Abstract
The purpose of this study was to examine the characteristics and treatment plans of patients who experienced fatal radiation pneumonitis after stereotactic body radiation therapy for primary or oligometastatic lung cancer. Records of 1789 patients treated with stereotactic body radiation therapy for primary or oligometastatic lung cancer were retrospectively reviewed to identify those who developed fatal radiation pneumonitis. Twenty-three (1.3%; 18 men and 5 women) patients developed fatal radiation pneumonitis after stereotactic body radiation therapy for lung cancer; their median age was 74 years. The mean Krebs von den Lungen-6 level and percent vital capacity were 1320 U/mL and 82%, respectively. Prestereotactic body radiation therapy computed tomography revealed pulmonary interstitial change in 14 (73.7%) of 19 patients in whom computed tomography data could be reviewed. Seven (30.4%) of 23 patients had regularly used steroids. The median time duration between stereotactic body radiation therapy commencement and pneumonia symptom appearance was 75 (range: 14-204) days. Median survival time following pneumonia symptom appearance was 53 (range: 4-802) days. The 6- and 12-month overall survival rates were 34.8% and 13.0%, respectively. The 6-month overall survival rates in patients with and without heart disease were 50.0%, 16.7%, and 46.7% for heart disease existence, respectively. There were 4 patients in whom fatal radiation pneumonitis occurred within 2 months after stereotactic body radiation therapy and who died within 1 month. Three of them had no pulmonary interstitial change before stereotactic body radiation therapy, but had heart disease. In summary, the survival time in this case series was generally short but varied widely. More than half of the patients had pulmonary interstitial change before stereotactic body radiation therapy, although immediately progressive fatal radiation pneumonitis was also observed in patients without pulmonary interstitial change. True risk factors for fatal radiation pneumonitis should be examined in a prospective study with a larger cohort.
Keywords: stereotactic body radiation therapy, lung cancer, radiation pneumonitis, pulmonary interstitial change, heart disease, mortality
Introduction
Hypofractionated stereotactic body radiation therapy (SBRT) is commonly prescribed to patients with small-sized primary or oligometastatic lung tumors as a radical and minimally invasive treatment. Stereotactic body radiation therapy has the advantage of administration of a high-dose concentration to the tumor with limited toxicity to normal structures and is therefore well utilized in patients who are medically inoperable owing to poor pulmonary function with chronic lung disease. Stereotactic body radiation therapy is generally safe and has no severe adverse effects in most patients; however, fatal radiation pneumonitis (FRP) occasionally occurs. Fatal radiation pneumonitis shows various patterns of inflammatory shadows inside or outside the irradiated volume and sometimes progresses rapidly to death with accompanying lung edema or infection. Identification of risk factors for FRP after SBRT is important to identify and manage candidates requiring this treatment properly; however, this has not been clarified. Therefore, the purpose of the study was to explore the characteristics, treatment plans, and clinical courses of patients who developed FRP in a Japanese multiinstitutional SBRT study group.
Materials and Methods
We based our study on a retrospective study design, and received approval from the institutional review board at University of Yamanashi Hospital (approval number 961). Patients who developed FRP after SBRT for primary or metastatic lung cancer were identified from a total of 1789 patients attending 8 institutions that are members of the Japanese multiinstitutional SBRT study group. The SBRT techniques and methods differed among the institutions. The gross target volume (GTV) was delineated on computed tomography (CT) images using a lung window level setting. The clinical target volume (CTV) enlarged the GTV by 0 to 5 mm as judged by the individual radiation oncologist. The internal margin was calculated and set at 2 to 5mm around the CTV according to the individual measurements for respiratory motion determined at each institution. The internal margin caused by respiratory motion was reduced by gating, tracking, the breath-hold technique, or abdominal compression. The planning target volume (PTV) comprised the CTV, a proper internal margin measured for each patient, and a 5 mm safety margin. The irradiated port marginally exceeded the PTV by 3 to 5 mm to secure the surface dose of the PTV. Dose calculation was mainly performed using the Clarkson algorithm with heterogeneity correction. A total dose of 35 to 72.5 Gy (mean, 58.7Gy) at the isocenter in 1 to 10 fractions with a single dose of 7.25 to 35 Gy was administered with 6 MV X-rays. Typical dose/fractionation schedules were 48 Gy in 4 fractions. No chemotherapy regimens were administered before or during radiotherapy. A board-certified thoracic radiologist in each institution examined the CT images of these patients to determine the presence of pulmonary interstitial change. Survival rate was calculated using the Kaplan-Meier method. Statistical difference was calculated with a generalized Wilcoxon test. All statistical analyses were performed using Statview software (SAS, Cary, North Carolina).
Results
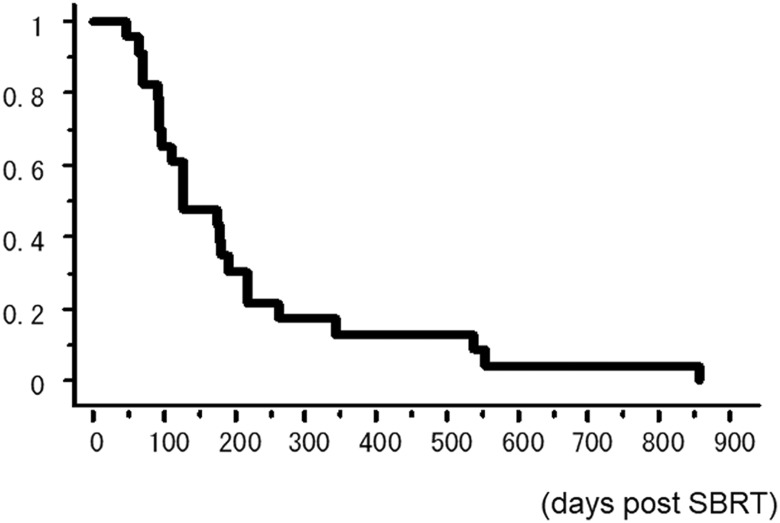
Twenty-three of the 1789 patients treated with SBRT for lung cancer (1.3%) developed FRP after SBRT, among whom 18 were men and 5 were women. Detailed characteristics and SBRT of all patients are shown in Table 1. One patient was treated for double lesions simultaneously. It is notable that 14 (73.7%) of 19 cases in which CT could be reviewed showed pulmonary interstitial change. Abnormally high Krebs von den Lungen-6 (KL-6) level was found in 72.2% of the total cases. The median time duration between the start of SBRT and pneumonia symptom appearance was 75 (range: 14-204) days. The median survival time following pneumonia symptom appearance was 53 (range: 4-802) days. Ten (43.5%) of 23 patients died within 1 month of the appearance of pneumonia symptoms. The overall survival (OS) rate for all patients is shown in Figure 1. The 6-and 12-month OS rates were 34.8% and 13.0%, respectively.
Table 1.
Characteristics and Treatment Detail of 23 Patients With Fatal Radiation Pneumonitis.a
| Age | 60-92 (median 74) years |
| Sex | 18 male, 5 female |
| Disease status | Primary 17, metastases 4, postoperative recurrence 2 |
| Tumor size | 15-62 mm (median: 32 mm) |
| Number of treated lesion | 1:22, 2:1 |
| Brinkman index | 0-1880 (median 840) |
| Pulmonary interstitial change | Positive 14, negative 5, not assigned 4 |
| Pulmonary emphysema | Positive 9, negative 10, not assigned 4 |
| Regular use of steroid before SBRT | Positive 7, negative 15 not assigned 1 |
| Chronic cardiac disease | Positive 6, negative 15, not assigned 2 |
| LDH (118-213 IU/L) | 183-795 (median: 255) IU/L |
| CRP (0-0.3 mg/dl) | 0.03-2.60 (mean: 0.40) mg/dL |
| KL-6 (0-499 U/mLL) | 222-9000 (median: 1320) U/mL |
| %VC | 50-114 (median: 82) % |
| FEV 1.0% | 36-135 (median: 83) % |
| Size of PTV (mL) | 17-210 (median: 56.5) mL |
| Dose of SBRT | 35-75Gy / 1-10 fraction |
| BED (α/β = 3 Gy) | 173-433 (median: 240) Gy |
| V20 (lung-GTV)* | 3.8-18.2 (median: 8.0) % |
| Mean (lung-GTV) dose | 2.6-12.9 (median: 5.0) Gy |
Abbreviations: BED, biological effective dose; CRP, C-reactive protein; FEV 1.0%: forced expiratory volume percent in 1 second; GTV, gross target volume; KL-6: Krebs von den Lungen-6; LDH, lactate dehydrogenase; PTV, planning target volume; SBRT, stereotactic body radiation therapy; %VC, percent vital capacity; V20, ratio of the volume receiving 20Gy or more to the normal lung volume.
a Parenthesis: normal range
Figure 1.
Overall survival rate for all patients.
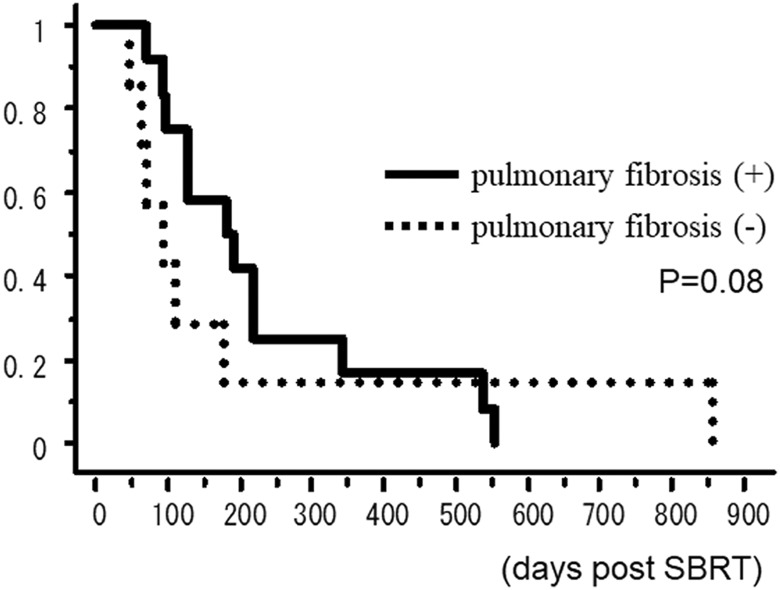
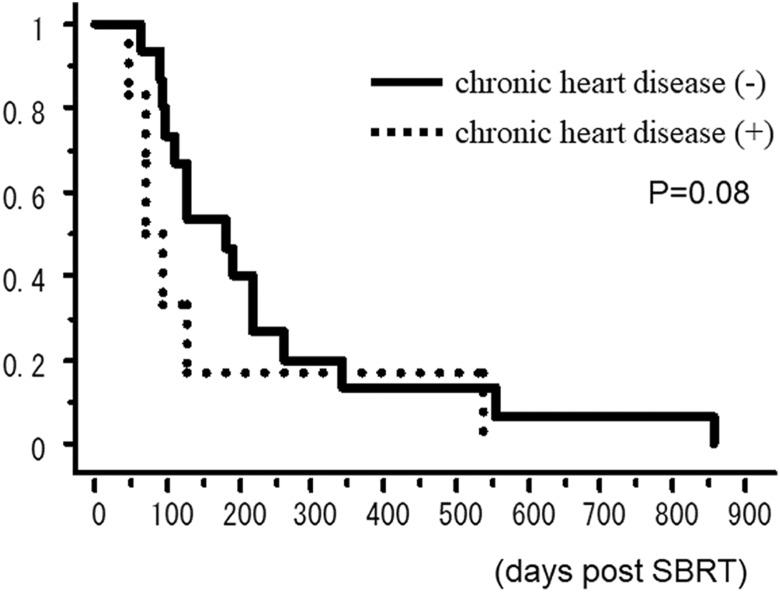
The OS rate curves according to the existence of pulmonary interstitial change are shown in Figure 2. The 6-month OS rates of patients with and without interstitial change on the CT before SBRT were 50.0% and 14.3%, respectively. The OS rate curves according to the existence of heart disease are shown in Figure 3. The 6-month OS rates of patients with and without interstitial change on the CT before SBRT were 16.7% and 46.7%, respectively.
Figure 2.
Overall survival rate according to the existence of pulmonary interstitial change.
Figure 3.
Overall survival rate according to the existence of heart disease.
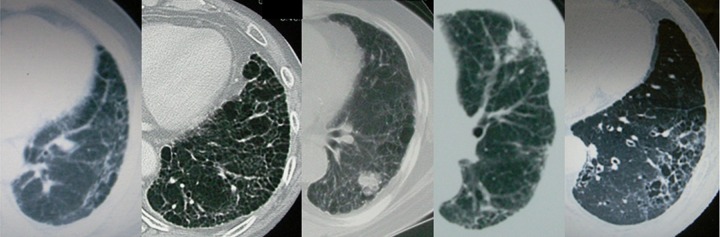
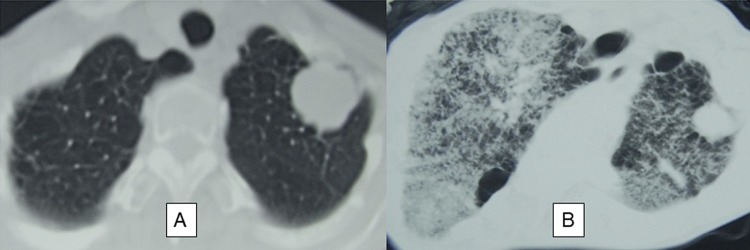
There were 4 patients in whom FRP occurred within 2 months after the start of SBRT and who died within 1 month thereof; their characteristics are described in Table 2. Three of these patients had no pulmonary interstitial change before SBRT, but had heart disease. Figure 4 shows the CT findings of the 5 patients who had pulmonary interstitial change before SBRT. Figure 5 shows FRP that appeared early in a patient and showed rapid progression.
Table 2.
Characteristics of the 4 Patients in Whom FRP Occurred Within 2 Months After the Start of SBRT and Resulted in Death in 1 Month.
| Case No. | Duration From SBRT to Onset of FRP, days | Duration From Onset of FRP to Death, days | Age (years) | Sex | Tumor Size (mm) | Brinkman Index | Pulmonary Interstitial Change Before SBRT | Administration of Steroid Before SBRT | Chronis Cardiac Disease | KL-6 | %VC | FEV 1.0% | SBRT Dose (Gy/fraction) | V20 (Lung-GV) | MLD, Gy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 42 | 4 | 64 | Female | 30 | Unknown | Negative | Positive | Positive | NA | 60% | 83.0% | 75/10 | 20.5% | 3.0 |
| 14 | 44 | 19 | 72 | Male | 15 | 300 | Negative | Positive | Negative | 835 | 79% | 68.9% | 48/4 | 6.3% | 4.9 |
| 16 | 56 | 15 | 85 | Male | 38 | 800 | Positive | Negative | Positive | 989 | 103% | 71.8% | 48/4 | 6.3% | 4.3 |
| 20 | 42 | 30 | 63 | Male | 30 | 840 | Negative | Negative | Positive | NA | 65% | 73.7% | 60/8 | 18.2% | 9.8 |
Abbreviation: FEV1.0%: forced expiratory volume percent in one second; FRP, fatal radiation pneumonitis; KL-6, Krebs von den Lungen-6; MLD: mean dose of normal lung; NA: not assigned; SBRT, stereotactic body radiation therapy; %VC: percent vital capacity; V20, ratio of the volume receiving 20Gy or more to the normal lung volume.
Figure 4.
Computed tomography images from 5 patients with fatal radiation pneumonitis in whom pulmonary interstitial change were detected before stereotactic body radiation therapy.
Figure 5.
Computed tomography (CT) images from a 64-year-old woman with T2N0M0 adenocarcinoma who developed early fatal radiation pneumonitis that showed rapid progression. The patient had chronic heart failure, and CT performed before stereotactic body radiation therapy (SBRT) showed no pulmonary interstitial change. (A) The tumor shrank (a partial response), but strong dyspnea with diffuse interstitial pneumonitis appeared 40 days after the start of SBRT (B); the patient died 4 days later. The total (normal) lung volume receiving 20 Gy or more (V20) was 5.0%, and the mean dose to the normal lung was 3.0 Gy.
Discussion
A 2008 survey of Japanese patients who underwent SBRT indicated that treatment-related deaths occurred in 11 (0.46%) of 2390 patients who underwent SBRT for pulmonary lesions.1 In the present study, FRP was observed in 1.3% of the patients investigated; the higher ratio in our study may be attributable to differences in patients’ characteristics compared to those in the Japanese survey.
It is important to clarify the mechanisms of FRP as well as its risk factors. Current evidence suggests that numerous factors, as well as various types of lung parenchymal cells, contribute to the pathogenesis of radiation lung damage.2 The progression of radiation-induced damage is a result of the early activation of inflammatory reactions leading to the expression and maintenance of an elevated cytokine cascade.3
With respect to the relationship between radiation pneumonitis and radiotherapy planning, Yamashita et al reported a high incidence of FRP (3 of 25 patients) in their early experience with SBRT, including in patients with poor respiratory function, interstitial pneumonitis (IP), and recurrence after surgery. They considered that the reason for the high incidence of FRP included patient background and large volumes of lung parenchyma irradiated at high doses.4 Timmerman et al 5 reported a higher incidence of FRP after SBRT in tumors that were located more centrally. Hope et al 6 and Matsuo et al 7 found that FRP occurrence is correlated with the volume of the higher dose region; although, Takeda et al 8 claimed that severe radiation pneumonitis post-SBRT for lung cancer was not related to lung dose.
Concerning the relationship with patient background in the development of FRP in this study, it is notable that more than half (63%) of patients who experienced FRP had CT findings of IP. Interstitial pneumonitis is considered a critical potential complication following surgery, chemotherapy, or radiotherapy for lung malignancies.9 Takeda et al 10 reported acute exacerbation of subclinical idiopathic pulmonary fibrosis triggered by SBRT in a patient with slight focal honeycomb changes of the lung, and argued that attention must be paid to the presence of comorbid IP even if minimal. Table 3 summarizes the incidences of severe radiation pneumonitis categorized based on whether they were accompanied by pulmonary interstitial changes after SBRT for lung cancer.7,11–14 Grade 3 or higher radiation pneumonitis was observed in 10% to 40% of patients with pulmonary interstitial changes; grade 5 was observed in more than 5% of such patients. These rates may even be higher in prospective settings.
Table 3.
Frequency of Radiation Pneumonitis Post-SBRT for Lung Cancer According to Presence of Pulmonary Interstitial Change.
| Pulmonary Interstitial Change | Author (Ref) | Study Design | Patient Number | Dose/Fraction | Grade of Radiation Pneumonitis | Frequency | Risk Factor |
|---|---|---|---|---|---|---|---|
| No pulmonary interstitial change | Matsuo et al 7 | Retrospective | 74 | 48 Gy/4 fr | 3 | 10.6% | V25 |
| 4 | 1.9% | ||||||
| Nagata et al 11 | Prospective | 104 (inoperable) | 48 Gy/4 fr | 3 | 6.2% | ||
| 65 (operable) | 48 Gy/4 fr | 3 | 3.6% | ||||
| Yamaguchi et al 12 | Retrospective | 86 | 48 Gy/4 fr | 4-5 | 0.0% | ||
| Ueki et al 13 | Retrospective | 137 | 48-60 Gy/4-8 fr | 3-5 | 1.4% | ||
| Yoshitake et al 14 | Retrospective | 242 | 48 Gy/4 fr | 3 | 1.2% | ||
| 4-5 | 0.0% | ||||||
| With pulmonaryinterstitial change | Yamaguchi et al 12 | Retrospective | 16 | 48 Gy/4 fr | 3 | 6.3% | V5-25, MLD |
| 4 | 6.3% | ||||||
| 5 | 6.3% | ||||||
| Ueki et al 13 | Retrospective | 20 | 40-60Gy/4-8 fr | 3-5 | 10.0% | ||
| Yoshitake et al 14 | Retrospective | 18 | 48 Gy/4 fr | 3 | 16.7% | KL-6, V5, V10, MLD | |
| 4-5 | 22.3% |
Abbreviations: KL-6, Krebs von den Lungen-6; MLD, mean dose of normal lung; SBRT, stereotactic body radiation therapy
As for other risk factors, Kong et al 15 reported that blood biomarkers such as transforming growth factor (TGF)- β1, interleukin-6, KL-6, surfactant proteins, and interleukin-1 receptor agonist could accurately predict radiation-induced lung damage. In this study, KL-6 was abnormally high in more than 70% of the cases. Additionally, other studies have shown a relationship between the severity of radiation pneumonitis and various genetic variations or polymorphisms in microRNA-related genes,16 vascular endothelial growth factor,17 and TGF-β1.18 Notably, 7 of 19 patients with FRP had no IP detected on CT. Coexisting heart disease might be possibly another important risk factor for FRP; however, the incidences of heart failure should be investigated in patients with no severe FRP outbreaks for comparison. To our knowledge, no published studies exist on the relationship between radiation pneumonitis and coexisting heart disease. However, the existence of such a relationship, as well as between radiation pneumonitis and other possible risk factors, is very much worth investigating. Our data suggest that patients with risk factors such as pulmonary interstitial change or coexisting heart disease would be better to receive special attention following SBRT; such individuals may require higher dose-concentrated methods and should be monitored carefully after SBRT. Ebara et al 19 reported that particle therapy may be useful for sparing the background lung from high radiation doses.
There were some limitations in our study. First, we could not definitely show the true proportion of patients with FRP in all patients because this was just a retrospective case series study.
Second, particular risk factors of FRP were not examined because we had no comparison of the factors between the 2 groups, with or without FRP. We remarked that coexisting pulmonary interstitial change and heart disease are major risk factors in this study; however, these remain to be confirmed. Therefore, we will further examine these issues in case–control or prospective studies; in addition to this, we aim to obtain serum or tissue for key genetic factors or inflammatory mediators, in future.
In summary, the survival intervals following the appearance of pneumonia symptoms in 23 patients who developed FRP after SBRT for lung cancer were generally short but exhibited a wide range. More than half of the patients had pulmonary fibrosis on CT before SBRT, although immediately progressive FRP was also observed among patients without such pulmonary interstitial change. Coexisting heart disease might possibly exacerbate radiation pneumonitis. Further prospective study with a large cohort is mandatory to confirm it by a comparison of these factors between patient cohorts with or without them.
Acknowledgment
We would like to thank Editage (www.editage.jp) for English-language editing.
Abbreviations
- BED
biological effective dose
- CRP
C-reactive protein
- CT
computed tomography
- CTV
clinical target volume
- FEV1%
forced expiratory volume percent in one second
- FRP
fatal radiation pneumonitis
- GTV
gross target volume
- IP
interstitial pneumonitis
- KL-6
Krebs von den Lungen-6
- LDH
lactate dehydrogenase
- OS
overall survival
- PTV
planning target volume
- SBRT
stereotactic body radiation therapy
- TGF
transforming growth factor
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number JP16H05389.
ORCID iD: Hiroshi Onishi, MD, PhD  http://orcid.org/0000-0002-3512-1166
http://orcid.org/0000-0002-3512-1166
Yukinori Matsuo, MD, PhD  http://orcid.org/0000-0002-4372-8259
http://orcid.org/0000-0002-4372-8259
References
- 1. Nagata Y, Hiraoka M, Mizowaki T, et al. Survey of stereotactic body radiation therapy in Japan by the Japan 3-D conformal external beam radiotherapy group. Int J Radiat Oncol Biol Phys. 2009;75(2):343–347. [DOI] [PubMed] [Google Scholar]
- 2. Kong FM, Ten Haken R, Eisbruch A, Lawrence TS. Non-small cell lung cancer therapy-related pulmonary toxicity: an update on radiation pneumonitis and fibrosis. Semin Oncol. 2005;32(2 suppl 3):S42–S54. [DOI] [PubMed] [Google Scholar]
- 3. Rubin P, Johnston CJ, Williams JP, McDonald S, Finkelstein JN. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys. 1995;33(1):99–109. [DOI] [PubMed] [Google Scholar]
- 4. Yamashita H, Nakagawa K, Nakamura N, et al. Exceptionally high incidence of symptomatic grade 2-5 radiation pneumonitis after stereotactic radiation therapy for lung tumors. Radiat Oncol. 2007;2:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24(30):4833–4839. [DOI] [PubMed] [Google Scholar]
- 6. Hope AJ, Lindsay PE, El Naqa I, et al. Modeling radiation pneumonitis risk with clinical, dosimetric and spatial parameters. Int J Radiat Oncol Biol Phys. 2006;65(1):112–124. [DOI] [PubMed] [Google Scholar]
- 7. Matsuo Y, Shibuya K, Nakamura M, et al. Dose–volume metrics associated with radiation pneumonitis after stereotactic body radiation therapy for lung cancer. Int J Radiat Oncol Biol Phys. 2012;83(4):e545–e549. [DOI] [PubMed] [Google Scholar]
- 8. Takeda A, Ohashi T, Kunieda E, et al. Early graphical appearance of radiation pneumonitis correlates with the severity of radiation pneumonitis after stereotactic body radiotherapy (SBRT) in patients with lung tumors. Int J Radiat Oncol Biol Phys. 2010;77(3):685–690. [DOI] [PubMed] [Google Scholar]
- 9. Collard HR, Moore BB, Flaherty KR, et al. ; Idiopathic Pulmonary Fibrosis Clinical Research Network Investigators. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176(7):636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takeda A, Enomoto T, Sanuki N, et al. Acute exacerbation of subclinical idiopathic pulmonary fibrosis triggered by hypofractionated stereotactic body radiotherapy in a patient with primary lung cancer and slightly focal honeycombing. Radiat Med. 2008;26(8):504–507. [DOI] [PubMed] [Google Scholar]
- 11. Nagata Y, Hiraoka M, Shibata T, et al. Prospective trial of stereotactic body radiation therapy for both operable and inoperable T1N0M0 non-small cell lung cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys. 2015;93(5):989–996. [DOI] [PubMed] [Google Scholar]
- 12. Yamaguchi S, Ohguri T, Ide S, et al. Stereotactic body radiotherapy for lung tumors in patients with subclinical interstitial lung disease: the potential risk of extensive radiation pneumonitis. Lung Cancer. 2013;82(2):260–265. [DOI] [PubMed] [Google Scholar]
- 13. Ueki N, Matsuo Y, Togashi Y, et al. Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival after stereotactic body radiation therapy for lung cancer. J Thorac Oncol. 2015;10(1):116–125. [DOI] [PubMed] [Google Scholar]
- 14. Yoshitake T, Shioyama Y, Asai K, et al. Impact of interstitial changes on radiation pneumonitis after stereotactic body radiation therapy for lung cancer. Anticancer Res. 2015;35(9):4909–4913. [PubMed] [Google Scholar]
- 15. Kong FM, Ao X, Wang L, Lawrence TS. The use of blood biomarkers to predict radiation lung toxicity: a potential strategy to individualize thoracic radiation therapy. Cancer Control. 2008;15(2):140–150. [DOI] [PubMed] [Google Scholar]
- 16. Wen J, Liu H, Wang Q, et al. Genetic variants of the LIN28B gene predict severe radiation pneumonitis in patients with non-small cell lung cancer treated with definitive radiation therapy. Eur J Cancer. 2014;50(10):1706–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yin M, Liao Z, Yuan X, et al. Polymorphisms of the vascular endothelial growth factor gene and severe radiation pneumonitis in non-small cell lung cancer patients treated with definitive radiotherapy. Cancer Sci. 2012;103(5):945–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yuan X, Liao Z, Liu Z, et al. Single nucleotide polymorphism at rs1982073:T869C of the TGFbeta 1 gene is associated with the risk of radiation pneumonitis in patients with non-small-cell lung cancer treated with definitive radiotherapy. J Clin Oncol. 2009;27(20):3370–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ebara T, Shimada H, Kawamura H, et al. Dosimetric analysis between carbon ion radiotherapy and stereotactic body radiotherapy in stage I lung cancer. Anticancer Res. 2014;34(9):5099–5104. [PubMed] [Google Scholar]