Abstract
Background:
A number of elements of the pivotal ‘cladribine tablets treating multiple sclerosis orally’ (CLARITY) trial have remained unpublished.
Objective:
To report the impact of cladribine on health-related quality of life (QoL) in people with relapsing multiple sclerosis (pwRMS).
Methods:
QoL data from the phase III trial of two different doses (3.5 and 5.25 mg/kg) of oral cladribine in pwRMS were acquired from the European Medicines Agency through Freedom of Information. Spearman’s rank correlation was used to analyse the relationship between baseline QoL scores and baseline Expanded Disability Status Scale (EDSS) scores. Responses of the Euro Quality of Life 5 Dimensions (EQ-5D) and Multiple Sclerosis Quality of Life-54 (MSQOL-54) questionnaires were compared between treatment and control groups using univariate analyses of covariance.
Results:
In total, n = 5148 EQ-5D responses and n = 894 MSQOL-54 physical, mental health and dimension scores were extracted. Baseline EQ-5D indices correlated with EDSS scores. After 2 years, pwRMS taking 3.5 (p = .001) and 5.25 mg/kg (p = .022) reported significantly improved EQ-5D index scores compared with placebo. Positive, yet non-significant, differences were detected in MSQOL-54 scores between cladribine and placebo.
Conclusion:
Analysis of the CLARITY dataset suggests that, over and above its established clinical efficacy, cladribine leads to improved QoL over 96 weeks. ClinicalTrials.gov identifier: NCT00213135.
Keywords: Cladribine, multiple sclerosis, quality of life, EQ-5D
Introduction
Multiple sclerosis (MS) is a major demyelinating and neurodegenerative disease resulting in the accumulation of disability and significant loss of quality of life (QoL) to the affected individual and their carers.1,2 QoL may be retained or improved by use of effective disease-modifying treatments (DMT) that stop or slow the accumulation of disability.3
Cladribine is a deoxyadenosine analogue, which is phosphorylated to 2-chlorodeoxyadenosine triphosphate that is selectively cytotoxic for lymphocytes in humans.4,5 Cladribine has activity as an injectable, generic agent6 or as an oral pro-drug.5,7 This was particularly well demonstrated in the phase III ‘Cladribine tablets treating multiple sclerosis orally’ (CLARITY) trial, where cladribine markedly inhibited (1) lesion accumulation as detected on magnetic resonance imaging (MRI), (2) relapses and (3) 3-month sustained disability progression.5,7 For a number of reasons, including a perceived increase in cancer risk,5 and in the absence of additional trial data, the regulators rejected licence applications prompting the manufacturer of oral cladribine to halt their programme for commercial development of the drug in 2011.8 Following resubmission in 2015, however, the Committee for Medicinal Products for Human Use of the European Medicines Agency (EMA) has now adopted a positive opinion with licensing of oral cladribine (Mavenclad®) likely in the third quarter 2017 (http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/004230/WC500229786.pdf).
Based on meeting abstracts prior to 2011, the termination of the oral cladribine programme meant a significant cache of data gathered during CLARITY never underwent peer-reviewed publication. Given the excellent efficacy of cladribine demonstrated in people with relapsing multiple sclerosis (pwRMS),5 as well as people with a first demyelinating event suggestive of MS,9 and following a meta-analysis suggesting the cladribine-associated cancer frequency was no different to natural ageing or that found in pivotal trials of other multiple sclerosis–DMTs,10 we saw a future in developing cladribine for people with MS (pwMS) further.11,12 We therefore obtained the entire CLARITY dataset using a Freedom of Information (FoI) request to the EMA, which included data on health-related QoL.
As QoL indices are particularly sensitive to fatigue, cognitive impairment, emotional changes and social isolation,13,14 they enable a complementary assessment of the impact of DMT over and above clinical scales, such as the Expanded Disability Status Scale (EDSS), which suffers from bias, notably due to the dominant contribution of ambulation at scores between 3.5 and 8.15 Furthermore, QoL indices may be more sensitive to adverse effects of treatments.16,17 Given the efficacy of cladribine to inhibit relapsing disease,5–7 we hypothesized a positive impact on QoL in pwRMS.
Materials and methods
Trial registration and data acquisition
After the apparent termination of the commercial development of oral cladribine in 2011, and subsequent discussions with the UK Medicines and Healthcare products Regulatory Agency about approaches to develop generic cladribine, the full regulatory submission of the 96-week CLARITY trial (NCT00213135)5 was obtained through FoI request to the EMA (submitted May 2013; obtained November 2013). While the original trial was undertaken following R&D approval and pwRMS recruited after informed consent,5 no R&D approval was required to use the ‘public domain’ documents used for the current analysis of anonymized datasets. Information regarding study design, setting, participants, eligibility, variables, randomization, blinding, study size, bias reduction, flow diagrams of participants and the Consolidated Standards of Reporting Trials (CONSORT) and Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines can be obtained from the original trial publication.5
The dataset from the EMA was provided in portable document format (pdf). Files containing relevant data were identified and converted into Microsoft Excel spreadsheets using a pdf parser developed on a Python 2.7 platform at the MidPlus computational facilities at Queen Mary University of London (code available on request). The converted data were validated by comparing sample records between pdf and spreadsheet versions of the files. Scores listed as ‘unscheduled’ or ‘99999’ were excluded from analysis.
Trial design
The full details of the trials have been reported previously.5 Briefly, 1326 pwRMS were randomized 1:1:1 (n = 1184 completed the 96-week study) to receive either placebo (n = 437; n = 380 completed) or one of the two doses of oral cladribine pro-drug. Patients were given tablets containing either 10 (60–69.9-kg body weight) or 20 mg/day (70–79-kg body weight) cladribine administered for 4–5 days in weeks 0 and 5 (year 1) and weeks 48 and 52 (year 2) to result in a total cumulative dose of 3.5 mg/kg (n = 433; n = 398 completed). Those randomized to the 5.25-mg/kg arm were given additional doses in weeks 9 and 13 (n = 456; n = 406 completed).5 Clinical efficacy was comparable for both doses of cladribine.5,7 QoL scores were collected at baseline and at weeks 24, 48, 72 and 96.
QoL indices
The regulatory submission included the EuroQol five-dimension three-level (EQ-D5-3L)18 and the Multiple Sclerosis Quality of Life-54 (MSQOL-54)1,19 questionnaires.
The EQ-5D-3L, a self-completed questionnaire, addresses five distinct dimensions: (1) mobility, (2) self-care, (3) usual activities, (4) pain or discomfort and (5) anxiety, where participants had a choice of three responses per dimension: no problems, moderate problems or severe problems.18 A scoring algorithm was implemented to convert responses into a summary index, known as the EQ-5D index,18 where 1 represents the best QoL. Values below 0 were not excluded. Values marked on a self-rating Visual Analogue Scale (VAS), known as EQ-5D-VAS, were also collected. EQ-5D-VAS scores on a scale 0–100, where 0 represented the worst QoL. The EQ-5D was collected at baseline and at weeks 24, 48, 72 and 96.
The MSQOL-54 is a 54-item questionnaire that measures 12 domains: physical function, role limitations-physical, role limitations-emotional, pain, emotional well-being, energy, health perceptions, social function, cognitive function, health distress, overall QoL and sexual function.1 Subscale and summary scores were calculated as described previously.19 For each domain, relevant item scores were totalled and then divided by the number of completed responses. This gave a score for each of the 12 domains. Physical health (PHS) and mental health (MHS) component scores were calculated from weighting relevant domains and summing the resultant products. Male and female sexual function domain scores were added together to provide a single physical component summary score. The MSQOL-54 scores ranged between 0 and 100, where 100 represented the highest QoL. Changes from baseline scores were calculated for each time point. The MSQOL-54 was collected at baseline and at weeks 24, 48, 72 and 96.
Statistical analysis
The relationship between baseline QoL scores and baseline EDSS was assessed using Spearman’s rank correlation. Univariate analyses of covariance (ANCOVA) tests, adjusted for baseline score, age at baseline, centre, ethnic group and gender were conducted to compare mean QoL scores at baseline and at weeks 24, 48, 72 and 96. This method was in line with EMA guidelines20 and similar to those used in previous QoL studies.21,22 The impact of relapses on QoL was also assessed using ANCOVA, comparing pwRMS who had a relapse, requiring steroid use as a reflection of the severity, between treatment and placebo-control groups. Relapses listed as ‘unscheduled’ were not included as relapses up to week 48, because it could not be safely assumed that these relapses occurred during this time. However, all relapses including those listed as ‘unscheduled’ were included as relapses up to week 96 and were totalled for each treatment arm. To investigate whether changes in EQ-5D index were independent of relapse reduction, two linear regression models were compared. The first model set high-dose groups, low-dose groups and their baseline index scores as predictors for changes in EQ-5D index at week 96. The second model also adjusted for whether participants relapsed at any point during the trial (a binary variable).
Changes in EQ-5D were examined using the index of ‘minimal clinically important difference’ (MCID),23 defined as the smallest QoL change that pwRMS considered important. This is because changes greater than the MCID are more likely to translate to real-world patient benefit.24 Previously, based on data from the North American Research Committee on Multiple Sclerosis (NARCOMS) registry, the EQ-5D-3L MCID was between 0.050 and 0.084.23 The likelihood that a patient experienced a QoL change greater than the MCID, set at 0.08, was assessed using a logistic regression model. The covariates were high-dose groups, low-dose groups and index scores at baseline. The dependent variable was whether or not individuals achieved an MCID in change EQ-5D index. The sample population came from the entire trial period (from week 24 to week 96). All statistical analyses were performed using SPSS version 23. No imputation was made for missing data.
Results
Analysis of respondent demography showed that the age and the gender ratio in the respondent sample matched the overall demography of the CLARITY trial,3 with no significant imbalances between treatment groups (Supplementary Table S1). At baseline, pwRMS with higher EDSS scores had a lower EQ-5D index (rs = −.472, n = 430, p < .001) and EQ-5D-VAS score (rs = −.442, n = 434, p < .001).
Effect of cladribine on EQ-5D index
At baseline, differences in EQ-5D index and EQ-5D-VAS scores between groups were not statistically significant. Participants receiving placebo experienced worsening in mean QoL over the duration of the trial reaching the lowest point by week 96 (Figure 1). In weeks 24, 72 and 96, the mean index change between groups was significantly different. In week 24 (F(2, 950) = 5.514, p = .004), participants treated with the low dose of cladribine had significantly higher index scores compared with placebo, likewise for the high dose of cladribine (p = .014 for low dose, p = .003 for high dose). In week 72, only the low-dose cladribine group had significantly higher scores compared with placebo (F(2, 899) = 4.340, p = .013, p = .003 for low dose). Finally, in week 96 (F(2, 945) = 5.639, p = .004), participants treated with either dose of cladribine had significantly higher EQ-5D index scores compared to placebo (planned contrast analysis; p = .001 for low dose, p = .022 for high dose).
Figure 1.
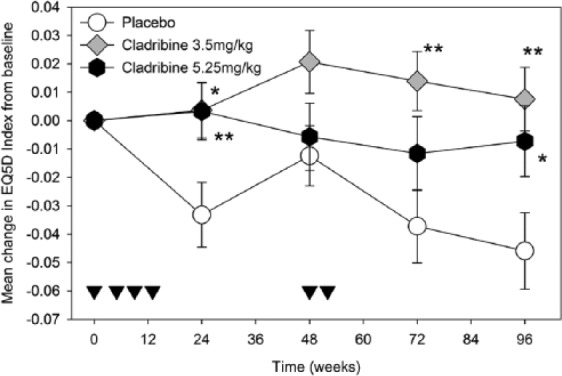
The impact of cladribine on EQ-5D.
People with relapsing MS were treated with either placebo (circle; n = 281–310) or 3.5-mg/kg cladribine (diamond; n = 306–319) on weeks 0, 5, 48 and 52 or treated with 5.25 mg/kg (hexagon; n = 320–329) by receiving additional oral doses in weeks 9 and 13. The results represent the mean ± standard error of the mean. *p < .05, **p < .01 compared to placebo. Inverse triangles indicate time points of drug administration.
Impact of cladribine on EQ-5D-VAS
While the 3.5-mg/kg cladribine group experienced consistent improvement in mean VAS scores throughout the trial, the placebo group reported worse than baseline VAS scores throughout the trial. However, none of these differences were statistically significant at any time point over the duration of the trial (Supplementary Table S1).
Impact of cladribine on EQ-5D index after relapse
An ANCOVA adjusted for age, gender, centre, baseline score, ethnicity and treatment group was used to assess the impact of relapses on the mean observed EQ-5D index and VAS scores. This analysis was performed on scores reported by pwRMS at the end of weeks 48 and 96. The analysis showed that EQ-5D-VAS scores in pwRMS who had at least one relapse up to week 48 (mean (M) = .60, standard deviation (SD) = .21) was significantly lower than in patients who did not relapse (M = .71, SD = .21; F(1, 923) = 19.178, p < .001). Similarly, pwRMS who relapsed by week 48 of the trial (M = .63, SD = .26) had significantly lower EQ-5D index scores compared with pwRMS who did not relapse (M = .73, SD = .22; F(1, 913) = 13.440, p < .001). Similarly, participants who relapsed by week 96 also had a significantly lower EQ-5D index compared to non-relapsed participants (0.60 vs 0.73; F(1, 945) = 29.052, p < .001). This effect was also evident for EQ-5D-VAS scores up to week 96 (0.61 vs 0.71; F(1, 956) = 29.644, p < .001), suggesting that relapses had a detrimental effect on QoL.
ANCOVA adjusted for age, gender, centre, baseline score and ethnicity was also used to assess the impact of cladribine on QoL scores in pwRMS experiencing a relapse. There was no statistically significant difference in EQ-5D index between groups within those who relapsed at week 48 or week 96. However, there were differences in the EQ-5D-VAS (F(2, 101) = 3.124, p = .048). The VAS score was significantly greater (better QoL) in relapsed placebo patients (M = .62, SD = .22) compared with relapsed low-dose patients (M = .52, SD = .20; planned contrast analysis, p = .039). Thus, if they had a relapse, pwRMS treated with cladribine had no better QoL compared to pwRMS on placebo.
Two linear regression models were compared to explore the influence of relapse reduction on changes in QoL further. The first model, which set doses and baseline scores as independent variables, showed that both high- (p = .028) and low-dose (p = .001) groups were significantly higher than placebo at week 96. However, when relapses up to week 96 were added as another independent variable in a second regression model, the high-dose improvement was reduced and no longer significant (p = .161). The low-dose effect remained significant (p = .016) even after adjustment for relapses.
Impact of cladribine on EQ-5D dimensions
The proportion of pwRMS reporting at least one point increase at week 96, which indicated worsening of their EQ-5D dimensions, was compared between treatment and control groups (Table 1). It was found that only the self-care dimension reached significance (p < .01) for both of the cladribine treatment groups (Table 1). Furthermore, a larger proportion of pwRMS on placebo worsened in mobility and anxiety dimensions compared with people treated with cladribine (chi-square; mobility: χ2 = 7.911, p = .019 (2df, n = 964); self-care: χ2 = 7.104, p = .029 (2df, n = 964), anxiety: χ2 = 6.389, p = .041 (2df, n = 964)).
Table 1.
Mean change in EQ-5D for cladribine and placebo groups.
| Treatment | Mobility | Self-care | Activity | Pain | Anxiety |
|---|---|---|---|---|---|
| Placebo | 0.04 ± 0.46 | 0.15 ± 0.50 | 0.03 ± 0.60 | 0.00 ± 0.59 | 0.08 ± 0.66 |
| 3.5-mg/kg cladribine | −0.05 ± 0.41 NS; p = .013 |
0.05 ± 0.43 p = .008 |
−0.02 ± 0.53 NS; p > .05 |
−0.05 ± 0.59 NS; p > 0.05. |
−0.03 ± 0.59 NS; p = .028 |
| 5.25-mg/kg cladribine | −0.02 ± 0.45 NS; p > .05 |
0.05 ± 0.45 p = .003 |
0.04 ± 0.60 NS; p > .05. |
0.00 ± 0.62 NS; p > .05. |
−0.02 ± 0.62 NS; p = .029 |
EQ-5D: Euro Quality of Life 5 Dimensions; NS: not significant.
EQ-5D was established at baseline and 96 weeks following treatment with either 3.5 or 5.25 mg/kg. The results represent the mean ± standard deviation. Differences between treatment and placebo were assessed using ANCOVA. p < .01 was considered significant for multiple comparisons involving five outcomes.
Impact of cladribine on pwRMS reporting an MCID in EQ-5D
Proportions of pwRMS achieving MCID in their EQ-5D index score change at each time point are detailed in Table 2. Our logistic model (χ2 = 368.7, p < .001 (3df, n = 4076)) showed that individuals on a low dose had an odds ratio of 1.26 (p = .019) compared to placebo. Those on high dose had an odds ratio of 1.06 (not statistically significant).
Table 2.
Counts and proportions of participants achieving MCID for each treatment arm.
| Timepoint | Group | Number within MCID ≥ 0.08 (%) |
|---|---|---|
| Week 24 | Cladribine 3.5 | 77 (36.8) |
| Cladribine 5.25 | 70 (33.5) | |
| Placebo | 62 (29.7) | |
| Week 48 | Cladribine 3.5 | 98 (39.5) |
| Cladribine 5.25 | 77 (31.0) | |
| Placebo | 73 (29.4) | |
| Week 72 | Cladribine 3.5 | 75 (33.6) |
| Cladribine 5.25 | 82 (36.8) | |
| Placebo | 66 (29.6) | |
| Week 96 | Cladribine 3.5 | 84 (35.7) |
| Cladribine 5.25 | 87 (37.0) | |
| Placebo | 64 (27.2) |
MCID: minimal clinically important difference.
Impact of cladribine on MSQOL-54 scores
The number of MSQOL-54 responses was small (n = 45–73). Baseline MSQOL-54 physical and mental health component scores between groups were not significantly different (Supplementary Table S2). Univariate ANCOVA adjusted for baseline score, age, gender, centre and ethnicity were conducted at each time point to assess the impact of oral cladribine on MSQOL-54 physical and mental health component scores failed to show significant improvements compared to placebo (Supplementary Table S2) at any point.
Discussion
Following refusal by the EMA to license oral cladribine in 2011, the drug was withdrawn from markets, where it had been licensed (Russia and Australia), and ongoing trials were terminated.8,9
However, the high efficacy and modest adverse effect profile of cladribine in pwRMS5,7,10 led us to seek information that could help inform on the merits of preparations other than the one used in CLARITY. Parenteral cladribine, which is available as a generic drug for treatment of people with hairy cell leukaemia, may have benefits for personalized dosing due to its consistent bioavailability of 100% compared to the oral route with a mean of 42% bioavailability25 prompting a study exploring combination of the acid sensitive oral cladribine pro-drug with the proton pump inhibitor pantoprazole (NCT00938366; the study has yet to be reported). However, a successful FoI request to the EMA for documentation placed in the public domain in 2009 enabled us to independently analyse the trial data. Here, we present class II evidence that cladribine has QoL benefits in addition to the clinical benefits previously reported.5,7,9
The key result of this analysis is that cladribine, at both doses used in CLARITY, 3.5 and 5.25 mg/kg orally,5 significantly improved QoL as measured using the widely accepted EQ-5D index. The benefits in EQ-5D for pwRMS treated with both doses were particularly significant for self-care, although influences on mobility could be detected and may be related to the clinical efficacy of cladribine in reducing relapses and delaying progression.5,7 It is plausible these clinical effects also translate into reduced anxiety.
The data collected during CLARITY are in line with previous studies suggesting that relapses have a significant impact on QoL.16,26 The implication from linear regression is that the beneficial effect on QoL using the higher dose was mediated by the relapse-reducing effect of cladribine, whereas at the lower dose, cladribine may also lead to improved QoL independent of its relapse-reducing effect. Given there was no difference in efficacy between the two doses during CLARITY, we hypothesize the lower incidence of adverse effects in the low-dose group as the potential cause for the ‘relapse-independent’ QoL benefit on the lower dose.
The magnitude of QoL benefits was sufficiently robust to reflect in the MCID, suggesting improved QoL was noticeable at the level of the individual patient. While the EQ-5D-VAS score change was consistent with the EQ-5D index, differences between groups were small and did not reach statistical significance. The reason for this may be the lower sensitivity of this QoL measure, where there may be issues with completion and coding implementation and where health improvements are less likely to be reported.27 Furthermore, no difference was detected using the MSQOL-54, likely due to the small number of respondents not providing a statistically meaningful sample.
Given only few fully blinded head-to-head studies have been undertaken so far, it is difficult to compare QoL indices between different interventions for pwRMS directly. Not all DMT have had an impact on QoL indices;28,29 however, improvements have been detected with treatments including dimethyl fumarate,21 natalizumab22 and alemtuzumab.30 Recently, alemtuzumab was shown to improve the EQ-5D index and the EQ-5D-VAS and physical and mental health components of short form-36 questionaire.30 It should be kept in mind, however, that pwRMS receiving alemtuzumab were unblinded to treatment allocation due to the infusions and infusion-related reactions,31 which precludes accounting for placebo effects.
On the basis of the data collected during CLARITY,5,7 cladribine was highly effective in controlling MRI lesion accrual, relapses and deterioration of disease-related disability,5,7 further corroborated by reduction in brain atrophy.32 As a rule, high-efficacy DMT are currently associated with a higher frequency of treatment-related adverse effects, such as opportunistic infections, notably progressive multifocal leukoencephalopathy and the development of secondary autoimmunities,33,34 which do not appear to be a feature of cladribine induction therapy5,7,9
Limitations
Reviewing the protocol and amendments of CLARITY between 2005 and 2008 suggests measuring QoL was not considered as important as in more recent trials. The EQ-5D was the only index included in the first version (January 2005) of the CLARITY protocol, while MSQOL-54, 36-Item Short Form Survey (data not included due to small number of responses) and an analysis plan were only added later in 2005 and 2006. As a result, the EQ-5D remained the only index for which the number of responses enabled robust analysis. While the number of MSQOL-54 responses was limited, making it less straightforward to interpret how cladribine affected specific domains, the EQ-5D results indicate that cladribine broadly improved symptoms associated with self-care. The original analysis plan of CLARITY included comparative analysis between the two cladribine groups and the placebo group using Hochberg’s step-up multiple comparison. However, we used ANCOVA methodology and planned contrast analysis, which are not significantly different.
In conclusion, we independently analysed QoL data collected during the largest ever trial of cladribine versus placebo in pwRMS. Over and above the established efficacy of cladribine on clinical outcomes,5,7 the treatment led to significant improvement in QoL. These results further underpin the potential of cladribine as a DMT for pwMS, be it as a drug licensed for MS, as is currently being sought once again in Europe,35 or using an off-label preparation.6,12,36
Supplementary Material
Acknowledgments
This research utilized MidPlus computational facilities at Queen Mary University of London, supported by QMUL Research IT and funded by EPSRC grant EP/K000128/1. The authors thank the European Medicines Agency for supplying the trial documents.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: None considered relevant. D.A. has nothing to declare, C.A. has nothing to declare, L.Z. has nothing to declare, D.R.A. has nothing to declare. D.B. has nothing relevant to declare. K.S. has been a PI of trials sponsored by Novartis, Roche, Teva, Medday, involved in trials sponsored by Biogen, Sanofi-Genzyme, BIAL, Cytokinetics and Canbex and has received speaking honoraria for lecturing and advisory activity and meeting support from Biogen, Merck Inc., Merck Serono, Novartis, Roche, Sanofi-Genzyme and Teva.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Contributor Information
Dayo Afolabi, Blizard Institute (Neuroscience), Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London, UK.
Christo Albor, Blizard Institute (Neuroscience), Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London, UK.
Lukasz Zalewski, ITS Research, Queen Mary University of London, London, UK.
Dan R Altmann, Blizard Institute (Neuroscience), Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London, UK/London School of Hygiene & Tropical Medicine, London, UK.
David Baker, Blizard Institute (Neuroscience), Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London, UK.
Klaus Schmierer, Blizard Institute (Neuroscience), Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London, UK/Emergency Care and Acute Medicine Clinical Academic Group Neurosciences, The Royal London Hospital, Barts Health NHS Trust, London, UK.
References
- 1. Benito-Leon J, Morales JM, Rivera-Navarro J, et al. A review about the impact of multiple sclerosis on health-related quality of life. Disabil Rehabil 2003; 25: 1291–1303. [DOI] [PubMed] [Google Scholar]
- 2. Miller DM, Allen R. Quality of life in multiple sclerosis: Determinants, measurement, and use in clinical practice. Curr Neurol Neurosci Rep 2010; 10: 397–406. [DOI] [PubMed] [Google Scholar]
- 3. Rudick RA, Miller DM. Health-related quality of life in multiple sclerosis: Current evidence, measurement and effects of disease severity and treatment. CNS Drugs 2008; 22: 827–839. [DOI] [PubMed] [Google Scholar]
- 4. Warnke C, Leussink VI, Goebels N, et al. Cladribine as a therapeutic option in multiple sclerosis. Clin Immunol 2012; 142: 68–75. [DOI] [PubMed] [Google Scholar]
- 5. Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med 2010; 362: 416–426. [DOI] [PubMed] [Google Scholar]
- 6. Beutler B, Sipe JC, Romine JS, et al. The treatment of chronic progressive multiple sclerosis with cladribine. Proc Natl Acad Sci U S A 1996; 93: 1716–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giovannoni G, Cook S, Rammohan K, et al. Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: A post-hoc and subgroup analysis. Lancet Neurol 2011; 10: 329–337. [DOI] [PubMed] [Google Scholar]
- 8.http://www.ema.europa.eu/docs/en_GB/document_library/Medicine_QA/2011/02/WC500102304.pdf (accessed 12 March 2017).
- 9. Leist TP, Comi G, Cree BA, et al. ; Oral Cladribine for early MS (ORACLE MS) Study Group. Effect of oral CLAD on time to conversion to clinically definite multiple sclerosis in patients with a first demyelinating event (ORACLE MS): A phase 3 randomised trial. Lancet Neurol 2014; 13: 257–267. [DOI] [PubMed] [Google Scholar]
- 10. Pakpoor J, Disanto G, Altmann DR, et al. No evidence for higher risk of cancer in patients with multiple sclerosis taking CLAD. Neurol Neuroimmunol Neuroinflamm 2015; 2(6): e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014; 83: 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alvarez-Gonzalez C, Adams A, Mathews J, et al. Cladribine to treat disease exacerbation after fingolimod discontinuation in progressive multiple sclerosis. Ann Clin Transl Neurol 2017; 4: 506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lysandropoulos AP, Havrdova E. ‘Hidden’ factors influencing quality of life in patients with multiple sclerosis. Eur J Neurol 2015; 22: 28–33. [DOI] [PubMed] [Google Scholar]
- 14. Benedict RH, Wahlig E, Bakshi R, et al. Predicting quality of life in multiple sclerosis: Accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. J Neurol Sci 2005; 231: 29–34. [DOI] [PubMed] [Google Scholar]
- 15. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 16. Nickerson M, Cofield SS, Tyry T, et al. Impact of multiple sclerosis relapse: The NARCOMS participant perspective. Mult Scler Relat Disord 2015; 4: 234–240. [DOI] [PubMed] [Google Scholar]
- 17. Balak DM, Hengstman GJ, Hajdarbegovic E, et al. Prevalence of cutaneous adverse events associated with long-term disease-modifying therapy and their impact on health-related quality of life in patients with multiple sclerosis: A cross-sectional study. BMC Neurol 2013; 13: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rabin R, de Charro F. EQ-5D: A measure of health status from the EuroQol Group. Ann Med 2001; 33: 337–343. [DOI] [PubMed] [Google Scholar]
- 19. Vickrey BG, Hays RD, Harooni R, et al. A health-related quality of life measure for multiple sclerosis. Qual Life Res 1995; 4: 187–206. [DOI] [PubMed] [Google Scholar]
- 20. Committee for Medicinal Products for Human Use (CHMP). Guideline on adjustment for baseline covariates in clinical trials, 2015, http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/03/WC500184923.pdf (accessed 12 Jan 2017).
- 21. Kappos L, Gold R, Arnold DL, et al. Quality of life outcomes with BG-12 (dimethyl fumarate) in patients with relapsing-remitting multiple sclerosis: The DEFINE study. Mult Scler 2014; 20: 243–252. [DOI] [PubMed] [Google Scholar]
- 22. Rudick RA, Miller D, Hass S, et al. Health-related quality of life in multiple sclerosis: Effects of natalizumab. Ann Neurol 2007; 62: 335–346. [DOI] [PubMed] [Google Scholar]
- 23. Kohn CG, Sidovar MF, Kaur K, et al. Estimating a minimal clinically important difference for the EuroQol 5-dimension health status index in persons with multiple sclerosis. Health Qual Life Outcomes 2014; 12: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brożek JL, Guyatt GH, Schünemann HJ. How a well-grounded minimal important difference can enhance transparency of labelling claims and improve interpretation of a patient reported outcome measure. Health Qual Life Outcomes 2006; 4: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liliemark J, Juliusson G. Cellular pharmacokinetics of 2-chloro-2′-deoxyadenosine nucleotides: Comparison of intermittent and continuous intravenous infusion and subcutaneous and oral administration in leukemia patients. Clin Cancer Res 1995; 1: 385–390. [PubMed] [Google Scholar]
- 26. Mäurer M, Comi G, Freedman MS, et al. Multiple sclerosis relapses are associated with increased fatigue and reduced health-related quality of life – A post hoc analysis of the TEMSO and TOWER studies. Mult Scler Relat Disord 2016; 7: 33–40. [DOI] [PubMed] [Google Scholar]
- 27. Feng Y, Parkin D, Devlin NJ. Assessing the performance of the EQ-VAS in the NHS PROMs programme. Qual Life Res 2014; 23: 977–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13: 545–556. [DOI] [PubMed] [Google Scholar]
- 29. National Institute for Health and Care Excellence. NICE technology appraisal guidance (TA303), 2014, https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-technology-appraisal-guidance [PubMed]
- 30. Arroyo González R, Kita M, Crayton H, et al. Alemtuzumab improves quality-of-life outcomes compared with subcutaneous interferon beta-1a in patients with active relapsing-remitting multiple sclerosis. Mult Scler; 2017: 23: 1367–1376. [DOI] [PubMed] [Google Scholar]
- 31. Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: A randomised controlled phase 3 trial. Lancet 2012; 380: 1829–1839. [DOI] [PubMed] [Google Scholar]
- 32. De Stefano N, Giorgio A, Battaglini M, et al. Reduced brain atrophy rates are associated with lower risk of disability progression in patients with relapsing multiple sclerosis treated with cladribine tablets. Mult Scler; 24: 222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martin R, Sospedra M, Rosito M, et al. Current multiple sclerosis treatments have improved our understanding of MS autoimmune pathogenesis. Eur J Immunol 2016; 46: 2078–2090. [DOI] [PubMed] [Google Scholar]
- 34. Berger JR. Classifying PML risk with disease modifying therapies. Mult Scler Relat Disord 2017; 12: 59–63. [DOI] [PubMed] [Google Scholar]
- 35. http://www.merckgroup.com/en/media/extNewsDetail.html?newsId=57F6B32E5CD8E3AAC1257FF100307005&;newsType=1 (accessed 12 March 2017).
- 36. Alvarez-Gonzalez C, Allen-Philbey K, Mathews J, et al. Treating multiple sclerosis with generic cladribine. J Neurol Neurosurg Psychiatry 2016; 22(suppl 3): 604–605. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


