contributes greater diversity in xanthine based derivatisation. With highlighting the existing challenges in chemical synthesis, the present review focuses the probable solution to fill existing lacuna. The review summarizes the available knowledge of xanthine based drugs development along with exploring new xanthine led chemical synthesis path for bringing diversification in xanthine based research. The main objective of this review is to explore the immense potential of xanthine as scaffold in drug development.
Keywords: Pharmaceutical chemistry, Natural product chemistry
1. Introduction
Since ancient time natural products from plants, animals, microbes and minerals have been the repertoire for drug development. Drugs may be either directly extracted from natural sources or synthetically modified using a lead compound of natural origin. Alkaloids are one such class of compounds from natural origin [1]. Early 19th century was the beginning of isolation and characterization of many important plant alkaloids such as xanthine (1817), atropine (1819), quinine (1820), caffeine (1820) etc [1]. These alkaloids are known for their diverse roles including self preserving action, inhibitor for various enzymes of cell signaling, autoinducer, allelochemicals, sidophore, anti depressants, antibacterial activity, metabolic activities, quenching activities, etc [2, 3]. Among these alkaloids, xanthine and its natural derivatives occupy prominent place in traditional medicine. Natural xanthine derivatives such as caffeine, theophylline and theobromine are purine based nitrogenous compounds which possess certain medicinal properties that are being exploited in broader ways. These derivatives are usually known as methyl xanthine derivative commonly produced by both plants and animals [4, 5]. Synthesis of these derivatives naturally in plant is the response of defense against pathogen and predators [6]. Xanthine derivatives are widely known for their non-specific inhibition properties toward phosphodiesterase enzymes [7, 8, 9]. In recent years, the scale of clinical applications on these derivatives has been augmented. This has helped in exploring the significance of these derivatives in various therapeutics applications such as adenosine receptor antagonists, inducers of histone deacetylase activity, antitumor drugs, anti asthmatic drug, psycho-stimulant drug etc [10, 11, 12, 13, 14]. The wider pharmaceutical applications of natural xanthine derivatives has prompted medicinal chemist or pharmaceutical companies to go for certain changes over lead molecule to develop more specific compounds using synthesis methodologies [15, 16, 17, 18, 19]. In drug development process, one of the major challenges is deciding the lead molecule which would act as a scaffold to provide avenue for achieving molecular diversity. Xanthine with versatile and structurally rigid scaffold provides highest possibility for molecular diversity in constructing xanthine derivatives for combinatorial chemistry [15, 20, 21, 22, 23].
Despite wider pharmaceutical applications and belongingness to purine family, xanthine based research has not taken up full pace resulting in very few synthesized xanthine based molecules. The most prominent reason for this is unfavorable synthesis methodologies such as ring closure synthetic mechanism and classical condensation route for generation of new derivatives [12, 21, 24, 25, 26, 27, 28]. These methods do not support large scale diversity in xanthine based drug development resulting in urgent need to develop viable synthesis methods so that new xanthine based drug candidates can be introduced. Till date, there has been diminutive effort for compilation of related research works in the form of review [29]. This review emphasizes details of xanthine and its existing derivatives in terms of structure, mechanism of action, therapeutic disease target, pharmacokinetics and biological effects of substitution, biological implications of different substitution sites and existing synthesis methods. It further explores the future perspectives of xanthine based research by coming up with innovative approach for novel synthetic method for synthesis of xanthine derivatives carried out by our research group ([30]; Singh et al., article not published). Interestingly, we explored the potential of ‘xanthine’ both as ‘scaffold’’ and as ‘reaction initiator’ for synthesis of biologically active xanthine derivatives.
2. Main text
2.1. Xanthine and its molecular structure
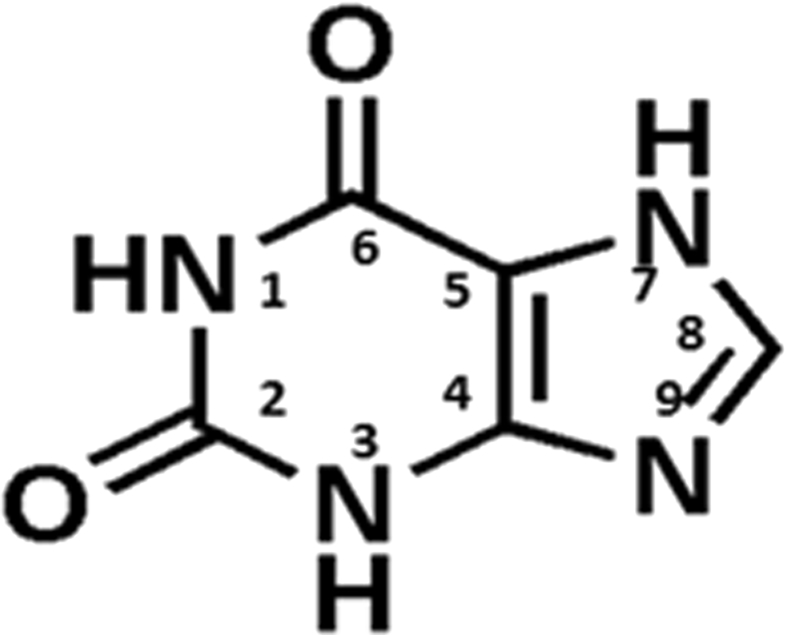
Xanthines (1H-purine-2,6(3H,7H)-diones) are purine based natural heterocyclic alkaloids. They were first discovered in 1817 by German chemist Emil Fisher and later the name ‘xanthine’ was coined in 1899 [13]. Under the umbrella of purine family, xanthines are considered as point of convergence because both adenine and guanine converge during metabolism at this common intermediate [6, 31]. In fact, xanthine and its derivatives act as intermediate molecules in generation of GMP, GDP and GTP via the salvage pathway inside the cells [32]. In addition, xanthine plays imperative role in catabolism of nucleotides and nucleic acids as xanthine acts as precursor of uric acid. Therefore, xanthine contains similar skeleton as that of purines which forms the building blocks of unit of life i.e. ribonucleotides (RNA) and deoxyribonucleotides (DNA). The structural resemblance with two of the important purine derivatives Adenine and Guanine; makes xanthine a good therapeutic molecule. Fig. 1 depicts the chemical structure of xanthine. The structure of xanthine consists of two fused rings-one ring is six membered and another is five membered. Theoretically, two types of tautomerism are displayed in xanthine molecule. First is annular i.e. migration of proton of imidazole ring between N7 and N9 positions. Second is lactim-lactam i.e. migration of proton between N1 and N3 and oxygen of carbonyl group at C2 position [29]. Despite of annular tautomerism in xanthine, 7H form predominates over 9H form [29]. Xanthine provides maximum possibility of substitutions. Five types of mono substitutions (1-, 3-, 7-, 8- and 9-), eight di-substitutions (1,3-, 1,7-, 1,8-, 1,9-, 3,7-, 3,8-, 3,9- and 7,8-), three types of tri- substitutions (1,3,7-, 1,3,8-, 1,3,9-) [12, 21, 24, 26, 28, 29, 33, 34, 35]. Most of these substitutions are readily obtainable, but substitution at N9 position becomes difficult because low nucleophilicity of N9 position, electrophiles can only attack under special circumstances reference [29]. Thus, the broad understanding over reactivity pattern of the different –NH groups of xanthine have paramount importance to utilize the full potential of xanthine ring in development of xanthine based compounds.
Fig. 1.
Structure of xanthine.
2.2. Availability of xanthines
Numerous types of bioactive xanthine derivatives have been reported so far. These derivatives have been made available by various means including natural sources (e.g. plants, microbes, algae, animals etc), biotranformation process (via bacteria, fungi and enzyme), transmethylation process (in plants) and chemical synthesis [32]. The following section discusses about some naturally derived xanthine molecules and their sources.
2.2.1. Natural xanthine derivatives: an overview
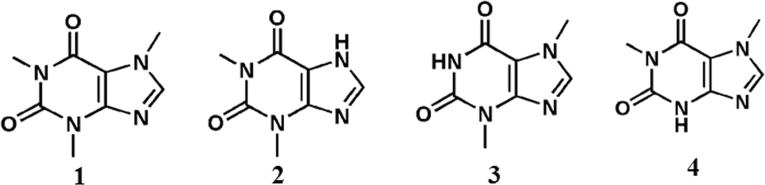
The natural source of xanthine and its derivatives are plants as tea, coffee, cocoa seeds, etc. The presence of natural xanthine derivatives in plant is good for human health but their precise biological role in plants still needs exploration [36]. However, there are two key established hypotheses. One is their presence in young leaves and fruits act as chemical defense system for protecting plants from predators [6, 36]. Second hypothesis is allelopathic or autotoxic theory according to which the release of natural xanthine derivatives from the seed coat prevents germination of other seeds [36]. Thus, along with bearing pharmaceutical importance for others, these derivatives act as defense system for their origin too [37, 38]. Fig. 2 depicts the chemical structure of naturally found xanthine derivatives.
Fig. 2.
Chemical structure of natural xanthine derivatives.
Caffeine (1) (1, 3, 7- trimethylxanthine) is the more thoroughly studied natural xanthine alkaloid to date. It acts as a stimulant in humans and occurs naturally in beans, leaves, and fruit of more than 60 plants. It acts as a natural pesticide [37, 38, 39, 40, 41]. Caffeine acts as central nervous system (CNS) stimulator and plays important role in warding off drowsiness and restoring mental alertness, improves clear thinking and attenuated fatigue [6]. The presence of caffeine in beverages such as coffee, tea, chocolate, soft drinks and energy drinks, give it a popular recognition as world's most consumed psychoactive substance [42]. Caffeine is easily absorbed from the gastrointestinal tract [40]. The principle mode of action of caffeine is in antagonization of adenosine receptor and phosphodiesterase inhibition [43, 44]. Thus, reduction in adenosine activity leads to increase in activity of the neurotransmitter dopamine. Caffeine can enhance the stimulation of epinephrine, epinephrine-like drugs including amphetamine, methamphetamine, or methylphenidate, etc, by regulating the catalytic action cAMP-phosphodiesterase (cAMP-PDE) and thus it leads to make cAMP available for the messaging cascade for various cellular functions [45]. Caffeine increases the levels of serotonin which causes positive mood changes [46, 47]. Caffeine has received lots of attention from sport regulatory institutions and even its use has been regulated by International Olympic Committee [6, 48].
Theophylline (2) (1, 3- dimethylxanthine) is the second widely researched natural xanthine derivative after caffeine. Its natural sources are black tea and green tea. In 1888, theophylline was first time extracted from tea leaves by German biologist Albrecht Kossel. Clinical use of theophylline was taken up for treating asthma in the 1950s. Theophylline is known for its therapeutics importance. It is widely known for its pharmaceutically active properties such as diuretic, cardiac stimulator, smooth muscle relaxant, bronchodilator and CNS stimulator and stabilizer of the mast cells [49, 50, 51, 52, 53]. Adenosine- stimulated release of mediators from mast cells, neutrophil activation, induction of 1L-1β and 1L-1α, synthesis and release of tumour necrosis factor (TNF-α) and cytokine release from T-lymphocytes are inhibited by theophylline [54]. Theophylline possesses anti-inflammatory, anti-tumour, and immunomodulatory activities [55]. Theophylline has also been reported as potent inhibitor for regulating the catalytic activity of alkaline phosphatase (Al-P) [56, 57].
Theobromine (3) (3, 7-dimethylxanthine) is derived from Theobroma, the genus of the cacao tree [58, 59]. It is the primary alkaloid found in cocoa and chocolate. The presence of theobromine in chocolate is one of the important causes for mood-elevating effects. The natural sources of theobromine are cacao, lesser extent in tea and coffee, and as a human metabolite of caffeine. In therapeutics, it is used as a diuretic, vasodilator, a myocardial stimulant, antitussive, antiangiogenic and anti tumour agent [60, 61, 62, 63].
Paraxanthine (4) (1,7-diimethylxanthine) is a major metabolite obtained as a result of biotransformation of caffeine. In human, after intake, nearly 80% of caffeine is demethylated at the N3 position to yield paraxanthine. It acts as a potent, antagonist of adenosine receptors A1, A2A, and A2B [34, 64]. As a result, paraxanthine triggers an elevated diastolic blood pressure, stimulation of thermogenesis, and an increase in plasma epinephrine [34]. Furthermore, paraxanthine shows lipolytic properties and its presence in the blood causes an increase in concentration of serum free fatty acid. Paraxanthine, unlike caffeine, acts as an enzymatic effector of Na+/K+ATPase. Thus, it increases the transport of potassium ions into skeletal muscle tissue. Similarly, the compound also stimulates increase in calcium ion concentration in muscle [65].
2.2.2. Biotransformation
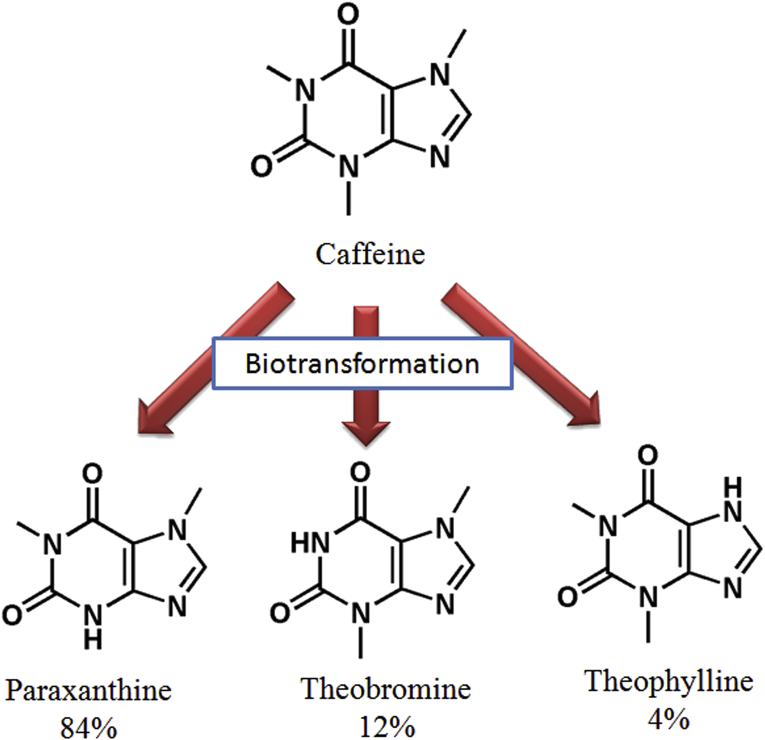
Biotransformation is a process of conversion of compounds from one form to another using biological system (such as bacteria, fungi and enzyme) [66]. It is a process of alteration in molecular and atomic form of compounds under enzyme catalytic action. Among natural xanthine derivatives caffeine is a major component for biotransformation into its different metabolites. In mammalian system, natural xanthine derivative caffeine is biotransformed in liver cells by the catalytic action of cytochrome P450 enzyme into three primary metabolites: paraxanthine (84%), theobromine (12%), and theophylline (4%) as shown in Fig. 3 [67,68]. The caffeine biotransformation in plant parts like coffee fruits and leaves proceeds via theobromine, theophylline, 3-methylxanthine, 7-methylxanthine, xanthine, allantoin, allantoic acid and urea [58, 69, 70, 71]. Likewise, biotransformation of caffeine is also carried out in various other systems such as microorganism including bacteria, fungi, nematodes etc. Biotransformation of caffeine produces theobromine and paraxanthine in bacterial systems whereas theophylline is produced by fungi [41, 72, 73, 74]. Knowing the pathway of the organism for complete metabolism, inhibitors can be used to block a particular step of the pathway to truncate the fermentation process at a desired time. This may help in accumulating the desired compounds. Biotransformation acts significantly in providing avenue for large scale synthesis of metabolites and their use in further drug development process [66]. Therefore, biotransformation may be a viable alternate method and an emerging field for attaining xanthine derivatives. It is cost-effective, non-hazardous, eco-friendly and less energy demanding process which utilizes the raw materials at lower investment costs [75].
Fig. 3.
Biotransformation of caffeine into other natural xanthine derivatives in mammalian system.
2.2.3. Transmethylation
Another process for the formation xanthine derivatives naturally in plants is transmethylation process. In transmethylation process methyl transferase enzymes plays important role in transferring methyl groups for conversion of one form of xanthine or xanthine derivatives to another form. In plants, the main biosynthesis pathway of caffeine consists of four steps. Biosynthesis starts with xanthosine, a purine nucleotide which gives xanthine skeleton to caffeine. The biosynthesis process follows the sequence of xanthosine→ 7-methylxanthosine→ 7-methylxanthine→ theobromine→ caffeine [76]. The precursor molecule xanthosine is derived mainly from different pathways including de novo purine biosynthesis (de novo route), the degradation pathways of guanine nucleotides (GMP route) and adenine nucleotides (AMP route), and the S-adenosyl-L-methionine (SAM) cycle (SAM route) [76]. Xanthine is another precursor molecule for transmethylation. The transmethylation of xanthine in living system occurs in the sequence of xanthine→7-methylxanthine →3,7-dimethylxanthine (theobromine)→ caffeine [29]. Thus, transmethylation can be exploited as a process to develop methylated derivatives of precursor molecule. Using transmethylation process, obtained resultant derivatives can be used as starting materials for synthesis of various manually designed derivatives.
2.2.4. Chemical synthesis
Very few xanthine derivatives have been reported from plant and other natural sources. However, the pharmaceutical active nature of natural xanthine derivatives has incentivized researchers to use these derivatives as lead molecule for synthesizing number of xanthine derivatives. Along with new xanthine derivatives, researchers have synthesized natural xanthine derivatives as well because their extraction from plant sources is uneconomical in large scale. For instance, the commercial availability of all methyl xanthines including theophylline is mostly through chemical synthesis. The commercial production of theophylline is commonly carried out by chemical synthesis which uses materials such as dimethyl urea and ethyl cyanoacetate [77]. Various methods have been reported for synthesis of xanthine derivatives. Ring closure mechanism, classical condensation and use of natural xanthine derivatives as starting material are some of them [12, 21, 22, 26, 28]. Traube synthesis mechanism is the widely used industrial method for synthesis in which 5, 6-diaminouracils formed from urea or N-substituted urea and followed by cyclization i,e. ring closure [11, 29, 78, 79]. Orthogonal safety-catch protection has been another strategy for synthesizing these derivatives [12, 80]. By using these schemes numbers of xanthine derivatives have been synthesized and used as therapeutics. Thus, chemical synthesis is a flexible route to escape complicated extraction methods from medicinal plants. However, this route has also many challenges that will be discussed in upcoming section. By bridging the existing lacuna, chemical synthesis can surely become a viable method for large scale diversification in derivatisation of xanthine derivatives.
2.2.5. Synthetic xanthine derivatives: an overview
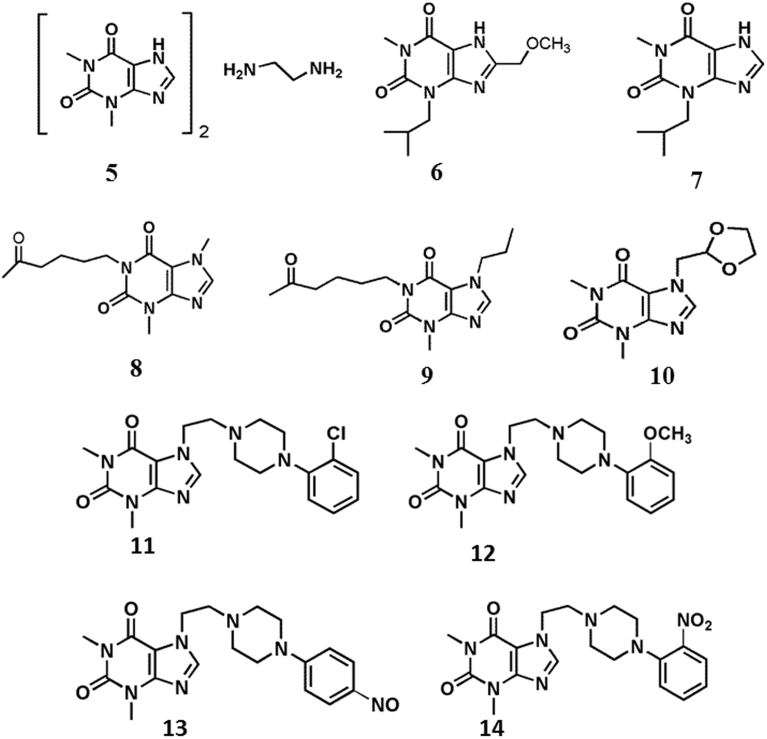
With recognition of xanthine as a lead molecule in natural xanthine derivatives, it incentivized researchers to look at the derivatisation aspect to fetch more potent and selective compounds. Potency and selectivity of compounds towards their targets are imperative in drug development process. The structure of xanthine gives maximum possibility for diversification in derivatisation because of the presence of N1, N3, N7 and C8 positions for substitution. Fig. 4 illustrates the chemical structure of some of the key pharmaceutically active synthetically derived xanthine derivatives (5–14). Following paragraphs provide the description of existing known synthetically derived xanthine derivatives.
Fig. 4.
Structure of synthetically derived xanthine derivatives.
Aminophylline (5), a combination of theophylline and ethylenediamine in 2:1 ratio, is a bronchodilatating and antiasthmatic compound. Aminophylline have been widely used for the treatment of bronchial asthma, chronic obstructive pulmonary disease, and cardiovascular problems. It is commonly known as potent peripheral vasodilator [81, 82, 83]. Due to phosphodiesterase inhibitory properties aminophylline possess antioxidant effects in human neutrophils. Despite antioxidant properties, it generates sufficient amount of free radical which are the responsible factor for seizures inducing potential of aminophylline [83].
Another widely used xanthine derivative in therapeutics is IBMX (7) (1-Methyl-3-isobutylxanthine). It is a potent inhibitor of cyclic nucleotide phosphodiesterase. It increases cyclic AMP and cyclic GMP in tissue and thereby activates cyclic nucleotide-regulated protein kinases. IBMX acts as bronchodilator. IBMX non-specifically inhibits most of the phosphodiesterases except PDE9 and PDE8 [84, 85, 86]. It acts as calcium liberator by releasing calcium ions from Ca2+-gated ion channels of neurons [87]. In HSP70-overexpressing cells, IBMX play imperative role in suppression of transcriptional activation of the tyrosinase gene [88] One similar xanthine derivative, 8-methoxymethyl-1-methyl-3- (2-methylpropyl) xanthine (6) specifically inhibits calmodulin-sensitive cGMP phosphodiesterase and it selectively inhibits Ca2+/calmodulin-dependent phosphodiesterase [89, 90]. Thus IBMX and other similar derivatives are sometimes shows non-pharmaceutical properties because of their small size. Various researches have been aligned towards increasing size of xanthine derivatives to enhance the pharmaceutical properties.
The cellular and molecular effect of one another synthetically derived compound, pentoxifylline (8) is widely known in various pharmaceutical applications. It is a methyl xanthine derivative, which acts as haemorheological agent [91]. In the United States, pentoxifylline has been widely in use for the treatment of intermittent claudication [91, 92]. It improves blood flow by decreasing its viscosity and increasing fibrinolytic activity in plasma. Thus, pentoxifylline is used for the treatment of peripheral vascular disease and cerebrovascular disease [93]. Pentoxifylline administration reduces peritoneal adhesion formation by altering peritoneal fibrinolytic activity [94]. Pentoxifylline has been found as a potent inhibitor of primary post-traumatic adhesion formation in a rodent model [95]. Thus, pentoxifylline has been widely used as safe, cost-effective therapeutic drug for various dermatological disease [96].
Propentofylline (9) (HWA285) is a unique xanthine derivative which exhibits various biochemical and pharmacological activities due to its neuroprotective, antioxidant and anti-inflammatory effects [97]. It acts as combined inhibitor of both adenosine transporter and phosphodiesterases. Propentofylline plays significant role in increasing the cerebral concentration of γ-aminobutyric acid (GABA) and adenosine while treating ischemia [98, 99]. It acts as neuroprotective glial cell modulator which suppresses the neurotoxic effects of microglial cells and thus restores the functions of astrocytes [100, 101]. It increases the blood flow in heart, brain and skeletal muscle [102]. Propentofylline has also been reported as a drug for significant protection from nerve cell damage which brain tumor, vascular dementia (VaD) and Alzheimer's disease (AD) [102, 103]. Thus wider mode of action, various biochemical and pharmaceutical activities make pentoxifylline a good drug for various therapeutic diseases.
In pharma, doxofylline (10) (7-(1,3-dioxalan-2-ylmethyl) theophylline), a 1,3,7-tri-substituted xanthine derivative has been proved as good therapeutic molecule. The mode of action of doxofylline differs with theophylline because of substitution at N7 position with dioxalane group in molecular structure. Like theophylline it act as inhibitor for phosphodiesterase but it shows poor antagonist activities for adenosine A1 and A2 receptors [104]. Doxofylline may participate in reducing various side effects such as gastric acid secretion. This suggests that the drug can be used safely and effectively without encountering the side effect which most of the xanthine derivatives are facing while treating particular disease [105]. Additionally, doxofylline has been reported with various pharmacological effects such as anti-inflammatory and bronchodilator activities [106]. Thus, the administration of drug is safe and cost-effective with diminutive side effects.
Another group of compounds commonly known as, KMUPs are unique xanthine and piperazine derivative widely used pharmaceutically active compounds [107, 108, 109, 110, 111]. Till date four KMUP derivatives (KMUP-1 (11) (7-[2-[4-(2-chlorophenyl) piperazinyl]ethyl]-1,3-dimethylxanthine), KMUP-2 (12) (7-[2-[4-(2-methoxy) piperazinyl]ethyl]-1,3-dimethylxanthine), KMUP-3 (13) (7-[2-[4-(4-nitro) piperazinyl]ethyl]-1,3-dimethylxanthine) and KMUP-4 (14) (7-[2-[4-(2-nitro) piperazinyl]ethyl]-1,3-dimethylxanthine) are reported with their therapeutic activities. KMUP derivatives, act as inhibitor for PDE3, PDE4 and PDE5 thus maintaining the level of second messengers which in turn activate protein kinase A (PKA), protein kinases G (PKG) and K+ channels which results tracheal smooth muscle relaxation [19, 112, 113]. KMUP-1 and KMUP-3 acts as eNOS/cGMP-enhancer which increases the level of nitric oxide (NO), gaseous second messenger, through endothelium nitric-oxide synthase (eNOS) [109, 114, 115]. It plays significant role in increasing eNOS expression, inhibiting iNOS expression, and inhibiting pulmonary hypertension [116]. KMUP derivatives can attenuate isoprenaline-induced cardiac hypertrophy and inhibit right ventricular hypertrophy induced by pulmonary artery hypertension. Thus, KMUP derivatives have been known for their multifunctional roles which include anti-inflammatory, anti-proliferation, neuroprotective, cardioprotective, anti-osteoclastogenic and anti-resorptive activities [109, 113].
As seen from the above examples, xanthine as scaffold has given number of therapeutically active drug candidates. These molecules have been used mainly to treat diseases related to dysfunction of cellular signaling pathway by targeting key enzymes of pathway confirming the scope and potency of the xanthine scaffold.
2.3. Xanthine derivatives and their therapeutic avenues
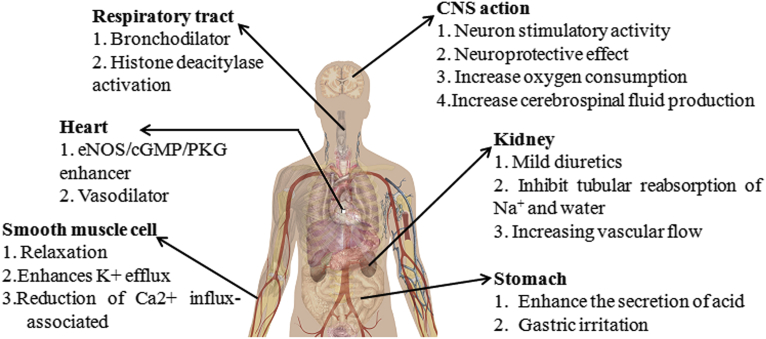
In recent years, xanthine derivatives have brought various attentions because of their dietary effects and their wider natural presence. Various natural and synthetic xanthine derivatives have been recognized as therapeutically potent compounds and reported for targeting various diseases. In terms of potential health benefits, interests towards these compounds have been increasing from the scientific communities, industries and consumers. The following section of the review addresses the various diseases/pathophysiological conditions which have been attended by xanthine based molecules. Fig. 5 illustrates the therapeutic course of action of xanthine derivatives in different parts of human body.
Fig. 5.
Therapeutic disease targets of xanthine derivatives.
2.3.1. Respiratory tract diseases
For many years xanthine derivatives have been widely used for the treatment of respiratory diseases. They are best known for their bronchodilator action. Earlier in the 20th century theophylline was particularly used as bronchodilator but years later the aminophylline (combination of theophylline and ethylenediamine) and glycine theophyllinate were taken up as effective bronchodilator to treat acute asthma [117]. Since then several bronchodilators have been developed. Traditionally, xanthines derivatives such as caffeine, theobromine, theophylline aminophylline, diprophylline, acephylline piperazine, etophylline, proxyphylline, 3-isobutyl-1-methylxanthine, paraxanthine, pentoxifylline, etc., are reported for their bronchodilator activity for treating asthma [31, 118, 119]. One of the major reasons for respiratory tract diseases is reduction in activity of histone deacetylase (HDAC). Xanthine derivatives as caffeine and theophylline are reported for increasing the activation of HDAC [120, 121]. Hence, xanthine derivatives present in the coffee, cocoa are act as relief system in asthmatic patient.
2.3.2. Neurodegenerative disease
Neuro-stimulatory actions of caffeine are well-known [32]. Adenosine acts as modulator in various physiological and pathophysiological processes in central nervous system (CNS). The structural similarity of xanthine derivatives play imperative role in the blocking of adenosine receptors to maintain the concentration of extracellular adenosine, thus, make available signaling molecule for adenylate cyclase mediated heart cell signaling pathway. Propentofylline and KMUP-1 are widely used as neuro-protecting xanthine derivatives [111, 113, 122, 123, 124]. Likewise various other xanthine derivatives show neuroprotective effect because of their course of action as phosphodiesterase inhibitor, blocker of adenosine uptake in neuron and glial cells and antagonist of adenosine receptors [122, 123, 125, 126]. The neuro-active effect of xanthine derivatives increases the life of brain cells. Higher neural activity may lead to increased oxygen consumption and cerebrospinal fluid production in brain. Because of these reasons xanthine derivatives have been used for the treatment of various neurodegenerative disease including Alzheimer disease, Parkinson disease, ischemia, etc [32].
2.3.3. Hypertension and cardiovascular diseases
Xanthine derivatives have various effects in heart and blood vessels. Conventional xanthine derivatives such as caffeine, theophylline, theobromine and aminophylline are reported as vasodilators [32]. In a group of middle age population, cocoa drinks enriched with theobromine significantly increases the ambulatory systolic blood pressure within 24 hours while the central and peripheral systolic blood pressure come down to normal level after two hour of consumption [32]. Xanthine derivatives are also reported as eNOS/cGMP/PKG enhancer. Endothelial nitric oxide synthase (eNOS) and inducible NOS (iNOS) are important enzymes play important role in production of nitric oxide (NO) and cyclic guanosine monophosphate (cGMP). NO and cGMP act as second messenger in heart cell signaling pathway. Xanthine derivatives increase the expression of eNOS and thus maintaining the appropriate level of second messengers for various heart cellular functioning. KMUP-1, a xanthine derivative, shows antihypertrophic effect to treat left ventricular hypertrophy (LVH) which develops due to cardiac stress [116]. Therefore, in cardiovascular diseases, xanthine derivatives effectively show therapeutics effect on blood pressure, arterial stiffness, circulating catecholamine, and endothelial dependent vasodilation [127].
2.3.4. Renal disease treatment
Natural xanthine derivatives such as caffeine and theophylline have been reported for treatment of renal disease since 1864. It is widely known that natural xanthine derivative have been used to increase urine output until the development of more potent diuretic [128]. The diuretic potency of natural xanthine derivatives are reported as theophylline > caffeine > paraxanthine > theobromine [128]. Though diuretic and natriuretic effect of natural xanthine derivatives are well established but the mechanism behind this activity is still not clear. A1 receptor blockade by xanthine derivatives could be most probable reason for diuretic effect of these derivatives because they act as antagonist of adenosine which is key regulator of kidney function by regulating the level of glomerular filtration rate (GFR), medullary blood flow, and renal water and electrolyte transport. Here, xanthine derivatives act as A1 receptor antagonists which increase renal fluid and Na+ excretion by blocking the adenosine receptor. Therefore, diuresis and natriuresis has been observed primarily due to selective A1 receptor blockade which is caused by inhibition of renal reabsorption [129].
2.3.5. Smooth muscle relaxation
The phosphodiesterase inhibition properties of xanthine derivatives have been related to their tracheal relaxant activities [7, 33, 130]. Smooth muscle cell relaxation is carried out through the activation of Adenylyl cyclase (AC) and soluble Guanylyl cyclase (GC) which leads to synthesis of second messengers - cAMP and cGMP respectively. The level of these second messengers is regulated by cyclic nucleotide phosphodiesterases. The phosphodiesterase inhibitory action of xanthine derivatives leads to accumulation of cAMP/cGMP which further activates PKA/PKG and enhances K+ efflux. It leads to reduction of Ca2+ influx-associated contractility in tracheal smooth muscle (TSM) [131]. KMUP-1, KMUP-3 and KMUP-4 are well studied smooth muscle relaxant [108, 112, 131].
2.3.6. Gastric acid secretion
Gastric distress is one of the most frequent side effects in course of treatment with xanthine derivatives. It has been reported that the xanthine derivative which are antagonist of adenosine are mostly responsible for gastric acid and pepsin secretion. Theophylline, aminophylline, caffeine etc come under this category because of their potency towards the adenosine receptor as antagonists [132]. However, xanthine derivatives such as doxofylline and enprofylline, are poor antagonist of adenosine and do not stimulate gastric acid and pepsin secretion [104, 132]. Doxofylline may participate in reducing various side effects such as gastric acid secretion. Thus, the administration of these selective drugs can make significant difference towards the specific target without encountering the side effect which are the most common issue with other xanthine derivatives facing while treating particular disease [105].
Thus, with creating difference towards the specificity for adenosine receptor, xanthine derivatives can be used as safer drug for various other targets such as PDE inhibition, anti tumor activity, anti-inflammatory activities etc.
2.4. Mechanism of action of xanthine derivatives in mammalian cells
Xanthine derivatives go through various regulatory courses of action insides the cellular system by targeting some of the important components of cell signaling such as Adenosine receptor antagonism, phosphodiesterase enzyme inhibition, and histone deacetylase activation.
2.4.1. Antagonizing activity for adenosine receptor
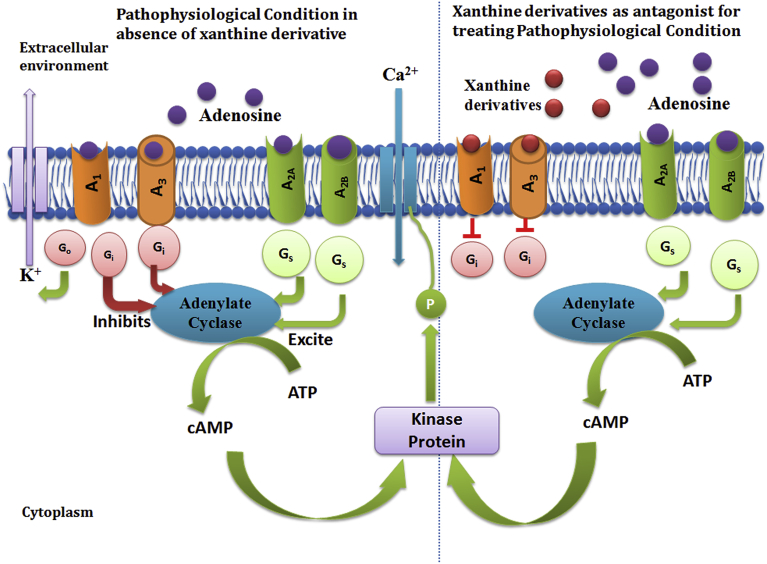
Adenosine is an endogenous nucleoside, an essential component for life dispersed in various mammalian tissues. It acts as a physiological regulator in variety of cellular signaling pathways and synaptic processes. It involves in multitudinous physiological functions including synthesis of nucleic acids, reduces tissue injury and promotes repair, control the level of neurotransmitters in the central nervous system (CNS), etc [133]. Multiple actions carried out by adenosine depend on the activation of adenosine receptors. In mammalian cells, four types of adenosine receptors A1, A3, A2A and A2B are reported. Adenosine and its agonist regulate the activity of adenylate cyclase by activation of all four adenosine receptor in which activation of A1 and A3 lead to Gi mediated inhibition of adenylate cyclase while activation of A2A and A2B lead to Gs mediated activation of adenylate cyclase as shown in Fig. 6. The activation of adenylate cyclase further leads to increase the concentration of cAMP, a second messenger plays vital role in various cellular functions [134]. In pathophysiological condition the activation of A1 and A3 receptors are detrimental for normal cellular functioning and can cause various diseases. The role of antagonist of adenosine becomes essential to regulate the catalytic action of A1 and A3. Generally, over expression of A2A and A2B receptor causes various dysfunctions such as vasodilation, mast cell degranulation, chloride secretion in epithelial cells, smooth muscle contraction, increase cytokines, increase of glucose production, etc [21]. Hence, antagonist of A2A and A2B receptors are required to treat such situation. Due to structural similarity with adenosine, xanthine derivatives act as both agonist and antagonist on adenosine receptors [21, 135, 136]. Caffeine and theophylline are the earliest compounds indentified with antagonist activity for adenosine receptors but with moderate selectivity [32, 136, 137]. Thus, structural modification might have significant impact in improving the potency of xanthine derivatives towards adenosine receptors. Fig. 6 illustrates the mechanism of action of xanthine derivatives in cell signaling by antagonizing activity on adenosine receptors.
Fig. 6.
Activation of adenosine receptors in normal physiological condition and role of xanthine as antagonist for adenosine receptor in pathophysiological condition to regulate the activation of adenosine receptor.
2.4.2. Phosphodiesterase inhibitory activity
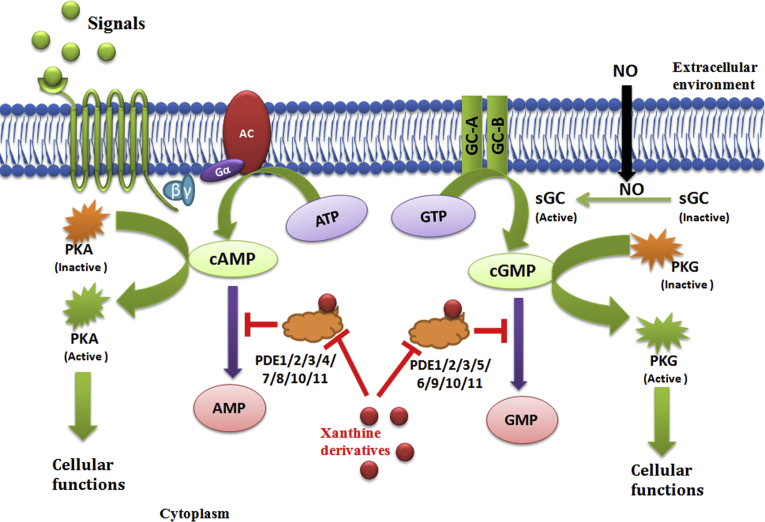
In mammalian signal transduction pathways, second messengers play prominent role in processing the signals coming from the extracellular environment through membrane bound receptors for various cellular functions [138]. Cyclic nucleotides Phosphodiesterases (PDEs) regulate the level of second messengers - cAMP and/or cGMP and thus regulate the various cellular process. PDEs are superfamily of 11 enzymes (PDE1-11) encoded by 21 genes in human genome [139, 140, 141, 142, 143]. In normal physiological condition, regular functioning of PDEs are required to maintain appropriate level of cAMP or/and cGMP which act as mediator to process the signals for various cellular functions. In various pathophysiological conditions, the level of second messengers lowered either due to dysfunction of receptor protein or due to low level of available signals. In such situation regular functioning of PDEs further decreases the level of cAMP or/and cGMP. All these in turn may affect the normal functioning of pathway [144, 145]. Here, inhibitor plays imperative role in regulating the catalytic action of PDEs [145]. Xanthine derivatives are known for their phosphodiesterase inhibition but most of these inhibitions are non-specific [7, 84, 85]. Theophylline shows bronchodilator effects, because of its ability to inhibit phosphodiesterase (PDEs) [51, 146]. Theophylline has been shown to inhibit tissue growth factor β (TGF-β) mediated conversion of pulmonary fibroblasts into myofibroblasts in COPD and asthma patients via cAMP-PKA pathway and suppresses COL1 mRNA, which codes for the protein collagen [147]. Fig. 7 depicts the role of xanthine derivatives in regulating the catalytic action of phosphodiesterases in pathophysiological condition to maintain the level of second messengers for maintaining the consistency in signal transduction pathway.
Fig. 7.
Action of xanthine derivatives as inhibitor for regulating the catalytic action of PDEs in signal transduction pathway.
2.4.3. Histone deacetylase activation
Histone deacetylase (HDAC) is an important enzyme that regulates the chromatin structure and thus affects inflammatory gene expression [120]. Acetylation and deacetylation of histone is important phenomenon for transcriptional activation and repression, respectively, of inflammatory gene expression. Histone deacetylase (HDAC) acts as repressor for activation of inflammatory genes. The activation of histone deacetylase depends on the level of glucocorticoids which is received by glucocorticoid receptor (GR). In cigarette smokers, due to oxidative stress, the glucocorticoid level reduces that leads to reduction in HDAC activity. Thus, in absence of HDAC activity, expression of inflammatory gene increases. Theophylline and other xanthine derivatives act as stimulator of HDAC activation [120, 121]. The activation of HDAC by xanthine derivatives shows their potential to act as novel anti-asthmatic drug. Thereafter, the activated HDAC are subsequently used to suppress inflammatory genes [120, 121].
Thus, targeting cell signaling pathway by different course of action ensures the multiple roles of xanthine derivatives inside the body which increases the potential of the xanthine as scaffold for future drug development.
2.5. Role of xanthine derivatives in pharmacology
For development of promising future drug candidates using the xanthine scaffold, pharmaceutical property analysis of existing drugs constructed over the same scaffold becomes imperative. This will ensure the suitability of constructing future drug candidates in the same line using the same scaffold. Antimicrobial, anti-inflammatory, anti-tumor, antioxidant properties are some of the important parameters to ensure the pharmaceutical properties of existing xanthine derivatives.
2.5.1. Antimicrobial activities of xanthine derivatives
In drug development process, antimicrobial activity of drugs becomes imperative to effectively combat microbial resistance. Xanthine derivatives such as caffeine, theophylline, aminophylline, pentoxifylline are reported for their antimicrobial effects as bactericidal, fungicidal and nematocidal [12, 148]. Caffeine is reported for enhancing the inhibitory effect of existing antibacterial agents such as Penicillin and tetracycline against Staphylococcus aureus [148]. Besides that, antimicrobial properties of caffeine have been reported against: human pathogens like Klebsiella pneumonia, Staphylococcus aureus and Pseudomonas aeruginosa [39]. For caffeine, the minimum inhibitory concentration (MIC) for the above mentioned microorganisms are reported between 5 to 20 mM whereas minimum bactericidal concentration (MBC) have been reported in range from 43 to 100 mM, respectively [39]. These derivatives act as antimicrobial agent through the action of nucleic acid synthesis [22]. It is reported bacterial infection may lead to asthma pathogenesis. Some derivatives are best known for treatment of asthma because of their antimicrobial action [22].
2.5.2. Anti inflammatory activities of xanthine derivatives
There are increasing evidence that xanthine derivatives such as caffeine, theophylline, pentoxifylline, KMUP-1, etc show anti-inflammatory effect [19]. Anti-inflammatory responses of these derivatives are the result of their non-selective phosphodiesterase inhibition and/or their non-selective adenosine receptor antagonist properties. PDE inhibition and/or adenosine antagonist role of xanthine derivatives leads to increase in cAMP concentration, activation of protein kinase A, inhibition of tumor necrosis factor (TNF-α) and leukotriene synthesis. With inhibition of leukotrienes synthesis, inflammation reduces [19, 31]. Leukotrienes increase inflammation by raising leukocyte infiltration, phagocyte microbial ingestion, and generation of pro-inflammatory cytokines such as IL-5, TNFα, and macrophage inflammatory protein-1β [31]. Pentoxifylline is one of the most used xanthine derivatives with anti-inflammatory effect by inhibiting the production of TNFα, therefore it has been found effective in both dermatological as well as non-dermatological conditions [96]. In patient with COPD, xanthine derivatives such as caffeine, theophylline, etc are reported for their effective role in reduction of leukotriene synthesis by reducing the proportion of neutrophils [31, 149].
2.5.3. Anti-oxidant activity of xanthine derivatives
There are various reports which say pathological changes may occur due to excessive accumulation of oxygen and nitrogen reaction product in body fluids including free radicals such as reactive oxygen species (ROS) and nitric oxide (NO) [150]. These changes may cause premature aging. ROS act as mediator in various cellular signaling pathways. The hyperproduction of ROS is limited by both enzymatic mechanisms and natural antioxidants such as uric acid, glutathione, vitamin C and E [150]. In the lack of appropriate regulation the level of ROS increases and leads to oxidative and nitrosative stresses. Thus, xanthine derivatives such as caffeine, theophylline, theobromine, etc, have been reported for their antioxidant activities [150, 151, 152].
2.5.4. Anti-tumour activities of xanthine derivatives
Several xanthine derivatives are reported for their inhibitory affinity of cell transformation. In lower eukaryotes and bacteria, these compounds induce gene mutations. They act as an antagonist of adenosine receptor and exert antiangiogenic properties in many types of tumors including ovarian cancer cells, prostate cancer [60, 61]. Xanthine derivatives such as caffeine, theophylline, pentoxifylline, theobromine, etc, are reported for inhibition of adriamycin and doxorubicin efflux from tumor cells. This inhibition leads to increase in concentration of doxorubicin in tumor enhancing anti tumor activity of doxorubicin [62, 153, 154, 155].xz.
Thus, by targeting the above key cellular mechanism, these derivatives help to reduce the mortality and morbidity rate significantly.
2.6. Pharmacokinetics of xanthines derivatives
Pharmacokinetics of therapeutic drug is imperative to ensure the safe and effective management of the drug molecule inside the body. Physiochemical parameters such as water solubility and lipid solubility may cause change in the pharmacokinetic parameters such as rate of absorption, distribution, metabolism, transport, excretion, therapeutic plasma level etc. With change in structure through substitution at different positions of xanthine changes the metabolic rate of xanthine derivatives. For instance, synthetic xanthine derivative pentoxifylline, is metabolized through its oxohexyl side chain. Whereas, in case of natural xanthine derivatives such as caffeine, theophylline, theobromine etc, metabolization is carried out via demethylation reaction. Pentoxifylline with polar oxohexyl side chain substitution at N1 position provides better tolerant potential than natural xanthine derivatives because demethylase enzymes do not break its N1 substituent [156]. Water solubility and partition co-efficient have been very important parameters for resorption and desorption in body. For many years n-octanol-water partition system has been satisfactory for comparing lipid solubility with biological distribution. Natural xanthine derivatives such as caffeine, theophylline, theobromine etc are with poor water solubility. One of the most important reason for such poor solubility is their strong inter-base hydrogen bond and base stacking [157]. Another important factor for poor solubility of xanthine derivate is existence of strong intra-molecular bond between N-H-groups [158]. It has been reported that the solubility of the natural derivatives improves in complexes. For instance, aminophylline (i.e. complex of theophylline and ethylenediamine) shows better solubility than theophylline [159]. Similarly, salts like choline theophyllinate or oxtriphylline possess better solubility. Substitution at different position of xanthine causes different potency. Along with potency substitutions also affect the physiochemical properties of compounds such as water solubility, metabolic rate, distribution and excretion [160]. Hydrophobic substituent at C8 position has been reported for increasing potency towards adenosine receptor as well as phosphodiesterase inhibition because of establishing the strong hydrophobic interaction with the target but these changes decrease water solubility [137].
2.7. Biological implications of different substitution sites of xanthine
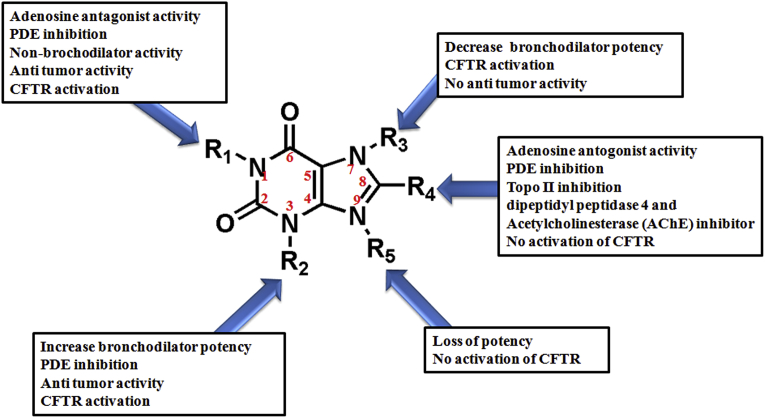
As Xanthine provides maximum possibility of substitution, Fig. 8 illustrates the pharmacological importance of different substitutions sites of the xanthine scaffold. Most of the substituted xanthine derivatives are reported as pharmacologically active compounds with well-known activity as adenosine receptor antagonists, phosphodiesterase inhibitors, cystic fibrosis transmembrane conductance Regulator (CFTR) activation and inducers of histone deacetylase activity [12, 120, 121, 161]. From the various in vitro analyses of natural xanthine derivatives such as theophylline, theobromine and caffeine it was found that N3 substitution has important role in generating bronchodilator properties of compounds [162]. N1 substitution of xanthine generates adenosine antagonism properties [162]. But N1 substitution along with N3 substitution further enhances the potency towards adenosine receptor. N7 substitution shows mixed impact. N7 substituted xanthine derivatives such as caffeine, theobromine, diprophylline, acephylline piperazine, etophylline and proxyphylline are reported for their bronchodilator activity [118, 119]. Though N7 substitution increases bronchoselectivity but it decreases the adenosine receptor affinity [137, 163]. Due to presence of methyl group at N7 position of caffeine, it is threefold less potent than theophylline towards adenosine receptor. Therefore, in most cases bulkiness of N7-substituents considerably decreases the affinity [137, 164]. With comparative studies of different substituent at different sites of xanthine it was found that N7 substitution shows comparatively lower potency as bronchodilator than C8 substituted compounds [119]. The C8 substitution has significant impact in increasing the pharmaceutical properties of compounds. The combined substitution at C8 position with N1 and N3 positions of xanthine are reported with better potency and anti allergic properties [165]. N1, N3 and C8 substitutions together are most promising sites of substitution on xanthine scaffold for generating compounds with selective potency towards subtypes of adenosine receptors [166]. Along with adenosine antagonist, xanthine derivatives with C8 substitution are also reported as PDE inhibitor, Topo II inhibitor, dipeptidyl peptidase 4 and acetylcholinesterase (AChE) inhibitor [34, 167, 168]. Aryl substitution at C8 position show immense affinity for increasing the inhibition potential of compounds towards PDEs as well as adenosine receptors. It is evident from the comparative studies of theophylline and C8 substituted 8-phenyl theophylline where 8-phenyl theophylline shows 100- and 30-fold more potency towards A1 and A2 receptors, respectively [137]. Increasing chain length at N1 and N3 site of xanthine increases the potency of compounds towards adenosine receptor and PDEs [137]. Substitution with alkyl groups at N1, N3 and N7 positions of xanthine are crucial for activation of CFTR. However, substitution at C8 and N9 positions does not activate CFTR. Thus, by manipulating C8 position of compounds, they can be made selective towards adenosine receptor. N7 position may activate CFTR except when N1 and N3 positions are occupied with methyl group [161]. The xanthine derivatives at N1 and N3 position with alkyl substitutions are reported for their antitumor activities. Here also N7 substitutions are reported with mixed response. Some cases of N7 substitution shows inhibitory affinity of cell transformation, however, most of the time N7 substitution has been non influential on cellular viability [169]. N3 substitution with increased alkyl chain length is more effective for inhibition of cell transformation [169]. Substitution at N9 position generally losses the bronchodilator potency of xanthine derivatives as well as substitution at this position does not activate CFTR [161].
Fig. 8.
Pharmaceutical significance of different substitution sites of xanthine scaffold to generate specificity and potency in drug development.
The basic structure of xanthine consists two rings- one ring is of six membered pyrimidinedione ring and second ring is of five membered imidazole ring. Xanthine basic structure itself has significance in generating pharmaceutically active nature. It was evident by studies on alternation of basic structure of xanthine ring including enlargement of xanthine ring, insertion of additional benzene ring, hybridization of xanthine with adenosine and synthesizing mesoionic derivatives of xanthine [15, 137, 170, 171]. Alternation in xanthine basic structure leads to decrease in biological potency. Enlargement of six-membered pyrimidinedione ring of xanthine to a seven-membered diazepinedione ring leads to weaker interaction with adenosine receptor. This was mainly because the enlarged ring system loss planarity [137, 172]. Thus, along with different substitution site basic structure of xanthine itself has significant role in generating biological potency towards the target.
2.8. Lacuna in the current synthesis methods of xanthine derivatives
Most of the earlier studies have used the ring closure synthetic route and classical condensation route for the synthesis of the xanthine derivatives [12, 21, 26]. The existing schemes are endured with several bottlenecks, such as use of toxic and expensive reagents/catalysts, use of acid/base or external oxidant, isolation of imine intermediate, high temperature, hazardous solvents, lengthened reaction time, tedious workup, by-products formation, low yields, expensive chemicals etc [25, 26, 173]. Use of existing xanthine based molecules (such as theophylline, caffeine, theobromine, IBMX etc) as reaction initiator has been reported as another widely used synthetic approach for the synthesis of new xanthine derivatives [24, 25, 27]. But in these derivatives, the availability of limited sites for substitution is the major issue. For instance, in case of theobromine, N3 and N7 positions are already occupied by methyl groups. Thus, only N1 and C8 positions are available for substitution [25]. Therefore, the existing methods are not favorable for the synthesis of large number of diverse xanthine derivatives.
2.9. Scope for molecular diversification using xanthine as scaffold in future drug development
Despite enormous ground available for substitution/modification still use of xanthine is limited in drug development process. Till date no research has been carried out using xanthine as starting material for xanthine derivatives. This is mainly because of the lack of proper understanding of the structural nature of xanthine. According to reports available till date, N3 position of xanthine has been considered as most reactive in xanthine and substitution follows the –NH group of N3>N7>N1 positions [29]. This analysis was contradictory to the transmethylation process of xanthine in living organism. The transmethylation of xanthine occurs in sequence of N7>N3>N1 i.e. xanthine→7-methylxanthine→3,7-dimethylxanthine (theobromine)→1,3,7-trimethylxanthine (caffeine) [29]. Thus, with considering N3 position most reactive site among all –NH groups on xanthine, most of the reports initiated synthesis with substitution at N3 position. However, this substitution was not directly carried out on the xanthine scaffold. N3 substitution in most of the reports starts with cyclization/condensation of substituted urea and this process is commonly known as ring closure mechanism and classical condensation process [174, 175]. In our research analysis we have found that the synthetic substitution process undoubtedly follows the natural transmethylation sequences i.e. N7>N3>N1 [data not published]. The reactivity difference is due to different atomic environment of three –NH groups. Among three –NH groups, –NH at N7 position faces less steric hinderance than other two –NH positions whereas –NH at N1 position faces highest steric hinderance. Therefore, based on the reactivity pattern, substitution follows the same order as it is found in living system i.e. N7>N3>N1. Therefore, by selective sequential substitution in order of N7>N3>N1, numbers of derivatives can be synthesized. The presence of three –NH groups makes the xanthine highly polar compound and that also could be a major reason for not using xanthine for synthesis of xanthine derivatives because separation of reaction product become difficult through column chromatography and also it becomes time consuming process with heavy use of costly solvents and end product obtained with less yield. To overcome this technical difficulty, concentration optimization of reactants can be a good way to achieve target substitution. The selective substitution can also be achieved by protecting higher reactive site if that site is not chosen for substitution. Using xanthine as starting material for synthesis would be cost effective, time saving, non-hazardous, less tedious and high yield producing because there would be no need to give extra effort for cyclization of xanthine ring. Abundance of xanthine naturally makes it a better alternative of any existing starting material. Thus, with considering the huge potential of xanthine in maximizing the derivatisation with structural diversification, it can become an ideal choice as ‘reaction initiator’ for future xanthine based drug developments. Fig. 9 summarizes the potentiality of xanthine as a prospective lead molecule for future drugs.
Fig. 9.
Xanthine as a lead molecule for future drugs for several therapeutic targets.
3. Conclusions
Xanthine derivatives are best known for their course of action by targeting regulatory enzymes of cell signaling pathway through various pharmaceutical applications such as phosphodiesterase inhibition, adenosine antagonizing activity, activation of histone deacetylase, etc. This review has covered the routes for availability of xanthine derivatives, their structural details, therapeutic disease targets, mechanism of action, implication of different substitution sites, current lacuna in xanthine based drug development process along with future perspectives for the development of viable synthesis method. By going through therapeutic importance and different course of action of existing xanthine derivatives, this review has also tried to resolve the existing challenges in this field of research. The availability of xanthines from various routes such as natural source, biotransformation, transmethylation and chemical synthesis; have been brought out with their corresponding advantages and challenges. Chemical synthesis is one of the most promising ways to synthesize diversity of compounds using any fragment of interest. But this is the most challenging route among all. Available synthesis routes are unable to deliver large scale diversity in derivatisation. The present review has tried to explore the possibility of xanthine to act as starting material for large scale diversification in derivatisation reaction because of the availability of all sites (N1, N3, N7, N9 and C8 sites) for the substitution. However, the presence of three –NH groups has been the most challenging part in xanthine based drug development process. Thus, clear understanding over the reactivity pattern of different –NH sites of xanthine is key to fetch full potential of xanthine in combinatorial drug development process. Thus, the subsequent understanding over the molecular and physiological behaviour of xanthine will be helpful in further research.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by Department of Biotechnology, Government of India (project BT/272/NE/TBP/2011).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Amirkia V., Heinrich M. Alkaloids as drug leads – a predictive structural and biodiversity-based analysis. Phytochem Lett. 2014;10 xlviii–liii. [Google Scholar]
- 2.Cushnie T.P.T., Cushnie B., Lamb A.J. Alkaloids: an overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents. 2014;44:377–386. doi: 10.1016/j.ijantimicag.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Perviz S., Khan H., Pervaiz A. Plant alkaloids as an emerging therapeutic alternative for the treatment of depression. Front. Pharmacol. 2016;7:1–7. doi: 10.3389/fphar.2016.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baraldi P.G., Fruttarolo F., Tabrizi M.A., Romagnoli R., Preti D. Novel 8-heterocyclyl xanthine derivatives in drug development - an update. Expet Opin. Drug Discov. 2007;2:1161–1183. doi: 10.1517/17460441.2.9.1161. [DOI] [PubMed] [Google Scholar]
- 5.S. Delarcina Jr., C. R. Ferrari, Process for obtaining extracts containing methylxanthine derivatives from cakes of plants of the genus Theobroma, as well as composition and use of said extract. US 9198848 B2 2009.
- 6.Monteiro J.P., Alves M.G., Oliveira P.F., Silva B.M. Structure-bioactivity relationships of methylxanthines: trying to make sense of all the promises and the drawbacks. Molecules. 2016;21:1–32. doi: 10.3390/molecules21080974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogawa K., Takagi K., Satake T. Mechanism of xanthine-induced relaxation of Guinea-pig isolated trachealis muscle. Br. J. Pharmacol. 1989;97:542–546. doi: 10.1111/j.1476-5381.1989.tb11983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt D.T., Watson N., Dent G., Rühlmann E., Branscheid D., Magnussen H. The effect of selective and non-selective phosphodiesterase inhibitors on allergen- and leukotriene C(4)-induced contractions in passively sensitized human airways. Br. J. Pharmacol. 2000;131:1607–1618. doi: 10.1038/sj.bjp.0703725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroeder J.A., Ruta J.D., Gordon J.S., Rodrigues A.S., Foote C.C. The phosphodiesterase inhibitor isobutylmethylxanthine attenuates behavioral sensitization to cocaine. Behav. Pharmacol. 2012;23:310–314. doi: 10.1097/FBP.0b013e3283536d04. [DOI] [PubMed] [Google Scholar]
- 10.Meskini N., Némoz G., Okyayuz-Baklouti I., Lagarde M., Prigent A.-F. Phosphodiesterase inhibitory profile of some related xanthine derivatives pharmacologically active on the peripheral microcirculation. Biochem. Pharmacol. 1994;47:781–788. doi: 10.1016/0006-2952(94)90477-4. [DOI] [PubMed] [Google Scholar]
- 11.Burbiel J.C., Hockemeyer J., Müller C.E. Microwave-assisted ring closure reactions : synthesis of 8-substituted xanthine derivatives and related pyrimido- and diazepinopurinediones. Beilstein J. Org. Chem. 2006;63:1–6. doi: 10.1186/1860-5397-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allwood M.B., Cannan B., van Aalten D.M.F., Eggleston I.M. Efficient synthesis of 1,3,7-substituted xanthines by a safety-catch protection strategy. Tetrahedron. 2007;63:12294–12302. [Google Scholar]
- 13.Suravajhala R., Poddar R., Nallapeta S., Ullah S. Agric. Bioinforma. Springer India; New Delhi: 2014. Xanthine derivatives: a molecular modeling perspective; pp. 283–291. [Google Scholar]
- 14.Van der Walt M.M., Terre'Blanche G. 1,3,7-Triethyl-substituted xanthines—possess nanomolar affinity for the adenosine A1 receptor. Bioorg. Med. Chem. 2015;23:6641–6649. doi: 10.1016/j.bmc.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Glennon R.A., Gaines J.J., Rogers M.E. Benz-fused mesoionic xanthine analogs as inhibitors of cyclic-AMP phosphodiesterase. J. Med. Chem. 1981;24:766–769. doi: 10.1021/jm00138a027. [DOI] [PubMed] [Google Scholar]
- 16.Wong E.H.-A., Ooi S.-O. Methylxanthine and non-xanthine phosphodiesterase inhibitors: their effects on adenosine uptake and the low Km cyclic AMP phosphodiesterase in intact rat adipocyte. Biochem. Pharmacol. 1985;34:2891–2896. doi: 10.1016/0006-2952(85)90012-7. [DOI] [PubMed] [Google Scholar]
- 17.Haginaka J., Wakai J., Yasuda H., Kimura Y. Determination of anticonvulsant drugs and methyl xanthine derivatives in serum by liquid chromatography with direct injection: column-switching method using a new internal-surface reversed-phase silica support as a precolumn. J. Chromatogr. B Biomed. Sci. Appl. 1990;529:455–461. doi: 10.1016/s0378-4347(00)83854-2. [DOI] [PubMed] [Google Scholar]
- 18.Constantin S., Lupascu F.G., Apotrosoaei M., Vasincu I.M., Lupascu D., Buron F. Synthesis and biological evaluation of the new 1,3-dimethylxanthine derivatives with thiazolidine-4-one scaffold. Chem. Cent. J. 2017;11:12. doi: 10.1186/s13065-017-0241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai Z.-K., Liu Y.-W., Hsu J.-H., Yeh J.-L., Chen I.-J., Wu J.-R. The xanthine derivative KMUP-1 attenuates serotonin-induced vasoconstriction and K+-Channel inhibitory activity via the PKC pathway in pulmonary arteries. Int. J. Biol. Sci. 2015;11:633–642. doi: 10.7150/ijbs.11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heizmann G., Eberle A.N. Xanthines as a scaffold for molecular diversity. Mol. Divers. 1997;2:171–174. doi: 10.1007/BF01682205. [DOI] [PubMed] [Google Scholar]
- 21.Hayallah A.M., Sandoval-Ramírez J., Reith U., Schobert U., Preiss B., Schumacher B. 1,8-Disubstituted xanthine derivatives: synthesis of potent A2B-selective adenosine receptor antagonists. J. Med. Chem. 2002;45:1500–1510. doi: 10.1021/jm011049y. [DOI] [PubMed] [Google Scholar]
- 22.Hayallah A.M., Elgaher W.A., Salem O.I., Alim A., Alim M.A. Design and synthesis of some new theophylline derivatives with bronchodilator and antibacterial activities. Arch Pharm. Res. (Seoul) 2011;34:3–21. doi: 10.1007/s12272-011-0101-8. [DOI] [PubMed] [Google Scholar]
- 23.Basu S., Barawkar D.A., Ramdas V., Patel M., Waman Y., Panmand A. Design and synthesis of novel xanthine derivatives as potent and selective A 2B adenosine receptor antagonists for the treatment of chronic inflammatory airway diseases. Eur. J. Med. Chem. 2017;134:218–229. doi: 10.1016/j.ejmech.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Sakai R., Konno K., Yamamoto Y., Sanae F., Takagi K., Hasegawa T. Effects of alkyl substitutions of xanthine skeleton on bronchodilation. J. Med. Chem. 1992;35:4039–4044. doi: 10.1021/jm00100a008. [DOI] [PubMed] [Google Scholar]
- 25.Kim D., Jun H., Lee H., Hong S.-S., Hong S. Development of new fluorescent xanthines as kinase inhibitors. Org. Lett. 2010;12:1212–1215. doi: 10.1021/ol100011n. [DOI] [PubMed] [Google Scholar]
- 26.Bandyopadhyay P., Agrawal S.K., Sathe M., Sharma P., Kaushik M.P. A facile and rapid one-step synthesis of 8-substituted xanthine derivatives via tandem ring closure at room temperature. Tetrahedron. 2012;68:3822–3827. [Google Scholar]
- 27.Chen Y., Wang B., Guo Y., Zhou Y., Pan L., Xiong L. Synthesis and biological activities of novel methyl xanthine derivatives. Chem. Res. Chin. Univ. 2014;30:98–102. [Google Scholar]
- 28.Lee D., Lee S., Liu K.H., Bae J.S., Baek D.J., Lee T. Solid-Phase synthesis of 1,3,7,8-tetrasubstituted xanthine derivatives on traceless solid support. ACS Comb. Sci. 2016;18:70–74. doi: 10.1021/acscombsci.5b00148. [DOI] [PubMed] [Google Scholar]
- 29.Gulevskaya A.V., Pozharskii A.F. Synthesis of N-substituted xanthines (review) Chem. Heterocycl. Comp. 1991;27:1–23. [Google Scholar]
- 30.Singh N., Saravanan P., Thakur M.S., Patra S. Development of xanthine based inhibitors targeting phosphodiesterase 9A. Lett. Drug Des. Discov. 2017;14:1–16. [Google Scholar]
- 31.Lee I., Kamba A., Low D., Mizoguchi E. Novel methylxanthine derivative-mediated anti-inflammatory effects in inflammatory bowel disease. World J. Gastroenterol. 2014;20:1127–1138. doi: 10.3748/wjg.v20.i5.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franco R., Oñatibia-Astibia A., Martínez-Pinilla E. Health benefits of methylxanthines in cacao and chocolate. Nutrients. 2013;5:4159–4173. doi: 10.3390/nu5104159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyamoto K., Yamamoto Y., Kurita M., Sakai R., Konno K., Sanae F. Bronchodilator activity of xanthine derivatives substituted with functional groups at the 1- or 7-position. J. Med. Chem. 1993;36:1380–1386. doi: 10.1021/jm00062a010. [DOI] [PubMed] [Google Scholar]
- 34.Müller C.E., Deters D., Dominik A., Pawlowski M. Synthesis of paraxanthine and isoparaxanthine analogs (1,7- and 1,9- substituted xanthine derivatives) Synthesis. 1998:1428–1436. [Google Scholar]
- 35.Bansal R., Kumar G., Gandhi D., Yadav R., Young L.C., Harvey A.L. Synthesis of 8-(cyclopentyloxy)phenyl substituted xanthine derivatives as adenosine A2A ligands. Arzneimittelforschung. 2010;60:131–136. doi: 10.1055/s-0031-1296261. [DOI] [PubMed] [Google Scholar]
- 36.McCarthy A.A., McCarthy J.G. The structure of two N-methyltransferases from the caffeine biosynthetic pathway. Plant Physiol. (Sofia) 2007;144:879–889. doi: 10.1104/pp.106.094854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frischknecht P.M., Ulmer-Dufek J., Baumann T.W. Purine alkaloid formation in buds and developing leaflets of Coffea arabica: expression of an optimal defence strategy? Phytochemistry. 1986;25:613–616. [Google Scholar]
- 38.Nathanson J.A. Caffeine and related methylxanthines: possible naturally occurring pesticides. Science. 1984;226:184–187. doi: 10.1126/science.6207592. [DOI] [PubMed] [Google Scholar]
- 39.Sledz W., Los E., Paczek A., Rischka J., Motyka A., Zoledowska S. Antibacterial activity of caffeine against plant pathogenic bacteria. Acta Biochim. Pol. 2015;62:605–612. doi: 10.18388/abp.2015_1092. [DOI] [PubMed] [Google Scholar]
- 40.Cappelletti S., Piacentino D., Daria P., Sani G., Aromatario M. Caffeine: cognitive and physical performance enhancer or psychoactive drug? Curr. Neuropharmacol. 2015;13:71–88. doi: 10.2174/1570159X13666141210215655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coelho A., Fraichard S., Le Goff G., Faure P., Artur Y., Ferveur J.-F. Cytochrome P450-dependent metabolism of caffeine in Drosophila melanogaster. PLoS One. 2015;10 doi: 10.1371/journal.pone.0117328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lisko J.G., Lee G.E., Kimbrell J.B., Rybak M.E., Valentin-Blasini L., Watson C.H. Caffeine concentrations in coffee, tea, chocolate, and energy drink flavored E-liquids. Nicotine Tob. Res. 2016;31:ntw192. doi: 10.1093/ntr/ntw192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fisone G., Borgkvist A., Usiello A. Caffeine as a psychomotor stimulant: mechanism of action. Cell. Mol. Life Sci. 2004;61:857–872. doi: 10.1007/s00018-003-3269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pennella S., Vittoria Mattioli A. Caffeine, energy drinks and atrial fibrillation: a Mini-Review. BAOJ Nutr. 2015;1:1–4. [Google Scholar]
- 45.Graham T.E., Rush J.W.E., van Soeren M.H. Caffeine and exercise: metabolism and performance. Can. J. Appl. Physiol. 1994;19:111–138. doi: 10.1139/h94-010. [DOI] [PubMed] [Google Scholar]
- 46.Chen M.D., Lin W.H., Song Y.M., Lin P.Y., Ho L.T. Effect of caffeine on the levels of brain serotonin and catecholamine in the genetically obese mice. Zhonghua Yixue Zazhi. 1994;53:257–261. [PubMed] [Google Scholar]
- 47.Szopa A., Poleszak E., Wyska E., Serefko A., Wośko S., Wlaź A. Caffeine enhances the antidepressant-like activity of common antidepressant drugs in the forced swim test in mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2016;389:211–221. doi: 10.1007/s00210-015-1189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chapman R.F., Mickleborough T.D. The effects of caffeine on ventilation and pulmonary function during exercise: an often-overlooked response. Phys. Sportsmed. 2009;37:97–103. doi: 10.3810/psm.2009.12.1747. [DOI] [PubMed] [Google Scholar]
- 49.Benfey B.G. Theophylline and phenylephrine effects on cardiac relaxation. Br. J. Pharmacol. 1977;59:75–81. doi: 10.1111/j.1476-5381.1977.tb06979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu G., Maskray V., Jackson S., Swift C., Tiplady B. A comparison of the central nervous system effects of caffeine and theophylline in elderly subjects. Br. J. Clin. Pharmacol. 1991;32:341–345. doi: 10.1111/j.1365-2125.1991.tb03909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rabe K.F., Magnussen H., Dent G. Theophylline and selective PDE inhibitors as bronchodilators and smooth muscle relaxants. Eur. Respir. J. 1995;8:637–642. [PubMed] [Google Scholar]
- 52.Bell M., Jackson E., Mi Z., McCombs J., Carcillo J. Low-dose theophylline increases urine output in diuretic-dependent critically ill children. Intensive Care Med. 1998;24:1099–1105. doi: 10.1007/s001340050723. [DOI] [PubMed] [Google Scholar]
- 53.Boison D. Methylxanthines, seizures, and excitotoxicity. Handb. Exp. Pharmacol. 2011;200:251–266. doi: 10.1007/978-3-642-13443-2_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spatafora M., Chiappara G., Merendino A.M., D’Amico D., Bellia V., Bonsignore G. Theophylline suppresses the release of tumour necrosis factor-alpha by blood monocytes and alveolar macrophages. Eur. Respir. J. 1994;7:223–228. doi: 10.1183/09031936.94.07020223. [DOI] [PubMed] [Google Scholar]
- 55.Foukas L.C., Daniele N., Ktori C., Anderson K.E., Jensen J., Shepherd P.R. Direct effects of caffeine and theophylline on p110δ and other phosphoinositide 3-kinases: differential effects on lipid kinase and protein kinase activities. J. Biol. Chem. 2002;277:37124–37130. doi: 10.1074/jbc.M202101200. [DOI] [PubMed] [Google Scholar]
- 56.Sugimura K., Mizutani A. The inhibitory effect of xanthine derivatives on alkaline phosphatase in the rat brain. Histochemistry. 1979;61:131–137. doi: 10.1007/BF00496525. [DOI] [PubMed] [Google Scholar]
- 57.Glogowski J., Danforth D.R., Ciereszko A. Inhibition of alkaline phosphatase activity of boar semen by pentoxifylline, caffeine, and theophylline. J. Androl. 2002;23:783–792. [PubMed] [Google Scholar]
- 58.Ashihara H., Crozier A. Biosynthesis and metabolism of caffeine and related purine alkaloids in plants. Adv. Bot. Res. 1999;30:117–205. [Google Scholar]
- 59.Naotoshi Katakura M., Matsuzaki K., Ohno-Shosaku T., Yachie A., Shido O. Vol. 30. Federation of American Societies for Experimental Biology; 2016. (Federation Proceedings). [Google Scholar]
- 60.Slattery M.L., West D.W. Smoking, alcohol, coffee, tea, caffeine, and theobromine: risk of prostate cancer in Utah (United States) Cancer Causes Control. 1993;4:559–563. doi: 10.1007/BF00052432. [DOI] [PubMed] [Google Scholar]
- 61.Barcz E., Sommer E., Janik P., Marianowski L., Skopinska-Rózewska E. Adenosine receptor antagonism causes inhibition of angiogenic activity of human ovarian cancer cells. Oncol. Rep. 2000;7:1285–1291. doi: 10.3892/or.7.6.1285. [DOI] [PubMed] [Google Scholar]
- 62.Kakuyama (nee Iwazaki) A., Sadzuka Y. Effect of methylxanthine derivatives on doxorubicin transport and antitumor activity. Curr. Drug Metabol. 2001;2:379–395. doi: 10.2174/1389200013338270. [DOI] [PubMed] [Google Scholar]
- 63.Usmani O.S., Belvisi M.G., Patel H.J., Crispino N., Birrell M.A., Korbonits M. Theobromine inhibits sensory nerve activation and cough. Faseb. J. 2005;19:231–233. doi: 10.1096/fj.04-1990fje. [DOI] [PubMed] [Google Scholar]
- 64.Orrú M., Guitart X., Karcz-Kubicha M., Solinas M., Justinova Z., Barodia S.K. Psychostimulant pharmacological profile of paraxanthine, the main metabolite of caffeine in humans. Neuropharmacology. 2013;67:476–484. doi: 10.1016/j.neuropharm.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hawke T.J., Allen D.G., Lindinger M.I. Paraxanthine, a caffeine metabolite, dose dependently increases [Ca2+]i in skeletal muscle. J. Appl. Physiol. 2000;89:2312–2317. doi: 10.1152/jappl.2000.89.6.2312. [DOI] [PubMed] [Google Scholar]
- 66.Toxicol J.D.M., Kebamo S., Tesema S., Geleta B. The role of biotransformation in drug discovery and development. Drug Metab. Toxicol. 2015;6:1–13. [Google Scholar]
- 67.Warren R.N. Metabolism of xanthine alkaloids in man. J. Chromatogr. 1969;40:468–469. doi: 10.1016/s0021-9673(01)96689-0. [DOI] [PubMed] [Google Scholar]
- 68.Chung W.-G., Cha Y.-N. Oxidation of caffeine to theobromine and theophylline is catalyzed primarily by flavin-containing monooxygenase in liver microsomes. Biochem. Biophys. Res. Commun. 1997;235:685–688. doi: 10.1006/bbrc.1997.6866. [DOI] [PubMed] [Google Scholar]
- 69.Kalberer P. Breakdown of caffeine in the leaves of coffea arabica L. Nature. 1965;205:597–598. [Google Scholar]
- 70.Suzuki T., Waller G.R. Biodegradation of caffeine: formation of theophylline and theobromine from caffeine in matureCoffea arabica fruits. J. Sci. Food Agric. 1984;35:66–70. [Google Scholar]
- 71.Suzuki T., Waller G.R. Biosynthesis and biodegradation of caffeine, theobromine, and theophylline in Coffea arabica L. fruits. J. Agric. Food Chem. 1984;32:845–848. [Google Scholar]
- 72.Asano Y., Komeda T., Yamada H. Microbial production of theobromine from caffeine. Biosci. Biotechnol. Biochem. 1993;57:1286–1289. [Google Scholar]
- 73.Babu V.R.S., Patra S., Thakur M.S., Karanth N.G., Varadaraj M.C. Degradation of caffeine by Pseudomonas alcaligenes CFR 1708. Enzym. Microb. Technol. 2005;37:617–624. [Google Scholar]
- 74.Schwimmer S., Kurtzman R.H., Heftmann E. Caffeine metabolism by Penicillium roqueforti. Arch. Biochem. Biophys. 1971;147:109–113. doi: 10.1016/0003-9861(71)90315-8. [DOI] [PubMed] [Google Scholar]
- 75.Retnadhas S., Gummadi S.N. Bioprocessing & biotechniques optimization of process conditions for biotransformation of caffeine to theobromine using induced whole cells of Pseudomonas sp. J. Bioprocess. Biotech. 2014;4 [Google Scholar]
- 76.Ashihara H., Sano H., Crozier A. Caffeine and related purine alkaloids: biosynthesis, catabolism, function and genetic engineering. Phytochemistry. 2008;69:841–856. doi: 10.1016/j.phytochem.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 77.BK Campbell, KN Campbell. Method of preparing xanthine and methylated xanthines. US Pat 1948; US2523496A.
- 78.Saldaña M.D.A., Mohamed R.S., Baer M.G., Mazzafera P., Han J., Deng W. Direct conversion of theophylline to 3-methylxanthine by metabolically engineered E. coli. J. Agric. Food Chem. 1999;47:3804–3808. [Google Scholar]
- 79.Aleksandrova E.V., Levich S.V., Romanenko N.I., Shkoda A.S., Mikhal′chenko E.K. Synthesis, transformations, and physicochemical properties of 3-(4′-Methylphenyl)-8-methylxanthine derivatives. Chem. Nat. Compd. 2014;49:1105–1109. [Google Scholar]
- 80.Algharrawi K.H.R., Summers R.M., Gopishetty S., Subramanian M. Direct conversion of theophylline to 3-methylxanthine by metabolically engineered E. coli. Microb. Cell Factories. 2015;14:203. doi: 10.1186/s12934-015-0395-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rutherford J.D., Vatner S.F., Braunwald E. Effects and mechanism of action of aminophylline on cardiac function and regional blood flow distribution in conscious dogs. Circulation. 1981;63:378–387. doi: 10.1161/01.cir.63.2.378. [DOI] [PubMed] [Google Scholar]
- 82.Skinhøj E., Paulson O.B. The mechanism of action of aminophylline upon cerebral vascular disorders. Acta Neurol. Scand. 1970;46:129–140. doi: 10.1111/j.1600-0404.1970.tb05612.x. [DOI] [PubMed] [Google Scholar]
- 83.Roy U., Pal M., Datta S., Harlalka S. Has oxidative stress any role on mechanisms of aminophylline – induced seizures? An animal study. Kathmandu Univ. Med. J. 2015;12:269. doi: 10.3126/kumj.v12i4.13733. [DOI] [PubMed] [Google Scholar]
- 84.Geisbuhler T.P., Schwager T.L., Ervin H.D. 3-Isobutyl-1-methylxanthine (IBMX) sensitizes cardiac myocytes to anoxia. Biochem. Pharmacol. 2002;63:2055–2062. doi: 10.1016/s0006-2952(02)00901-2. [DOI] [PubMed] [Google Scholar]
- 85.Huai Q., Wang H., Zhang W., Colman R.W., Robinson H., Ke H. Crystal structure of phosphodiesterase 9 shows orientation variation of inhibitor 3-isobutyl-1-methylxanthine binding. Proc. Natl. Acad. Sci. U. S. A. 2004;101:9624–9629. doi: 10.1073/pnas.0401120101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang X., Feng Q., Cote R.H. Efficacy and selectivity of phosphodiesterase-targeted drugs in inhibiting photoreceptor phosphodiesterase (PDE6) in retinal photoreceptors. Invest. Ophthalmol. Vis. Sci. 2005;46:3060–3066. doi: 10.1167/iovs.05-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Usachev Y., Verkhratsky A. IBMX induces calcium release from intracellular stores in rat sensory neurones. Cell Calcium. 1995;17:197–206. doi: 10.1016/0143-4160(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 88.Hoshino T., Matsuda M., Yamashita Y., Takehara M., Fukuya M., Mineda K. Suppression of melanin production by expression of HSP70. J. Biol. Chem. 2010;285:13254–13263. doi: 10.1074/jbc.M110.103051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wells J.N., Miller J.R. Methylxanthine inhibitors of phosphodiesterases. Methods Enzymol. 1988;159:489–496. doi: 10.1016/0076-6879(88)59048-1. [DOI] [PubMed] [Google Scholar]
- 90.Ho Sam Ahn, Crim W., Romano M., Sybertz E., Pitts B. Effects of selective inhibitors on cyclic nucleotide phosphodiesterases of rabbit aorta. Biochem. Pharmacol. 1989;38:3331–3339. doi: 10.1016/0006-2952(89)90631-x. [DOI] [PubMed] [Google Scholar]
- 91.Samlaska C.P., Winfield E.A. Pentoxifylline. J. Am. Acad. Dermatol. 1994;30:603–621. doi: 10.1016/s0190-9622(94)70069-9. [DOI] [PubMed] [Google Scholar]
- 92.Beebe H.G., Dawson D.L., Cutler B.S., Herd J.A., Strandness D.E., Bortey E.B. A new pharmacological treatment for intermittent claudication. Arch. Intern. Med. 1999;159:2041. doi: 10.1001/archinte.159.17.2041. [DOI] [PubMed] [Google Scholar]
- 93.Ward A., Clissold S.P. Pentoxifylline.Pentoxifylline. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic efficacy. Drugs. 1987;34:50–97. doi: 10.2165/00003495-198734010-00003. [DOI] [PubMed] [Google Scholar]
- 94.Tarhan O.R., Barut I., Sutcu R., Akdeniz Y., Akturk O. Pentoxifylline, a Methyl xanthine derivative, reduces peritoneal adhesions and increases peritoneal fibrinolysis in rats. Tohoku J. Exp. Med. 2006;209:249–255. doi: 10.1620/tjem.209.249. [DOI] [PubMed] [Google Scholar]
- 95.Steinleitner A., Lambert H., Kazensky C., Danks P., Roy S. Pentoxifylline, a methylxanthine derivative, prevents postsurgical adhesion reformation in rabbits. Obstet. Gynecol. 1990;75:926–928. [PubMed] [Google Scholar]
- 96.Hassan I., Dorjay K., Anwar P. Pentoxifylline and its applications in dermatology. Indian Dermatol. Online J. 2014;5:510–516. doi: 10.4103/2229-5178.142528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bondan E.F., De Fátima M., Martins M., Bernardi M.M. Propentofylline reverses delayed remyelination in streptozotocin- induced diabetic rats. Arch. Endocrinol. Metab. 2015;59:47–53. doi: 10.1590/2359-3997000000009. [DOI] [PubMed] [Google Scholar]
- 98.Vukadinovića V., Stefanovich V., Rakića L. Effect of propentofylline on the GABA system of a rat brain. Drug Dev. Res. 1986;7:87–94. [Google Scholar]
- 99.Gwak Y.S., Crown E.D., Unabia G.C., Hulsebosch C.E. Propentofylline attenuates allodynia, glial activation and modulates GABAergic tone after spinal cord injury in the rat. Pain. 2008;138:410–422. doi: 10.1016/j.pain.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rudolphi K., Park C.K., Rother M. Propentofylline (HWA 285), a neuroprotective glial cell modulator: pharmacologic profile. CNS Drug Rev. 1997;3:260–277. [Google Scholar]
- 101.Jacobs V.L., Landry R.P., Liu Y., Romero-Sandoval E.A., De Leo J.A. Propentofylline decreases tumor growth in a rodent model of glioblastoma multiforme by a direct mechanism on microglia. Neuro Oncol. 2012;14:119–131. doi: 10.1093/neuonc/nor194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stefanovich V. Effect of propentofylline on cerebral metabolism of rats. Drug Dev. Res. 1985;6:327–338. [Google Scholar]
- 103.Deleo J., Schubert P., Kreutzberg G.W. Protection against ischemic brain damage using propentofylline in gerbils. Stock. 1987;19:1535–1539. doi: 10.1161/01.str.19.12.1535. [DOI] [PubMed] [Google Scholar]
- 104.Dini F.L., Doxofylline Cogo R. A new generation xanthine bronchodilator devoid of major cardiovascular adverse effects. Curr. Med. Res. Opin. 2000;16:258–268. doi: 10.1185/030079901750120196. [DOI] [PubMed] [Google Scholar]
- 105.Gupta A., Yadav V., Yadav J.S., Rawat S. An analytical approach of doxofylline: a review. Asian J. Pharm. Anal. 2011;1:67–70. [Google Scholar]
- 106.van Mastbergen J., Jolas T., Allegra L., Page C.P. The mechanism of action of doxofylline is unrelated to HDAC inhibition, PDE inhibition or adenosine receptor antagonism. Pulm. Pharmacol. Ther. 2012;25:55–61. doi: 10.1016/j.pupt.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 107.Wu B.-N., Lin R.-J., Lin C.-Y., Shen K.-P., Chiang L.-C., Chen I.-J. A xanthine-based KMUP-1 with cyclic GMP enhancing and K + channels opening activities in rat aortic smooth muscle. Br. J. Pharmacol. 2001;134:265–274. doi: 10.1038/sj.bjp.0704231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin R.-J., Wu B.-N., Shen K.-P., Huang C.-H., Liu Z.-I., Lin C.-Y. Xanthine-analog, KMUP-2, enhances cyclic GMP and K+ channel activities in rabbit aorta and corpus cavernosum with associated penile erection. Drug Dev. Res. 2002;55:162–172. [Google Scholar]
- 109.Liu C.-P., Yeh J.-L., Wu B.-N., Chai C.-Y., Chen I.-J., Lai W.-T. KMUP-3 attenuates ventricular remodelling after myocardial infarction through eNOS enhancement and restoration of MMP-9/TIMP-1 balance. Br. J. Pharmacol. 2011;162:126–135. doi: 10.1111/j.1476-5381.2010.01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.I-J Chen. Processes for preparing piperazinium salts of KMUP and use thereof. United States Pat US 8,470 2010;805.
- 111.Liu C.-P., Yeh J.-L., Liou S.-F., Wu B.-N., Chen I.-J. Phosphodiesterase inhibitor KMUP-3 displays cardioprotection via protein kinase G and increases cardiac output via G-protein-coupled receptor agonist activity and Ca2+ sensitization. Kaohsiung J. Med. Sci. 2016;32:55–67. doi: 10.1016/j.kjms.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu B.-N., Chen I.-C., Lin R.-J., Chiu C.-C., An L.-M., Chen I.-J. Aortic smooth muscle relaxants KMUP-3 and KMUP-4, two nitrophenylpiperazine derivatives of xanthine, display cGMP-enhancing activity. J. Cardiovasc. Pharmacol. 2005;46:600–608. doi: 10.1097/01.fjc.0000180900.32489.f9. [DOI] [PubMed] [Google Scholar]
- 113.Liou S.-F., Hsu J.-H., Lin I.-L., Ho M.-L., Hsu P.-C., Chen L.-W. KMUP-1 suppresses RANKL-induced osteoclastogenesis and prevents ovariectomy-induced bone loss: roles of MAPKs, Akt, NF-κB and Calcium/Calcineurin/NFATc1 Pathways. PLoS One. 2013;8 doi: 10.1371/journal.pone.0069468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chung H.-H., Dai Z.-K., Wu B.-N., Yeh J.-L., Chai C.-Y., Chu K.-S. The xanthine derivative KMUP-1 inhibits models of pulmonary artery hypertension via increased NO and cGMP-dependent inhibition of RhoA/Rho kinase. Br. J. Pharmacol. 2010;160:971–986. doi: 10.1111/j.1476-5381.2010.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hong T.Y., Guh J.Y., Wu B.N., Chai C.Y., Huang H.T., Chen I.J. KMUP-1 protects kidney from streptozotocin-induced pro-inflammation in early diabetic nephropathy by restoring enos/pparγ and inhibiting MMP-9. Eur. J. Inflamm. 2014;12:89–100. [Google Scholar]
- 116.Yeh J.-L., Liu C.-P., Hsu J.-H., Tseng C.-J., Wu P.-J., Wang Y.-Y. KMUP-1 inhibits hypertension-induced left ventricular hypertrophy through regulation of nitric oxide synthases, ERK1/2, and calcineurin. Kaohsiung J. Med. Sci. 2012;28:567–576. doi: 10.1016/j.kjms.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cushley M.J., Holgate S.T. Bronchodilator actions of xanthine derivatives administered by inhalation in asthma. Thorax. 1985;40:176–179. doi: 10.1136/thx.40.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zuidema J., Merkus F.W.H.M. Pharmacokinetics and pharmacodynamics of diprophylline. Pharm. Weekbl. - Sci. Ed. 1981;3:1320–1325. [Google Scholar]
- 119.Hasegawa T., Nadai M., Apichartpichean R., Muraoka I., Nabeshima T., Takagi K. Pharmacokinetic characteristics of N7-substituted theophylline derivatives and their interaction with quinolone in rats. J. Pharm. Sci. 1991;80:962–965. doi: 10.1002/jps.2600801012. [DOI] [PubMed] [Google Scholar]
- 120.Cosio B.G., Tsaprouni L., Ito K., Jazrawi E., Adcock I.M., Barnes P.J. Theophylline restores histone deacetylase activity and steroid responses in COPD macrophages. J. Exp. Med. 2004;200:689–695. doi: 10.1084/jem.20040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ito K., Caramori G., Lim S., Oates T., Chung K.F., Barnes P.J. Expression and activity of histone deacetylases in human asthmatic airways. Am. J. Respir. Crit. Care Med. 2002;166:392–396. doi: 10.1164/rccm.2110060. [DOI] [PubMed] [Google Scholar]
- 122.Grome J.J., Hofmann W., Gojowczyk G., Stefanovich V. Effects of a xanthine derivative, propentofylline, on local cerebral blood flow and glucose utilization in the rat. Brain Res. 1996;740:41–46. doi: 10.1016/s0006-8993(96)00353-8. [DOI] [PubMed] [Google Scholar]
- 123.Mielke R., Kittner B., Ghaemi M., Kessler J., Szelies B., Herholz K. Propentofylline improves regional cerebral glucose metabolism and neuropsychologic performance in vascular dementia. J. Neurol. Sci. 1996;141:59–64. doi: 10.1016/0022-510x(96)00127-x. [DOI] [PubMed] [Google Scholar]
- 124.Müller C.E., Jacobson K.A. Xanthines as adenosine receptor antagonists. NIH Publ. Access. 2011 doi: 10.1007/978-3-642-13443-2_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Haghgoo S., Hasegawa T., Nadai M., Wang L., Ishigaki T., Miyamoto K.-I. Brain distribution characteristics of xanthine derivatives and relation to their locomotor activity in mice. J. Pharm. Pharmacol. 1995;47:412–419. doi: 10.1111/j.2042-7158.1995.tb05821.x. [DOI] [PubMed] [Google Scholar]
- 126.Spina D., Page C.P. Handb. Exp. Pharmacol. Springer; Cham: 2016. Xanthines and Phosphodiesterase Inhibitors; pp. 63–91. [DOI] [PubMed] [Google Scholar]
- 127.Zainab S., Djafarian K. Coffee consumption and coronary heart diseases: a mini-review. J. Clin. Nutr. Diet. 2016;2:1–7. [Google Scholar]
- 128.Osswald H., Schnermann J. Springer Berlin Heidelberg; 2011. Methylxanthines and the Kidney; pp. 391–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rieg T., Steigele H., Schnermann J., Richter K., Osswald H., Vallon V. Requirement of intact adenosine A1 receptors for the diuretic and natriuretic action of the methylxanthines theophylline and caffeine. J. Pharmacol. Exp. Ther. 2005;313:403–409. doi: 10.1124/jpet.104.080432. [DOI] [PubMed] [Google Scholar]
- 130.Bhatia M.S., Waghmare V.S., Choudhari P.B., Kumbhar S.S. Synthesis and biological activity of xanthene derivatives as antiasthamatic agents. RGUHS J. Pharm. Sci. 2016;6:26–31. [Google Scholar]
- 131.Lin R.J., Wu B.N., Lo Y.C., An L.M., Dai Z.K., Lin Y.T. A xanthine-based epithelium-dependent airway relaxant KMUP-3 (7-[2-[4-(4-nitrobenzene)piperazinyl]ethyl]-1,3-dimethylxanthine) increases respiratory performance and protects against tumor necrosis factor-alpha-induced tracheal contraction, involving nitri. J. Pharmacol. Exp. Ther. 2006;316:709–717. doi: 10.1124/jpet.105.092171. [DOI] [PubMed] [Google Scholar]
- 132.Lazzaroni M., Grossi E., Porro G.B. The effect of intravenous doxofylline or aminophylline on gastric secretion in duodenal ulcer patients. Aliment Pharmacol. Ther. 2007;4:643–649. doi: 10.1111/j.1365-2036.1990.tb00512.x. [DOI] [PubMed] [Google Scholar]
- 133.Sachdeva S., Gupta M. Adenosine and its receptors as therapeutic targets: an overview. Saudi Pharm. J. 2013;21:245–253. doi: 10.1016/j.jsps.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.St. Hilaire C., Carroll S.H., Chen H., Ravid K. Mechanisms of induction of adenosine receptor genes and its functional significance. J. Cell. Physiol. 2009;218:35–44. doi: 10.1002/jcp.21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Franchetti P., Messini L., Cappellacci L., Grifantini M., Lucacchini A., Martini C. 8-Azaxanthine derivatives as antagonists of adenosine receptors. J. Med. Chem. 1994;37:2970–2975. doi: 10.1021/jm00044a018. [DOI] [PubMed] [Google Scholar]
- 136.Jacobson K.A., Gao Z.-G. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.van Galen P.J.M., Stiles G.L., Michaels G., Jacobson K.A. Adenosine A1 and A2 receptors: structure–function relationships. Med. Res. Rev. 1992;12:423–471. doi: 10.1002/med.2610120502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Francis S.H., Blount M.A., Corbin J.D. Mammalian cyclic nucleotide phosphodiesterases: molecular mechanisms and physiological functions. Physiol. Rev. 2011;91:651–690. doi: 10.1152/physrev.00030.2010. [DOI] [PubMed] [Google Scholar]
- 139.Mehats C., Andersen C.B., Filopanti M., Jin S.-L.C., Conti M. Cyclic nucleotide phosphodiesterases and their role in endocrine cell signaling. Trends Endocrinol. Metab. 2002;13:29–35. doi: 10.1016/s1043-2760(01)00523-9. [DOI] [PubMed] [Google Scholar]
- 140.Omori K., Kotera J. Overview of PDEs and their regulation. Circ. Res. 2007;100:309–327. doi: 10.1161/01.RES.0000256354.95791.f1. [DOI] [PubMed] [Google Scholar]
- 141.Ke H., Wang H., Ye M. Structural insight into the substrate specificity of phosphodiesterases. Handb. Exp. Pharmacol. 2011:121–134. doi: 10.1007/978-3-642-17969-3_4. [DOI] [PubMed] [Google Scholar]
- 142.Maurice D.H., Ke H., Ahmad F., Wang Y., Chung J., Manganiello V.C. Advances in targeting cyclic nucleotide phosphodiesterases. Nat. Rev. Drug Discov. 2014;13:290–314. doi: 10.1038/nrd4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shao Y., Huang M., Cui W., Feng L., Wu Y., Cai Y. 2014. Discovery of a Phosphodiesterase 9A Inhibitor as a Potential Hypoglycemic Agent. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Andreeva S.G., Dikkes P., Epstein P.M., Rosenberg P.A. Expression of cGMP-specific phosphodiesterase 9A mRNA in the rat brain. J. Neurosci. 2001;21:9068–9076. doi: 10.1523/JNEUROSCI.21-22-09068.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Singh N., Patra S. Phosphodiesterase 9: insights from protein structure and role in therapeutics. Life Sci. 2014;106:1–11. doi: 10.1016/j.lfs.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 146.Boswell-Smith V., Cazzola M., Page C.P. Are phosphodiesterase 4 inhibitors just more theophylline? J. Allergy Clin. Immunol. 2006;117:1237–1243. doi: 10.1016/j.jaci.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 147.Yano Y., Yoshida M., Hoshino S., Inoue K., Kida H., Yanagita M. Anti-fibrotic effects of theophylline on lung fibroblasts. Biochem. Biophys. Res. Commun. 2006;341:684–690. doi: 10.1016/j.bbrc.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 148.Hosseinzadeh H., Fazly Bazzaz B.S., Moaddab Sadati M. In vitro evaluation of methylxanthines and some antibiotics: interaction against Staphylococcus aureus and Pseudomonas aeruginosa. Iran. Biomed. J. 2006;10:163–167. [Google Scholar]
- 149.Peter T., Barnes J. Theophylline. Pharmaceuticals. 2010;3:725–747. doi: 10.3390/ph3030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Katherine Aleksandrova, Belenichev Igor, Alexander Shkoda, Levich Sergey, Yurchenko Darja, Buchtiyarova Nina. Research of antioxidant properties of theophyllinyl-7-acetic acid derivatives. Med. Sci. 2014;3:187–194. [Google Scholar]
- 151.Vignoli J.A., Bassoli D.G., Benassi M.T. Antioxidant activity, polyphenols, caffeine and melanoidins in soluble coffee: the influence of processing conditions and raw material. Food Chem. 2011;124:863–868. [Google Scholar]
- 152.Yashin A., Yashin Y., Wang J.Y., Nemzer B. Antioxidant and antiradical activity of coffee. Antioxidants. 2013;2:230–245. doi: 10.3390/antiox2040230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sadzuka Y., Iwazaki A., Miyagishima A., Nozawa Y., Hirota S. Effects of methylxanthine derivatives on adriamycin concentration and antitumor activity. Jpn. J. Cancer Res. 1995;86:594–599. doi: 10.1111/j.1349-7006.1995.tb02439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li F., Zheng C., Xin J., Chen F., Ling H., Sun L. Enhanced tumor delivery and antitumor response of doxorubicin-loaded albumin nanoparticles formulated based on a Schiff base. Int. J. Nanomed. 2016;11:3875–3890. doi: 10.2147/IJN.S108689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang Y., Yu J., Zhang L., Cai J., Cai D., Lv C. Enhanced anti-tumor effects of doxorubicin on glioma by entrapping in polybutylcyanoacrylate nanoparticles. Tumor Biol. 2016;37:2703–2708. doi: 10.1007/s13277-015-4106-7. [DOI] [PubMed] [Google Scholar]
- 156.Hinze H.-J. Vieweg+Teubner Verlag; Methylxanthine, Wiesbaden: 1982. Analytic and pharmacokinetic data of natural methylxanthines and of the xanthine derivative pentoxifylline. Theophylline other Methylxanthines/Theophyllin und And; pp. 55–61. [Google Scholar]
- 157.Bruns R.F., Fergus J.H. Solubilities of adenosine antagonists determined by radioreceptor assay. J. Pharm. Pharmacol. 1989;41:590–594. doi: 10.1111/j.2042-7158.1989.tb06537.x. [DOI] [PubMed] [Google Scholar]
- 158.Andrei K. 2011. Solubility and Physical Stability Improvement of Natural Xanthine Derivates. [Google Scholar]
- 159.Aslaksen A., Bakke O., Vigander T. Comparative pharmacokinetics of theophylline and aminophylline in man. Br. J. Clin. Pharmacol. 1981;11:269–273. doi: 10.1111/j.1365-2125.1981.tb00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fredholm B.B., Jacobson K.A., Jonzon B., Kirk K.L., Li Y.O., Daly J.W. Evidence that a Novel 8-phenyl–substituted xanthine derivative is a cardioselective adenosine receptor antagonist in Vivo. J. Cardiovasc. Pharmacol. 1987;9:396–400. doi: 10.1097/00005344-198704000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chappe V., Mettey Y., Vierfond J.M., Hanrahan J.W., Gola M., Verrier B. Structural basis for specificity and potency of xanthine derivatives as activators of the CFTR chloride channel. Br. J. Pharmacol. 1998;123:683–693. doi: 10.1038/sj.bjp.0701648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Takagi K., Hasegawa T., Kuzuya T., Ogawa K., Watanabe T., Satake T. Structure-activity relationship in N3-alkyl-xanthine derivatives. Jpn. J. Pharmacol. 1988;46:373–378. doi: 10.1254/jjp.46.373. [DOI] [PubMed] [Google Scholar]
- 163.Miyamoto K., Kurita M., Ohmae S., Sakai R., Sanae F., Takagi K. Selective tracheal relaxation and phosphodiesterase-IV inhibition by xanthine derivatives. Eur. J. Pharmacol. 1994;267:317–322. doi: 10.1016/0922-4106(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 164.Schwabe U., Ukena D., Lohse M.J. Xanthine derivatives as antagonists at A1 and A2 adenosine receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 1985;330:212–221. doi: 10.1007/BF00572436. [DOI] [PubMed] [Google Scholar]
- 165.Abdulrahman L.K., Al-Mousilly M.M., Al-Halaibeh T.S., Al-Azzawii K.M. International research journal of pharmacy synthesis of new 1, 3, 7, 8-tetrasubstituted xanthenes analogue. Int. Res. J. Pharm. 2012;3:83–85. [Google Scholar]
- 166.Laddha S.S., Wadodkar S.G., Meghal S.K. CAMP-dependent phosphodiesterase inhibition and SAR studies on novel 6,8-disubstituted 2-phenyl-3-(substituted benzothiazole-2-yl)-4[3H]- quinazolinone. Med. Chem. Res. 2009;18:268–276. [Google Scholar]
- 167.Kadi A.A., El-Tahir K.E.H., Jahng Y., Rahman A.F.M.M. Synthesis, biological evaluation and Structure Activity Relationships (SARs) study of 8-(substituted)aryloxycaffeine. Arab J. Chem. 2015 [Google Scholar]
- 168.Jacobson K.A., de la Cruz R., Schulick R., Kiriasis L., Padgett W., Pfleiderer W. 8-Substituted xanthines as antagonists at A1- and A2-adenosine receptors. Biochem. Pharmacol. 1988;37:3653–3661. doi: 10.1016/0006-2952(88)90398-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rogozin E.A., Lee K.W., Kang N.J., Yu H., Nomura M., Miyamoto K. Inhibitory effects of caffeine analogues on neoplastic transformation structure–activity relationship.pdf. Carcinogenesis. 2008;29:1228–1234. doi: 10.1093/carcin/bgn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Daly J.W., Hong O., Padgett W.L., Shamim M.T., Jacobson K.A., Ukena D. Non-xanthine heterocycles: activity as antagonists of A1 and A2-adenosine receptors. Biochem. Pharmacol. 1988;37:655–664. doi: 10.1016/0006-2952(88)90139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schneller S.W., Ibay A.C., Christ W.J., Bruns R.F. Linear and proximal benzo-separated alkylated xanthines as adenosine-receptor antagonists. J. Med. Chem. 1989;32:2247–2254. doi: 10.1021/jm00130a004. [DOI] [PubMed] [Google Scholar]
- 172.Daly J.W., Hide I., Bridson P.K. Imidazodiazepinediones: a new class of adenosine receptor antagonists. J. Med. Chem. 1990;33:2818–2821. doi: 10.1021/jm00172a022. [DOI] [PubMed] [Google Scholar]
- 173.Ivanov E.I., Polishchuk A.A., Kalayanov G.D. Synthesis of crown-containing xanthine derivatives. Chem. Heterocycl. Comp. 1992;28:1266–1269. [Google Scholar]
- 174.Elzein E., Kalla R.V., Li X., Perry T., Gimbel A., Zeng D. Discovery of a novel A2B adenosine receptor antagonist as a clinical candidate for chronic inflammatory airway diseases. J. Med. Chem. 2008;51:2267–2278. doi: 10.1021/jm7014815. [DOI] [PubMed] [Google Scholar]
- 175.Soriano A., Ventura R., Molero A., Hoen R., Casado V., Corte A. Adenosine A2A receptor-antagonist/dopamine D2 receptor-agonist bivalent ligands as pharmacological tools to detect A 2A-D2 receptor heteromers. J. Med. Chem. 2009;52:5590–5602. doi: 10.1021/jm900298c. [DOI] [PubMed] [Google Scholar]