Abstract
Background and aims
This study aimed to evaluate the association between the severity of pathological gambling, serum brain-derived neurotrophic factor (BDNF) level, and the characteristics of quantitative electroencephalography (EEG) in patients with gambling disorder.
Methods
A total of 55 male patients aged 18–65 with gambling disorder participated. The severity of pathological gambling was assessed with the nine-item Problem Gambling Severity Index from the Canadian Problem Gambling Index (CPGI-PGSI). The Beck Depression Inventory and Lubben Social Network Scale were also assessed. Serum BDNF levels were assessed from blood samples. The resting-state EEG was recorded while the eyes were closed, and the absolute power of five frequency bands was analyzed: delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–30 Hz), and gamma (30–50 Hz).
Results
Serum BDNF level was positively correlated with theta power in the right parietal region (P4, r = .403, p = .011), beta power in the right parietal region (P4, r = .456, p = .010), and beta power in the right temporal region (T8, r = .421, p = .008). Gambling severity (CPGI-PGSI) was positively correlated with absolute beta power in the left frontal region (F7, r = .284, p = .043) and central region [(C3, r = .292, p = .038), (C4, r = .304, p = .030)].
Conclusions
These findings support the hypothesis that right-dominant lateralized correlations between BDNF and beta and theta power reflect right-dominant brain activation in addiction. The positive correlations between beta power and the severity of gambling disorder may be associated with hyperexcitability and increased cravings. These findings contribute to a better understanding of brain-based electrophysiological changes and BDNF levels in patients with pathological gambling.
Keywords: quantitative electroencephalography, resting state, BDNF, gambling disorder
Introduction
Gambling is a popular leisure enjoyed by 60%–90% of people at least once in their lives, pathological gambling results in health, financial, and social problems (Wölfling et al., 2011). Gambling disorder is defined by persistent and recurrent maladaptive gambling behavior that disrupts personal, family, and vocational pursuits (American Psychiatric Association [APA], 2013). The lifetime prevalence of gambling disorder is 0.4%–1.0% (APA, 2013). Gambling disorder is known to be highly comorbid with other psychiatric disorders, such as substance-use disorder, depressive disorder, and anxiety disorder (APA, 2013; Lorains, Cowlishaw, & Thomas, 2011).
Brain-derived neurotrophic factor (BDNF) is an important regulator of neural survival, development, function, and plasticity (Huang & Reichardt, 2001). BDNF is highly expressed in limbic structures and the cerebral cortex, and plays an important role in learning, memory, and reward-related processes (Boulle et al., 2012; Yamada & Nabeshima, 2003). It is associated with multiple mental disorders, including depression, anxiety, schizophrenia, and addiction (Boulle et al., 2012; Li & Wolf, 2015). Several studies have reported that BDNF is elevated in patients with pathological gambling (Angelucci et al., 2013; Choi et al., 2016; Geisel, Panneck, Hellweg, Wiedemann, & Müller, 2015). Researchers have suggested that the increased BDNF in pathological gamblers is related to altered dopaminergic transmission in the ventral tegmental area and nucleus accumbens, which are central components of the brain’s reward system (Geisel, Banas, Hellweg, & Müller, 2012; Pu, Liu, & Poo, 2006).
Quantitative electroencephalography (qEEG) involves power spectral analysis of frequency bands, such as delta (1–4 Hz), theta (5–7 Hz), alpha (8–13 Hz), and beta (14–30 Hz) (Houston & Ceballos, 2013). Pathological gamblers show dysfunctional EEG activity, especially in the frontoparietal area (Quintero, 2017). Some studies of the EEG correlates of gambling behavior have focused on reward sensitivity and decision-making (Houston & Ceballos, 2013). For example, theta and delta activities are correlated with various aspects of the decision-making process (Houston & Ceballos, 2013). Massar, Rossi, Schutter, and Kenemans (2012) reported that an increased theta–beta ratio in the resting-state EEG to be associated with risky or disadvantageous decision-making in the Iowa Gambling Task. Amoss (2009) reported that beta power asymmetry was associated with the number of risky decision-making behaviors.
Gambling disorder is closely related to impulsivity, which is a core feature of addictive disorders. In spite of some inconsistent findings (Lee et al., 2017), studies of EEG correlates in impulsive disorders, such as attention-deficit/hyperactivity disorder, substance-use disorder, and violence, reported relatively consistent findings of increases in the delta, theta, and beta bands (Kamarajan & Porjesz, 2012). Increased power of slow waves, such as the delta and theta bands, suggests low cortical arousal, whereas increased power in the beta band suggests hyperexcitability of the central nervous system.
A relationship between BDNF and qEEG has been described in patients with depressive disorder (Gatt et al., 2008; Zoon et al., 2013). However, there has been no study of the association between BDNF and qEEG power specifically in patients with gambling disorder. Thus, this study aimed to investigate the associations between serum BDNF level, qEEG power distribution, and the severity of pathological gambling in patients with gambling disorder.
Methods
Subjects
Individuals who visited the gambling disorder clinic were considered for inclusion in the study. The inclusion criteria were a diagnosis of gambling disorder, according to the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria, male sex, and age between 18 and 65 years. Subjects were excluded if any of the following applied: a diagnosis of substance-use disorder other than nicotine or caffeine, based on the DSM-5 criteria; use of psychotropic medications over the previous year; and presence of a physical, mental, or neurological disorder other than gambling disorder. Based on the inclusion and exclusion criteria, 55 male subjects (age: 38.42 ± 11.59 years) were enrolled in the study. None of the participants were taken any medications and all had completed at least 12 years of education (mean: 14.65 ± 1.89 years).
Measures
To assess serum BDNF level, a total of 10 ml of blood was drawn from each subject and placed in a serum separator tube. For analysis of serum, samples were allowed to clot for 30 min at room temperature before centrifugation for 15 min at approximately 1,000 × g, after which the serum was removed. All samples were stored at −80 °C. Serum BDNF levels were determined using an enzyme-linked immunosorbent assay protocol according to the manufacturer’s instructions (DBD00, R&D Systems, Europe). The BDNF level values were normally distributed, as verified by the Kolmogorov–Smirnov test.
The nine-item Problem Gambling Severity Index (PGSI) from the Canadian Problem Gambling Index (CPGI) (CPGI-PGSI) was selected to quantify gambling severity (Ferris & Wynne, 2001). The PGSI was used to assess problematic gambling behavior and adverse consequences during the previous 12 months. The response choices for each PGSI item are “never,” “sometimes,” “most of the time,” and “almost always,” with the total score ranging from 0 to 27. Participants were categorized as “non-problem-gamblers” (PGSI = 0), “low-risk” (PGSI = 1–2), “moderate-risk” (PGSI = 3–7), or “problem-gambler” (PGSI > 7). The psychometric properties of the PGSI have been examined in the Korean population; Cronbach’s α value was .94 (Kim, Cha, Kwon, & Lee, 2011). Beck Depression Inventory (BDI) and Lubben Social Network Scale were also used to assess the depressive mood and social activity of the participants.
EEG recording and preprocessing
The EEG recordings were performed using a SynAmps2 direct-current (DC) amplifier and a 10–20 layout 64-channel Quick-Cap electrode-placement system (Neuroscan Inc., NC, USA). The EEG data were digitally recorded from 19 gold cup electrodes placed according to the international 10–20 system. The impedances were maintained below 5 kΩ, and the sampling rate was 1,000 Hz. We used a linked-mastoid reference and two additional bipolar electrodes to measure horizontal and vertical eye movements. During the recordings, each participant laid down in a semi-darkened, electrically shielded, sound-attenuated room. Resting EEG samples were recorded after 3 min with the participant’s eyes closed.
We used Matlab 7.0.1 (Math Works, Natick, MA, USA) and the EEGLAB toolbox (Delorme & Makeig, 2004) to preprocess and analyze the EEG recordings. First, the EEG data were downsampled to 250 Hz. Next, the EEG data were detrended and mean-subtracted to remove the DC component. A 1-Hz high-pass filter and a 60-Hz notch filter were applied to remove eye movement artifacts and electrical noise. Independent component analysis (ICA) was then performed to remove well-defined sources of artifacts. ICA has been demonstrated to reliably isolate artifacts caused by eye and muscle movements and heart noise (Jung et al., 2000). Finally, clinical psychiatrists and EEG experts visually inspected the corrected EEGs. For the analysis, we selected at least 2 min of artifact-free EEG data from the 3-min recordings.
EEG analysis
Four frequency bands were defined for further analysis: delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), and beta (12–30 Hz). We investigated the power spectra of the EEG data from each subject using the short-time-interval Fourier transform “spectrogram.m” function from the Signal Processing Toolbox in MATLAB. Time windows of 1,000 ms with an 800-ms overlap and Hamming window were used for the spectral analysis. Outliers that were far from the spectral value distribution for each frequency band, at the 0.05 significance level, were removed. Finally, the absolute power for each frequency band was averaged over all the time windows for further analysis.
Statistical analysis
The MATLAB 7.0.1 Statistical Toolbox was used for the statistical analyses. All values were expressed as the mean and standard deviation (SD). To assess the relationship between gambling severity (CPGI-PGSI) and EEG recordings, we used a Pearson’s partial correlation analysis that controlled for age, education, BDI score, and Beck Anxiety Inventory (BAI) score. To assess the relationship between BDNF and the EEG recordings, we used the same analytic method. Statistical significance was defined as p < .05. To control for false positives from multiple comparisons, we used a false discovery rate (FDR) correction in which the p values were multiplied by the number of comparisons (Benjamini & Hochberg, 1995).
Ethics
The study procedure was performed in accordance with the Declaration of Helsinki (World Medical Association, 1964). The study protocols were approved by the Regional Ethical Review Board in Seoul. All participants gave written informed consent after receiving a complete description of the study and were not compensated for taking part in the study.
Results
Demographic and clinical characteristics
The total sample comprised 55 male individuals with gambling disorder [mean age (SD) = 38.42 (11.59) years]. The participants showed clinically higher scores compared with the general population on the BDI [mean (SD) = 16.64 (9.42)] and CPGI-PGIS [mean (SD) = 18.87 (6.18)] (Kim et al., 2011; Lasa, Ayuso-Mateos, Vazquez-Barquero, Dıez-Manrique, & Dowrick, 2000). Their scores on the Lubben Social Network Scale [mean (SD) = 24.71 (5.35)] were low level and reflected a limited social network (Lee et al., 2009). The demographic and clinical characteristics are summarized in Table 1.
Table 1.
Demographic and clinical characteristics of subjects
| Mean ± SD | GD (n = 55) |
|---|---|
| Age (years) | 38.42 ± 11.59 |
| Education (years) | 14.65 ± 1.89 |
| BDNF (pg/ml) | 32,177.69 ± 9,969.95 |
| BDI | 16.64 ± 9.42 |
| BAI | 12.58 ± 9.68 |
| BIS | 56.42 ± 8.28 |
| LSNS | 24.71 ± 5.35 |
| WURS | 30.07 ± 15.80 |
| CPGI-PGIS | 18.87 ± 6.18 |
| RTCQ | |
| Precontemplation | 2.09 ± 2.47 |
| Contemplation | 5.67 ± 2.04 |
| Action | 4.23 ± 2.58 |
Note. SD: standard deviation; GD: gambling disorder; BDNF: brain-derived neurotrophic factor; BDI: Beck Depression Inventory; BAI: Beck Anxiety Inventory; BIS: The Korean version of Barratt Impulsiveness Scale; LSNS: Lubben Social Network Scale; WURS: Wender–Utah Rating Scale; CPGI-PGIS: Canadian Problem Gambling Index–Problem Gambling Severity Index; RTCQ: Readiness To Change Questionnaire.
Correlation between serum BDNF level and absolute power of resting EEG
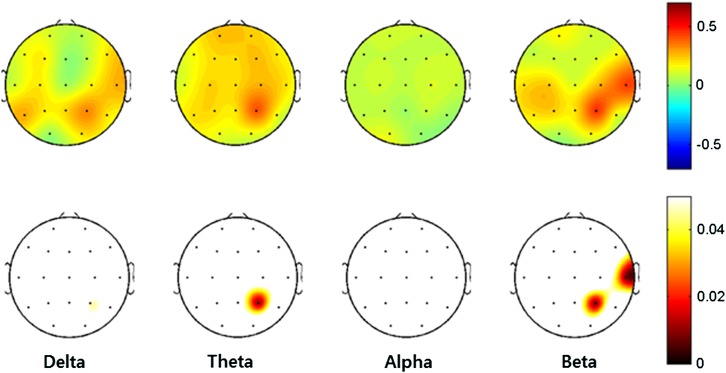
To investigate whether the serum BDNF levels were related to the absolute power of the resting EEG, we examined partial correlations corrected for age, education, BDI, and BAI. The partial correlation analysis for the absolute delta and alpha power and the serum BDNF levels showed no significant correlations, after imposing the FDR correction. The serum BDNF level was positively correlated with theta power in the right parietal region (P4, r = .403, p = .011), beta power in the right parietal region (P4, r = .456, p = .010), and beta power in the right temporal region (T8, r = .421, p = .008). All these significance assessments include FDR corrections (corrected p < .05). A topographical representation of the Pearson’s partial correlation coefficients and the associated p values is presented in Figure 1.
Figure 1.
Topographical representations of the Pearson’s partial correlations, corrected for age, education, BDI, and BAI, between the absolute powers and the serum BDNF levels. Scales show uV2 for absolute power. Red represents higher values and blue represents lower values. The upper topography denotes the Pearson’s partial correlation coefficients, and the lower topography denotes the p values after adjusting for the false discovery rate (corrected p < .05). BDI: Beck Depression Inventory; BAI: Beck Anxiety Inventory; BDNF: brain-derived neurotrophic factor
Correlation between gambling severity and absolute power of resting EEG
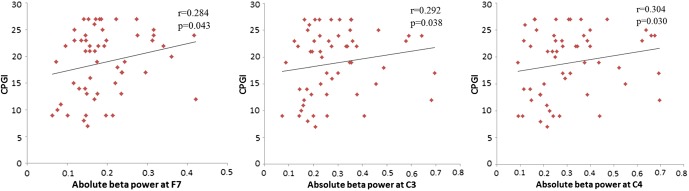
The partial correlation analysis, controlled for age, education, BDI, and BAI, for the absolute delta, theta, and alpha power and gambling severity (CPGI-PGSI) showed no significant relationships. However, gambling severity (CPGI-PGSI) was positively correlated with absolute beta power. The significant findings appeared in the left frontal region (F7, r = .284, p = .043) and central region [(C3, r = .292, p = .038), (C4, r = .304, p = .030)]. All significant differences were FDR-corrected (corrected p < .05). Scatter plots of the three significant (p < .01) partial correlations are presented in Figure 2.
Figure 2.
Correlation of the absolute beta power at the F7, C3, and C4 electrodes site with scores on the Canadian Problem Gambling Index (CPGI) in patients with gambling disorder
Discussion
This study investigated associations between the severity of pathological gambling, serum BDNF level, and the characteristics of qEEG power in patients with gambling disorder. Several previous studies have reported an association between BDNF and qEEG power. Gatt et al. (2008) reported that the BDNF Val66Met gene polymorphism was associated with EEG power in depressive patients: carriers of the methionine (Met) variant, who show phenotypic characteristics of depression, presented elevated relative theta and delta power coupled with reduced alpha power compared to carriers of the valine (Val) variant. Zoon et al. (2013) also reported that parietal–occipital alpha power was negatively associated with depression severity in depressive patients, and those Met/Met depressive patients showed lower global absolute alpha power compared to Val carriers.
However, those studies of the association between BDNF and qEEG were performed with depressive patients. In addition, those studies investigated the association between EEG power and BDNF gene polymorphism. Notably, a meta-analysis indicated that the BDNF Val66Met variant is not associated with serum BDNF level (Terracciano et al., 2013). This is the first study of the association between BDNF and qEEG power in patients with gambling disorder. Furthermore, we investigated the associations of qEEG band power and BDNF in terms of the serum BDNF level, not in terms of BDNF gene polymorphism. We identified significant positive correlations between serum BDNF level and beta and theta power in the right parietal and temporal regions of the gambling disorder patients.
BDNF is known to be involved in learning and memory, which are the functions of the hippocampus (Yamada & Nabeshima, 2003). Some researchers have reported that the BDNF Val66Met polymorphism is associated with the volume of the hippocampal formation (Pezawas et al., 2004; Szeszko et al., 2005). Grunwald, Hensel, Wolf, Weiss, and Gertz (2007) reported that an increase in EEG theta power was associated with decreased hippocampal volume. Thus, it is plausible that the correlations between BDNF and EEG power in the temporal and parietal lobes, which were identified in this study, might be associated with the anatomical location of the hippocampus. However, this explanation requires confirmation by further studies using MRI data.
With regard to the EEG asymmetry, multiple studies have reported a right-dominant resting frontal EEG asymmetry in alpha power in relation to depression and anxiety (Thibodeau, Jorgensen, & Kim, 2006). Bulgin et al. (2008) reported that theta EEG asymmetry in parietal brain regions was affected by the BDNF Val66Met polymorphism in patients with childhood-onset mood disorder. In a functional MRI study of the laterality of brain activation in addictive disorders, Gordon (2016) reported right dominance of brain activation for impulsivity tasks and left dominance for cue-induced craving in most addictive disorders, including addictions to cocaine, heroin, and food. However, cue-induced craving for Internet gaming, which is a behavioral addiction comparable to gambling, showed right-dominated brain activation. The right-favoring lateralized correlations between BDNF and beta and theta power identified in this study reflect consistent findings, which should be confirmed by further studies.
Another main finding of this study is the positive correlation between beta activity and the severity of pathological gambling, as measured by the CPGI-PGSI. The high-frequency bands (beta and gamma) are involved in local, short-range neural communication (Von Stein & Sarnthein, 2000), and increased beta power is considered to indicate hyperexcitability in the brain (Rangaswamy et al., 2002). Several psychiatric disorders characterized by impulsivity and hyperarousal, including intermittent explosive disorder and substance-use disorders, were reported to be associated with increased beta activity (Kamarajan & Porjesz, 2012). Herrera-Díaz et al. (2016) also reported increased absolute and relative beta power in alcohol-use disorder patients compared to controls.
Other researchers have focused on associations between craving and beta activity. Knott et al. (2008) reported that a scripted scene eliciting the desire to smoke-produced craving response and increased beta power in smokers. Arras (2017) reported that women with uncontrollable eating show increased reward sensitivity, which is correlated with elevated beta activity during the resting state. Tammela et al. (2010) reported that women with binge-eating showed higher resting-state beta power than women without binge-eating, and that pictures of food increased beta power in women both with and without binge-eating. Increased high beta power in response to positive feedback was also reported to be associated with activation in a largely subcortical network encompassing core areas of the reward network (Andreou et al., 2017).
It is plausible that the positive correlations between beta power and CPGI-PGSI identified in this study may be associated with hyperexcitability and increased craving in people with gambling disorders. Further studies are required to confirm these associations, including studies using other physiologic indices or experimental procedures to assess hyperexcitability and craving.
Interestingly, previous studies of Internet addiction, which is a behavioral addiction comparable to gambling disorder, have reported some inconsistent findings. An Internet gaming disorder group was reported to show lower absolute beta power than healthy control groups (Son et al., 2015). However, Park et al. (2017) reported that an Internet gaming disorder group showed increased gamma band (30–40 Hz) coherence compared to an alcohol-use disorder group and healthy controls. Choi et al. (2013) also reported that an Internet addiction group showed higher absolute power in the gamma band than controls, whereas the beta power of the Internet addiction group was lower than that of the controls. These reports may be consistent with the finding that increased activity in the higher frequency bands is associated with pathological hyperexcitability in behavioral addictions.
Notably, previous studies of serum BDNF levels in patients with gambling disorder have reported inconsistent findings. On one hand, the serum BDNF level has been reported to increase in patients with gambling disorder compared to healthy control (Choi et al., 2016; Geisel et al., 2012). On the other hand, although Choi et al. (2016) also reported a positive correlation between serum BDNF level and the severity of the gambling disorder, there was no significant correlation between BDNF serum level and the severity of pathological gambling in the study of Geisel et al. (2012). This study also did not find a significant correlation between serum BDNF level and the severity of pathological gambling.
Limitations
This study has several limitations that should be noted. First, this study did not include healthy controls and therefore does not allow comparisons between the patients and controls. A future case–control design is required to confirm our findings. Second, the age range of the participants was broad, that is, 18–65. The power of EEG frequency bands is known to vary according to age, although the changes in EEG power during adulthood are less remarkable than those in childhood (Dustman, Shearer, & Emmerson, 1999). The characteristics of gambling can also show age-dependent features (Kardos, Tóth, Boha, File, & Molnár, 2017). A future study with an age-matching design or narrow age span is warranted to overcome this limitation. Third, the severity of gambling was assessed by self-administered questionnaires alone. In addition, we did not collect information on the clinical history of gambling. Patients with gambling disorder often have poor insight, so more objective tools for the assessment of gambling severity and a thorough clinical history of the patients are required. Fourth, although qEEG could be affected by handedness, and studies for qEEG usually consider the handedness of the participants, we did not take handedness into account. However, only 4.2% of Koreans were reported to be left-handed in a study of Korean college students (Kang & Harris, 2000). Thus, the effect of handedness on the results of this study is expected to be minimal. Finally, only 19 electrodes were used in this study, which is not enough to evaluate topographical functions. To resolve topographical issues, EEG source reconstruction is required, but is not possible with the relatively small number of 19 channels. To compensate this limitation, previous studies have combined EEG with MRI or used more electrodes (Lei, Wang, Yuan, & Mantini, 2014; Mantini, Perrucci, Del Gratta, Romani, & Corbetta, 2007). Therefore, a future study with more electrodes is warranted.
Conclusions
This study extends the understanding of the associations between serum BDNF level, EEG characteristics in the resting state, and the severity of gambling disorder. The results showed a positive correlation between serum BDNF level and theta and beta power in the right temporoparietal region, and a positive correlation between the severity of pathological gambling and beta power in the frontal and central regions. The right-dominant lateralized correlations between BDNF and beta and theta power might reflect the right-dominant brain activation that is reported in most addictive disorders. The positive correlations between beta power and the severity of gambling disorder may be associated with hyperexcitability and increased cravings. These findings suggest the possibility of objective measurement of the severity of gambling disorder and neurophysiological state of the patients, and may lead to useful clinical information in the form of neurobiological markers for assessment and treatment planning for patients with gambling disorder.
Funding Statement
Funding sources: This work was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare, South Korea (no. A120157).
Authors’ contribution
All authors are accessible to the study data and responsible for preparing all contents of the article. All authors had authority over the decision to submit the manuscript for publication. KMK and S-WC contributed equally to this work.
Conflict of interest
The authors declare no conflict of interest.
References
- Amoss R. T. (2009). Frontal alpha and beta EEG power asymmetry and Iowa Gambling Task performance. Atlanta, GA: Georgia State University. [Google Scholar]
- Andreou C., Frielinghaus H., Rauh J., Mußmann M., Vauth S., Braun P., Leicht G., Mulert C. (2017). Theta and high-beta networks for feedback processing: A simultaneous EEG-fMRI study in healthy male subjects. Translational Psychiatry, 7(1), e1016. doi: 10.1038/tp.2016.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelucci F., Martinotti G., Gelfo F., Righino E., Conte G., Caltagirone C., Bria P., Ricci V. (2013). Enhanced BDNF serum levels in patients with severe pathological gambling. Addiction Biology, 18(4), 749–751. doi: 10.1111/j.1369-1600.2011.00411.x [DOI] [PubMed] [Google Scholar]
- Arras L. (2017). Reward sensitivity and self-control in uncontrolled eating: Analysis of EEG beta and theta dynamics. Tartu, Estonia: University of Tartu. [Google Scholar]
- American Psychiatric Association [APA]. (2013). Diagnostic and statistical manual of mental disorders (DSM-5). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 57(1), 289–300. doi: 10.2307/2346101 [DOI] [Google Scholar]
- Boulle F., van den Hove D. L., Jakob S. B., Rutten B. P., Hamon M., van Os J., Lesch K. P., Lanfumey L., Steinbusch H. W., Kenis G. (2012). Epigenetic regulation of the BDNF gene: Implications for psychiatric disorders. Molecular Psychiatry, 17(6), 584–596. doi: 10.1038/mp.2011.107 [DOI] [PubMed] [Google Scholar]
- Bulgin N. L., Strauss J. S., King N. A., Shaikh S. A., George C. J., Fox N. A., Barr C. L., Kovacs M., Kennedy J. L. (2008). Association study of theta EEG asymmetry and brain-derived neurotrophic factor gene variants in childhood-onset mood disorder. Neuromolecular Medicine, 10(4), 343–355. doi: 10.1007/s12017-008-8038-x [DOI] [PubMed] [Google Scholar]
- Choi J.-S., Park S. M., Lee J., Hwang J. Y., Jung H. Y., Choi S.-W., Oh S., Lee J.-Y. (2013). Resting-state beta and gamma activity in Internet addiction. International Journal of Psychophysiology, 89(3), 328–333. doi: 10.1016/j.ijpsycho.2013.06.007 [DOI] [PubMed] [Google Scholar]
- Choi S.-W., Shin Y.-C., Mok J. Y., Kim D.-J., Choi J.-S., Suk-Hyun Hwang S. (2016). Serum BDNF levels in patients with gambling disorder are associated with the severity of gambling disorder and Iowa Gambling Task indices. Journal of Behavioral Addictions, 5(1), 135–139. doi: 10.1556/2006.5.2016.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A., Makeig S. (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. doi: 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Dustman R., Shearer D., Emmerson R. (1999). Life-span changes in EEG spectral amplitude, amplitude variability and mean frequency. Clinical Neurophysiology, 110(8), 1399–1409. doi: 10.1016/S1388-2457(99)00102-9 [DOI] [PubMed] [Google Scholar]
- Ferris J. A., Wynne H. J. (2001). The Canadian Problem Gambling Index. Ottawa, ON: Canadian Centre on Substance Abuse. [Google Scholar]
- Gatt J. M., Kuan S. A., Dobson-Stone C., Paul R. H., Joffe R. T., Kemp A. H., Gordon E., Schofield P. R., Williams L. M. (2008). Association between BDNF Val66Met polymorphism and trait depression is mediated via resting EEG alpha band activity. Biological Psychology, 79(2), 275–284. doi: 10.1016/j.biopsycho.2008.07.004 [DOI] [PubMed] [Google Scholar]
- Geisel O., Banas R., Hellweg R., Müller C. A. (2012). Altered serum levels of brain-derived neurotrophic factor in patients with pathological gambling. European Addiction Research, 18(6), 297–301. doi: 10.1159/000338281 [DOI] [PubMed] [Google Scholar]
- Geisel O., Panneck P., Hellweg R., Wiedemann K., Müller C. A. (2015). Hypothalamic–pituitary–adrenal axis activity in patients with pathological gambling and Internet use disorder. Psychiatry Research, 226(1), 97–102. doi: 10.1016/j.psychres.2014.11.078 [DOI] [PubMed] [Google Scholar]
- Gordon H. W. (2016). Laterality of brain activation for risk factors of addiction. Current Drug Abuse Reviews, 9(1), 1–18. doi: 10.2174/1874473709666151217121309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald M., Hensel A., Wolf H., Weiss T., Gertz H.-J. (2007). Does the hippocampal atrophy correlate with the cortical theta power in elderly subjects with a range of cognitive impairment? Journal of Clinical Neurophysiology, 24(1), 22–26. doi: 10.1097/WNP.0b013e31802ed5b2 [DOI] [PubMed] [Google Scholar]
- Herrera-Díaz A., Mendoza-Quiñones R., Melie-Garcia L., Martínez-Montes E., Sanabria-Diaz G., Romero-Quintana Y., Salazar-Guerra I., Carballoso-Acosta M., Caballero-Moreno A. (2016). Functional connectivity and quantitative EEG in women with alcohol use disorders: A resting-state study. Brain Topography, 29(3), 368–381. doi: 10.1007/s10548-015-0467-x [DOI] [PubMed] [Google Scholar]
- Houston R. J., Ceballos N. A. (2013). Human neurophysiology: EEG and quantitative EEG in addiction research. In Miller P. (Ed.), Biological research on addiction (Chapter 38, pp. 379–390). Amsterdam, The Netherlands: Elsevier Inc. [Google Scholar]
- Huang E. J., Reichardt L. F. (2001). Neurotrophins: Roles in neuronal development and function. Annual Review of Neuroscience, 24(1), 677–736. doi: 10.1146/annurev.neuro.24.1.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T.-P., Makeig S., Humphries C., Lee T.-W., Mckeown M. J., Iragui V., Sejnowski T. J. (2000). Removing electroencephalographic artifacts by blind source separation. Psychophysiology, 37(2), 163–178. doi: 10.1111/1469-8986.3720163 [DOI] [PubMed] [Google Scholar]
- Kamarajan C., Porjesz B. (2012). Brain waves in impulsivity spectrum disorders. In Cyders M. A. (Ed.), Psychology of impulsivity (pp. 20–93). Hauppauge, NY: Nova Science Publishers. [Google Scholar]
- Kang Y., Harris L. (2000). Handedness and footedness in Korean college students. Brain and Cognition, 43(1–3), 268–274. [PubMed] [Google Scholar]
- Kardos Z., Tóth B., Boha R., File B., Molnár M. (2017). Age-dependent characteristics of feedback evaluation related to monetary gains and losses. International Journal of Psychophysiology, 122, 42–49. doi: 10.1016/j.ijpsycho.2016.05.007 [DOI] [PubMed] [Google Scholar]
- Kim A., Cha J., Kwon S., Lee S. (2011). Construction and validation of Korean version of CPGI. Korean Journal of Psychology, 30(4), 1011–1038. [Google Scholar]
- Knott V., Cosgrove M., Villeneuve C., Fisher D., Millar A., McIntosh J. (2008). EEG correlates of imagery-induced cigarette craving in male and female smokers. Addictive Behaviors, 33(4), 616–621. doi: 10.1016/j.addbeh.2007.11.006 [DOI] [PubMed] [Google Scholar]
- Lasa L., Ayuso-Mateos J., Vazquez-Barquero J., Dıez-Manrique F., Dowrick C. (2000). The use of the Beck Depression Inventory to screen for depression in the general population: A preliminary analysis. Journal of Affective Disorders, 57(1), 261–265. doi: 10.1016/S0165-0327(99)00088-9 [DOI] [PubMed] [Google Scholar]
- Lee J. Y., Park S. M., Kim Y. J., Kim D. J., Choi S.-W., Kwon J. S., Choi J.-S. (2017). Resting-state EEG activity related to impulsivity in gambling disorder. Journal of Behavioral Addictions, 6(3), 387–395. doi: 10.1556/2006.6.2017.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. W., Kim S. Y., Chung W., Hwang G. S., Hwang Y. W., Hwang I. H. (2009). The validity and reliability of Korean version of Lubben Social Network Scale. Korean Journal of Family Medicine, 30(5), 352–358. doi: 10.4082/kjfm.2009.30.5.352 [DOI] [Google Scholar]
- Lei X., Wang Y., Yuan H., Mantini D. (2014). Neuronal oscillations and functional interactions between resting state networks. Human Brain Mapping, 35(7), 3517–3528. doi: 10.1002/hbm.22418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Wolf M. E. (2015). Multiple faces of BDNF in cocaine addiction. Behavioural Brain Research, 279, 240–254. doi: 10.1016/j.bbr.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorains F. K., Cowlishaw S., Thomas S. A. (2011). Prevalence of comorbid disorders in problem and pathological gambling: Systematic review and meta-analysis of population surveys. Addiction, 106(3), 490–498. doi: 10.1111/j.1360-0443.2010.03300.x [DOI] [PubMed] [Google Scholar]
- Mantini D., Perrucci M. G., Del Gratta C., Romani G. L., Corbetta M. (2007). Electrophysiological signatures of resting state networks in the human brain. Proceedings of the National Academy of Sciences of the United States of America, 104(32), 13170–13175. doi: 10.1073/pnas.0700668104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massar S., Rossi V., Schutter D., Kenemans J. (2012). Baseline EEG theta/beta ratio and punishment sensitivity as biomarkers for feedback-related negativity (FRN) and risk-taking. Clinical Neurophysiology, 123(10), 1958–1965. doi: 10.1016/j.clinph.2012.03.005 [DOI] [PubMed] [Google Scholar]
- Park S. M., Lee J. Y., Kim Y. J., Lee J.-Y., Jung H. Y., Sohn B. K., Kim D. J., Choi J.-S. (2017). Neural connectivity in Internet gaming disorder and alcohol use disorder: A resting-state EEG coherence study. Scientific Reports, 7(1), 1333. doi: 10.1038/s41598-017-01419-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L., Verchinski B. A., Mattay V. S., Callicott J. H., Kolachana B. S., Straub R. E., Egan M. F., Meyer-Lindenberg A., Weinberger D. R. (2004). The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. The Journal of Neuroscience, 24(45), 10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu L., Liu Q.-S., Poo M.-M. (2006). BDNF-dependent synaptic sensitization in midbrain dopamine neurons after cocaine withdrawal. Nature Neuroscience, 9(5), 605–607. doi: 10.1038/nn1687 [DOI] [PubMed] [Google Scholar]
- Quintero G. C. (2017). A biopsychological review of gambling disorder. Neuropsychiatric Disease and Treatment, 13, 51–60. doi: 10.2147/NDT.S118818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaswamy M., Porjesz B., Chorlian D. B., Wang K., Jones K. A., Bauer L. O., Rohrbaugh J., O’Connor S. J., Kuperman S., Reich T., Begleiter H. (2002). Beta power in the EEG of alcoholics. Biological Psychiatry, 52(8), 831–842. doi: 10.1016/S0006-3223(02)01362-8 [DOI] [PubMed] [Google Scholar]
- Son K., Choi J., Lee J., Park S., Lim J., Lee J., Kim S., Oh S., Kim D., Kwon J. (2015). Neurophysiological features of Internet gaming disorder and alcohol use disorder: A resting-state EEG study. Translational Psychiatry, 5(9), e628. doi: 10.1038/tp.2015.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko P. R., Lipsky R., Mentschel C., Robinson D., Gunduz-Bruce H., Sevy S., Ashtari M., Napolitano B., Bilder R. M., Kane J. M., Goldman D., Malhotra A. K. (2005). Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Molecular Psychiatry, 10(7), 631–636. doi: 10.1038/sj.mp.4001656 [DOI] [PubMed] [Google Scholar]
- Tammela L. I., Pääkkönen A., Karhunen L. J., Karhu J., Uusitupa M. I., Kuikka J. T. (2010). Brain electrical activity during food presentation in obese binge-eating women. Clinical Physiology and Functional Imaging, 30(2), 135–140. doi: 10.1111/j.1475-097X.2009.00916.x [DOI] [PubMed] [Google Scholar]
- Terracciano A., Piras M. G., Lobina M., Mulas A., Meirelles O., Sutin A. R., Chan W., Sanna S., Uda M., Crisponi L. (2013). Genetics of serum BDNF: Meta-analysis of the Val66Met and genome-wide association study. The World Journal of Biological Psychiatry, 14(8), 583–589. doi: 10.3109/15622975.2011.616533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau R., Jorgensen R. S., Kim S. (2006). Depression, anxiety, and resting frontal EEG asymmetry: A meta-analytic review. Journal of Abnormal Psychology, 115(4), 715–729. doi: 10.1037/0021-843X.115.4.715 [DOI] [PubMed] [Google Scholar]
- Von Stein A., Sarnthein J. (2000). Different frequencies for different scales of cortical integration: From local gamma to long range alpha/theta synchronization. International Journal of Psychophysiology, 38(3), 301–313. doi: 10.1016/S0167-8760(00)00172-0 [DOI] [PubMed] [Google Scholar]
- Wölfling K., Mörsen C. P., Duven E., Albrecht U., Grüsser S. M., Flor H. (2011). To gamble or not to gamble: At risk for craving and relapse – Learned motivated attention in pathological gambling. Biological Psychology, 87(2), 275–281. doi: 10.1016/j.biopsycho.2011.03.010 [DOI] [PubMed] [Google Scholar]
- World Medical Association. (1964). Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. Helsinki, Finland: WMA General Assembly. [Google Scholar]
- Yamada K., Nabeshima T. (2003). Brain-derived neurotrophic factor/TrkB signaling in memory processes. Journal of Pharmacological Sciences, 91(4), 267–270. doi: 10.1254/jphs.91.267 [DOI] [PubMed] [Google Scholar]
- Zoon H. F., Veth C., Arns M., Drinkenburg W., Talloen W., Peeters P. J., Kenemans J. (2013). EEG alpha power as an intermediate measure between brain-derived neurotrophic factor Val66Met and depression severity in patients with major depressive disorder. Journal of Clinical Neurophysiology, 30(3), 261–267. doi: 10.1097/WNP.0b013e3182933d6e [DOI] [PubMed] [Google Scholar]