Abstract
MAPK pathways represent a unique extracellular signal response system. An important feature of such a multicomponent system appears to be the spatial intracellular organization of individual components. Recent studies demonstrate that the MAP kinases of such pathways are the molecular link between the plasma membrane sensors and the nuclear transcription factors. Stimulation of several MAPK pathways induces rapid and transient nuclear accumulation of MAP kinases. Investigations on the mode of regulation of this process using higher eukaryotes Erk2 and lower eukaryotes Hogl and Sty1/Spc1 have revealed that at least three events contribute to signal-induced nuclear localization of these MAP kinases: activation by phosphorylation, regulated nuclear import and export, and nuclear retention.
Keywords: MAPK, MEK, Nuclear import and export, Stress, Yeast
IN the eukaryotic kingdom, the response to a broad spectrum of extracellular stimuli is often mediated by well-conserved cascades of signaling molecules generally known as MAPK pathways. The core of MAPK pathway signaling modules consists of three protein kinases, which serve as acceptors of signal received via primary signal sensors located usually in the plasma membrane. The activated MAPK kinase kinase (MAPKKK or MEKK), the most upstream component of the MAPK module, phosphorylates and thereby activates the dual specificity MAPK kinase (MAPKK or MEK), which in turn activates MAP kinase (MAPK or ERK) by phosphorylation on closely spaced conserved tyrosine and threonine residues within T-X-Y motif, where X could be glutamic acid, proline, or glycine. This highly ordered sequence of phosphorylation events within MAPK module then helps to transmit extracellular stimulus into the signal-specific transcriptional output, which finally determines a cell/organism response. There are many examples that transcription of the response-specific genes requires MAP kinase-dependent modification of relevant targets such as transcription factors (38,41).
Naturally, when a signal is initially recorded by plasma membrane-associated sensors it has to cross cytoplasm and reach the nucleus in order to result into transcriptional response. This emphasizes the importance of dynamic intracellular localization of signaling components involved as a key determinant of proper response and leads to important questions: particularly, which signalling component enters the nucleus and how is this process regulated?
A variety of MAPK pathways have been identified both in mammals and yeasts that are involved in different cellular processes. Erk1/2 were the first members of the MAPK family characterized in mammals. Erk1/2 MAPK pathway integrates several upstream signals which have in common that they stimulate cell proliferation. The binding of growth factors to appropriate membrane-associated receptors leads to subsequent stimulation of Erk1/2 MAPK pathway activity (34). Erk1/2 pathway has also been shown to be important to trigger a cell differentiation program in neuronal cells (5). At least two additional MAPK pathways exist in mammals: the JNK/SAPK (6,24) and p38/RK/CSBP pathways (10,16,24,25,35). These MAPK pathways are activated by multiple environmental stresses, including osmotic stress, UV irradiation, heat stress, and lipopolysaccharides. In addition, both of these pathways accept input signals coming from the action of cytokines (33). In both budding and fission yeast, MAPK pathways are indispensable for control of a variety of cellular processes including mating differentiation, pseudohyphal development, resistance to hyperosmotic and hyposmotic stress, heat stress, oxidative stress, polarized growth, cell cycle progression, and sporulation [for review see (1,28,39)]. Studies on Erk1/2 (18,27) and JNK1 (3) localization revealed the exciting possibility that MAP kinases might function as signal messengers between the cytoplasmic and the nuclear compartments. However, the regulation of this process remained for a long period of time largely unknown. Recent studies on yeast and mammalian MAPKs have now contributed substantially to our understanding of regulation of MAP kinase nuclear transport events and have shed light on the complexity of this phenomenon.
THE SIGNAL FOR NUCLEAR ACCUMULATION OF MAP KINASES
Early studies on spatial distribution of MAPK pathway components in mammalian cells have concentrated on the closely related isoforms Erk1 and Erk2. They have revealed that they rapidly accumulate in the nuclear compartment and that this change in cellular location correlates with stimulation of Erk1/2 activity upon addition of growth stimulating factors (4,18,27). Increased concentration of activated Erk1/2 in the nucleus might result in phosphorylation of nuclear targets such as Elk1 [for review see (41)]. Indeed, it has been demonstrated that Erk1/2-dependent phosphorylation of Elk1 enhances its transcription activity (17,21). Thus, nuclear accumulation of MAP kinase elicits response-specific stimulation of gene expression (4). These results suggest that there might be a direct coordination between activation and nuclear accumulation of Erk1/2. There are at least two plausible models that would be in agreement with such a coordinated event. Phosphorylation of Erk1/2 might cause changes in their three-dimensional structure in the way that they become recognized by phosporylated form-specific nuclear transport machinery. Alternatively, Erk1/2 activity could be essential for the modification of certain protein(s) participating in their nuclear accumulation. Surprisingly, Lenormand et al. (27) have shown that corresponding phosphorylation site mutants accumulate in the nucleus as efficiently as a wild-type form of kinase. It is noteworthy that these experiments have been performed with transfected cells expressing relatively high levels of kinase and in the presence of the endogenous Erk1/2, which might interfere with the localization assay of ectopically expressed Erk1/2 (23). Khokhlatchev et al. (23) have demonstrated using microinjection technique that unphosphorylated form of Erk2 was able to accumulate in the nucleus when co-injected with phosphorylated form. These authors have shown that phosphorylated form of MAP kinase forms dimers with both phosphorylated and unphosphorylated kinase in vitro and suggested that homodimerization facilitates MAPK nuclear translocation. Thus, phosphorylation of endogenous form of MAP kinase might be able to stimulate in trans nuclear accumulation of ectopically expressed phosphorylation mutant form of kinase by induced protein dimerization. Importantly, these authors have clearly provided evidence that phosphorylation of Erk2 promotes its nuclear accumulation (23). Moreover, using yeast stress-activated MAP kinases as model systems, three groups have independently demonstrated that phosphorylation of conserved residues within the T-X-Y motifs of fission yeast Sty1/ Spc1 MAP kinase and budding yeast Hog1 MAP kinase is absolutely required and sufficient for its signal (i.e., hyperosmotic stress)-induced nuclear accummulation [(8,15), Reiser et al., submitted]. Yeast genetics has allowed to perform the localization studies in a strain background where endogenous chromosomal copy of the MAP kinase gene was replaced by tagged form of gene expressed at physiological levels [(8,15), Reiser et al., submitted]. Whether homodimerization plays a role in the nuclear accumulation of yeast MAP kinases remains to be addressed. However, the fact that activated form of Sty1/Spc1 and Hog1 MAP kinase does not induce nuclear accumulation of mutants deficient in threonine and tyrosine phosphorylation does not support such a hyphothesis [(15), Reiser et al., submitted].
Studies from both mammals and yeast demonstrate that MAP kinase mutants defective in catalytic activity are competent for nuclear accumulation [(8,18,23), Reiser et al., submitted]. Thus, MAP kinase activity-dependent modification of transport factors potentially involved in nuclear import of MAP kinase is therefore not required for nuclear accumulation of MAP kinase to take place.
How then is dual phosphorylated MAPK committed to accumulate in the nucleus? The fission yeast Sty1/Spc1 MAP kinase and its budding yeast counterpart Hog1 seem to be evenly distributed between cytoplasm and the nucleus of nonstressed cells [(8,15), Reiser et al., submitted]. This distribution pattern remains unchanged in mutants that lack the corresponding MAPK activator Wis1 and Pbs2, respectively [(15), Reiser et al., submitted], where no dual MAPK phosphorylation on threonine or tyrosine residues required for the activation of the kinase is supposed to occur. This observation implies that basal nuclear distribution does not require phosphorylation of MAP kinase. Because Hog1 molecular weight, when fused to GFP moiety, exceeds considerably the limit for diffusion through nuclear pore (31), it is reasonable to assume that also unphosphorylated Hog1 might enter the nucleus by an active process under basal conditions. It is not unlikely that Hog1 continuously shuttles between the cytoplasm and the nucleus. According to this picture, stress-induced phosphorylation of Hog1 and Sty1/Spc1, respectively, might then enhance nuclear import. Alternatively, it is intriguing to speculate that signal-induced modification of MAPK by dual phosphorylation makes such a form incompetent for nuclear export. Consistent with this model, when injected into the nuclear compartment, unphosphorylated Erk2 remains nuclear only for less than 5 min whereas the phosphorylated form resides in the nucleus for at least 2 h before being redistributed into the cytoplasm (23). Moreover, cytoplasmic redistribution of Hog1 correlates with its dephosphorylation [(8), Reiser et al., submitted]. Both these mechanisms, enhanced nuclear import or reduced nuclear export, would change MAP kinase intracellular distribution towards nuclear accumulation upon stimulation of the corresponding MAPK pathway.
In summary MAP kinases studied so far seem to have evolutionary conserved mode of regulation of their intracellular distribution; the phosphorylation of threonine and tyrosine residues in T-X-Y motif is both necessary and sufficient for nuclear accumulation of these MAP kinases whereas kinase activity is dispensable for this process. Crystallographic studies on nonactivated and activated Erk2 MAPK demonstrate that activation of Erk2 by phosphorylation of conserved amino acid residues causes conformational change in MAPK three-dimensional structure (2). Thus, it is possible that such a structural change is not only important for kinase enzymatic activity, but also provides conformation-build-in signal for nuclear accumulation. It remains to be clarified whether phosphorylation stimulates nuclear import, inhibits nuclear export, or acts in both ways.
CYTOPLASMIC ANCHORING OF MAPKs
Because MAP kinases apparently do not possess a classical nuclear localization signal (NLS), which could be identified in their primary sequence, a mechanism involving this type of element does not appear to contribute to nuclear transport of MAP kinases. However, it cannot be excluded that MAPKs have other types of signal sequences determining their nuclear localization. In line with such a model, these signals might be recognized by the nuclear import machinery only when MAPK is dissociated from a cytoplasmic anchor. MEK has been proposed to play such a role (14). According to this model, MAPK would be released from MEK in the cytoplasm upon MEK-dependent phosphorylation. Alternatively, the MAPK could be coimported with help of another molecule providing the signal for nuclear translocation and MEK could also have this type of function. Wild-type MEK appears to be constitutively localized in cytoplasm because of the presence of nuclear export signal (NES) (12,13). Jaaro et al. (22) have shown that deletion of NES results in nuclear localization of kinase-deficient mutant of MEK during Erk1/2 pathway stimulation. Thus, MAPK could be coimported with MEK into nucleus, where the MEK–MAPK complex disassembles upon MAPK phosphorylation, and MEK is rapidly exported to cytoplasm resulting in its cytoplasmic appearance.
Yeast MEKs seem not to have classical NES sequences; however, the observation that both MEKs are located in the cytoplasm is consistent with an assumption that some mechanism of active nuclear exclusion might exist also in this case. As already mentioned above, yeast Hog1 and Spc1 MAPKs are uniformly distributed in cells deleted for corresponding yeast MEK [(8,15), Reiser et al., submitted]. This observation indicates that, in contrast to mammalian cells, MAPK is not detained in the cytoplasm by MEK-dependent retention. However, one should not ignore the fact that no dual phosphorylation of MAPK, an essential precondition for MAPK nuclear accumulation, takes place in MEK-deficient cells. Thus, perhaps direct protein interaction between MEK and MAPK under basal conditions rather ensures that most of MAPK is localized at the vicinity of MEK in order to be rapidly “found” and activated by MEK.
NUCLEAR RETENTION OF MAPKs
The dual phosphorylation of conserved threonine and tyrosine residue is essential and sufficient for rapid accumulation of MAPK in the nucleus. In terms of physiology, not only rapid nuclear accumulation is important, but also duration of MAPK nuclear residence has relevant physiological importance: the time during which ERK2 remains nuclear determines cell fate. In PC 12 cells, prolonged activation and concentration of MAPK in the nucleus is correlated with cell differentiation rather than proliferation (40).
What determines the duration of MAPK nuclear residence? In fission yeast Gaits et al. (15) have introduced nuclear retention as a new important mechanism in regulation of MAPK intracellular distribution. These investigations were able to demonstrate that Sty1/Spc1 downstream targets, transcription factor Atf1 (the fission yeast homolog of mammalian ATF2), and another transcription factor, Pcr1, are required for nuclear accumulation of Sty1/Spc1 induced by hyperosmotic stress. Atf1 has been shown to be a direct target of Sty1/Spc1, and Sty1/Spc1-dependent phosphorylation increases Atf1 trancriptional activity (36,42). Atf1 appears to be localized constitutively in the nucleus and this distribution pattern depends on heterodimerization with Pcr1. The lack of nuclear accumulation of Sty1/Spc1 in atf1 mutants also suggests that, in fission yeast, Atf1 appears to be a major (if not exclusive) nuclear retention factor for Sty1/Spc1 MAPK. The situation in budding yeast is more complicated. There appears to be no functional homolog(s) of Atf1/ATF2 in this organism. Furthermore, up to now, no direct substrate of Hog1 MAPK has been identified. However, two closely homologous transcription factors, Msn2 and Msn4, which have been shown to bind to stress regulatory sequence (STRE) (29) located in promotors of many stress-regulated genes (30), play a role in this context. They are essential or important for transcription induction of these genes by a variety of environmental stresses, including the HOG pathway-dependent induction by high osmolarity, but their involvement in Hog1-dependent induction through STREs is unclear. Furthermore, two other factors, Msn1 and Hot1, have been recently identified, which together with Msn2 and Msn4 appear to be essential for hyperosmotic stress induction of some genes involved in protection against this type of stress, like GPD1 (M. Rep and V. Reiser, in preparation). When the localization of Hog1 MAPK fused to GFP was studied in the strain deficient for Msn1, Msn2, Msn4, and Hot1, the duration of Hog1 nuclear residence following hyperosmotic stress was strongly reduced. In msn2,4 mutant background, Hog1 becomes prematurely redistributed into the cytoplasm during stress adaptation. An additional deletion of MSN1 and HOT1 further reduces Hog1 half-time of nuclear residence to almost one third compared with wild-type strain, suggesting that these proteins might also function as nuclear retention factors (Reiser et al., submitted). Consistent with this view, Msn2/4 translocate into the nucleus following hyperosmotic stress (19) and Msn1, Hot1 appear nuclear (7) (M. Rep and V. Reiser, in preparation). Because protein synthesis is not required for nuclear accumulation of Hog1, an appealing model is that reduced duration of nuclear accumulation in msn1,2,4,hot1 mutants is due to absence of retention factors. However, one cannot entirely exclude the possibility, that the basal transcriptional activity of these factors is required for nuclear retention of Hog1.
Importantly, Hog1 accumulates in msn1,2,4,hot1 strain in the nucleus as rapidly and as efficiently as in wild-type strain, pointing to the fact that these putative retention factors do not affect the mechanism that ensures rapid accumulation of MAPK in the nucleus (Reiser et al., submitted). In the case of fission yeast it is not known whether the initial nuclear accumulation is affected in atf1 mutants.
Recent data suggest that MAPKs in higher eukaryotes are also retained in the nucleus. Erk1/2 rapidly accumulate in the nucleus by a process independent of protein synthesis. However, their further nuclear localization depends on de novo protein synthesis of nuclear anchors, which have not yet been identified (26).
Thus, it appears that one of the crucial determinants of MAPK nuclear localization is protein interactions with response-specific targets; the higher the number/concentration of nuclear substrates, the longer the kinase stays in the nucleus. These interactions may protect kinase against being grabbed by other factors involved in nuclear export of kinase. Interestingly, Ptp2, a major phosphatase responsible for dephosphorylation of the tyrosine residue essential for activation of Hog1 MAPK, has been found localized in the nucleus (Reiser et al., submitted). There is a clear correlation between cytoplasmic redistribution of Hog1 and its dephosphorylation [(8), Reiser et al., submitted]. Hogl tyrosine dephosphorylation requires its intrinsic enzyme kinase activity for activation of Ptp2 (43). It is therefore not unlikely that Hog1-dependent activation of Ptp2 phosphatase is required for its exit from the nucleus. In such case, the duration of nuclear residence of Hog1 might be determined by competition in kinase–substrate interactions between “positive” partners (such as transcription factors) and “negative” partners (such as phosphatase).
In summary, the studies of dynamics of MAPK localization in fission yeast and budding yeast have revealed that at least two, presumably independent, mechanisms operate on stress-induced intracellular distribution of MAPKs: the regulation of early nuclear transport, and nuclear retention during stress adaptation. It will be important to determine what are the physiological consequences of the modulation of the MAP kinase nuclear residence.
MAPK NUCLEAR TRANSPORT FACTORS
The nuclear accumulation of MAPKs is a very rapid and efficient process. In budding yeast, stress-induced MAPK nuclear localization is observable within 1 min (Reiser et al., submitted); mammalian Erk1/2 is found concentrated in the nucleus within 10 min upon mitogenic stimulation of cells (26). The fact that molecular weights of most MAPKs do not exceed the limit for free diffusion through channel formed by nuclear pore complex does not rule out the possibility that MAPKs’ rapid signal-dependent nuclear transport is an active process. This idea has been tested on yeast MAPK models. All active nuclear transport processes require the small GTPase protein Ran and its regulators (20,31). Ferrigno et al. (8) have investigated whether Ran is also required for budding yeast Hog1 MAPK nuclear transport, Hog1 has failed to accumulate in the nucleus of cells with temperature-sensitive allele of Gsp1, a yeast homolog of mammalian Ran at the restrictive temperature (8). There is a evidence that Sty1/Spc1 MAPK is unable to accumulate in the nucleus of pim1 mutants, which are defective in the guanine-nueleotide-exchange factor (GEF) for fission yeast Ran (F. Gaits and P. Russell, submitted), Ferrigno et al. (8) have identified a putative Hog1 MAPK import factor, Deletion of NMD5, a gene coding for a putative importin β homolog, shows defect in Hog1 stress-induced nuclear accumulation, This effect is specific, because mutants in all other known nuclear transport factors did not display such phenotype, It remains to be proven that Nmd5 is indeed the direct import factor for Hog1 by demonstrating in vivo interaction of these proteins under conditions that induce Hog1 nuclear import, We have discussed above that Hog1 and perhaps also other MAPK might continuously shuttle between cytoplasm and nucleus and that this movement does not require activation of MAPK, It could be that basal intracellular distribution of Hog1 requires other import factor(s) than Nmd5, because nmd5 mutants do not show Hog1 nuclear exclusion (8). Identification of such factors would be necessary to prove the hypothesis of basal nucleocytoplasmic shuttling of MAPKs.
MAPK nuclear accumulation is transient, and adaptation to stimulus-dependent activation involves its cytoplasmic reappearance [(8,26), Reiser et al., submitted]. This process is relatively slow if compared to the rapid nuclear accumulation upon MAPK stimulation. Protein synthesis inhibition has no influence on this process (Reiser et al., submitted). Thus, cytoplasmic redistribution of Hog1 is caused by a nuclear export mechanism rather than by its cytoplasmic resynthesis. Interestingly, the MAP kinase activity is necessary for redistribution. Hog1 catalytically inactive mutant remains nuclear and cannot be redistributed into the cytoplasm [(8), Reiser et al., submitted].
These data suggest that MAPK nuclear export might be an active process like nuclear import. Indeed, Ferrigno et al. (8) could demonstrate that cytoplasmic redistribution of Hog1 is impaired in xpo1/crm1 mutants. Xpo1/Crm1 is nuclear export factor for proteins containing nuclear export sequences (NES) (9,11,32,37). Similar evidence for Crm1-mediated export of MAPK has been obtained also from fission yeast; in addition, Sty1/Spc1 MAPK can be copurified with Crm1 during a time period of MAPK cytoplasmic redistribution (F. Gaits and P. Russell, submitted). Importantly, a small amount of Sty1/Spc1 can be found to be associated with Crm1 even in the absence of stress (F. Gaits and P. Russell, submitted). This finding is consistent with the idea of continuous nucleocytoplasmic shuttling of MAPKs. In this respect, a key experiment would be to find out whether a portion of Sty1/Spc1 associated with Crm1 is phosphorylated or not.
CONCLUDING REMARKS
The basic features of the MAPKs nuclear localization seem to be conserved in evolution within the eukaryotic kingdom. Generalization of our current knowledge of the regulation of MAPKs’ nuclear transport obtained from both higher and lower eukaryotes allows to envision the following “nucleocytoplasmic traffic” scenario (Fig. 1). MAPKs might continuously shuttle between the cytoplasmic and nuclear compartment. Activation of MAPK by dual phosphorylation causes its dissociation from the cytoplasmic MAPKK. MAPK translocates into the nucleus with the help of nuclear import factor(s). Phosphorylated MAPK serves either as a more efficient nuclear import cargo or a less efficient nuclear export cargo, both models causing its rapid nuclear accumulation. MAPKs targets like transcription factors retain the kinase in the nucleus. Adaptation to the signal induces MAPK dephosphorylation and its active nuclear export. Although active import as well as active export of MAPKs has been proven experimentally only in yeast, we hypothesize that it is the most likely to occur also in the case of higher eukaryotic MAPKs. The nature of the primary signal for nuclear export of MAPKs remains to be solved. Experimental data obtained so far suggest that dephosphorylation of MAPK might take place in the nucleus before actual nuclear export of MAPK. However, it is not known whether dephosphorylation of MAP kinase induces its nuclear export. MAP kinase activity-dependent modification of export factors might contribute to the stimulation of its nuclear export.
FIG. 1.
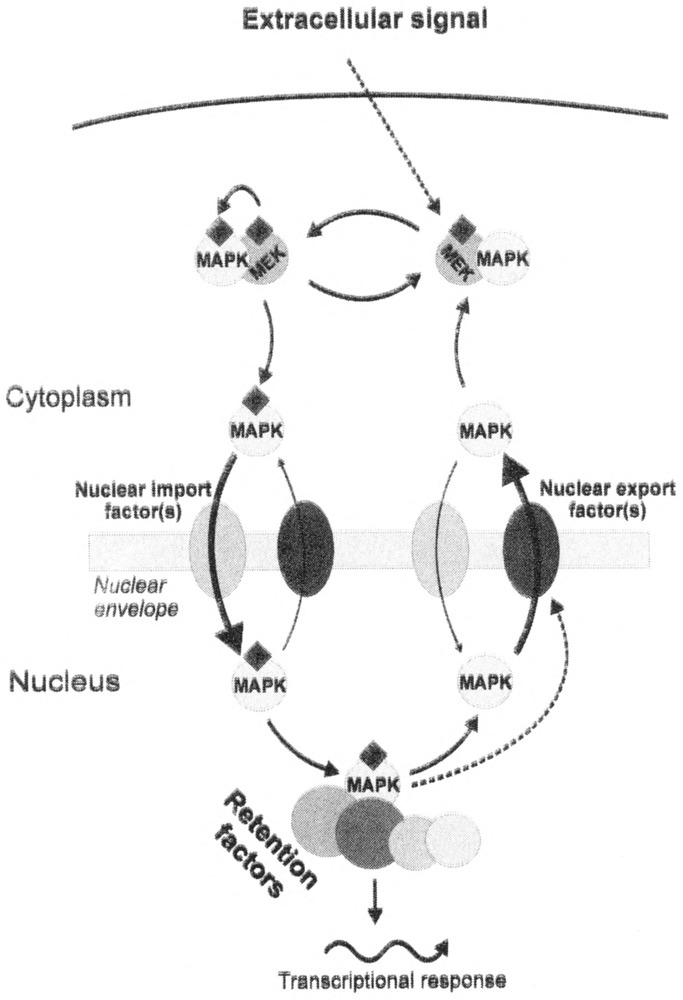
Generalized view on the regulation of MAP kinases nuclear accumulation. For details see Concluding Remarks, P: phosphate.
ACKNOWLEDGMENTS
We thank Paul Russell for communicating results prior to publication and G. Griffioen for helpful comments. Financial support of the work carried out from grants P1 2478 (to H.R.) and W001 (predoctoral fellowship to V.R.) from the Fonds zur Förderung der Wissenschaftlichen Forschung, Vienna is gratefully acknowledged.
REFERENCES
- 1. Banuett F. Signalling in the yeasts: An informational cascade with links to the filamentous fungi. Microbiol. Mol. Biol. Rev. 62:249–274; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Canagarajah B. J.; Khokhlatchev A.; Cobb M. H.; Goldsmith E. J. Activation mechanism of the MAP kinase ERK2 by dual phosphorylation. Cell 90:859–869; 1997. [DOI] [PubMed] [Google Scholar]
- 3. Cavigelli M.; Li W. W.; Lin A.; Su B.; Yoshioka K.; Karin M. The tumor promoter arsenite stimulates AP-1 activity by inhibiting a JNK phosphatase. EMBO J. 15:6269–6279; 1996. [PMC free article] [PubMed] [Google Scholar]
- 4. Chen R. H.; Sarnecki C.; Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol. Cell. Biol. 12:915–927; 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cowley S.; Paterson H.; Kemp P.; Marshall C. J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell 77:841–852; 1994. [DOI] [PubMed] [Google Scholar]
- 6. Derijard B.; Hibi M.; Wu I. H.; Barrett T.; Su B.; Deng T.; Karin M.; Davis R. J. JNK1: A protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 76: 1025–1037; 1994. [DOI] [PubMed] [Google Scholar]
- 7. Estruch F.; Carlson M. Increased dosage of the MSN1 gene restores invertase expression in yeast mutants defective in the SNF1 protein kinase. Nucleic Acids Res. 18:6959–6964; 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferrigno P.; Posas F.; Koepp D.; Saito H.; Silver P. A. Regulated nucleo/cytoplasmic exchange of HOG1 MAPK requires the importin beta homologs NMD5 and XPO1. EMBO J. 17:5606–5614; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fornerod M.; Ohno M.; Yoshida M.; Mattaj I. W. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 90:1051–1060; 1997. [DOI] [PubMed] [Google Scholar]
- 10. Freshney N. W.; Rawlinson L.; Guesdon F.; Jones E.; Cowley S.; Hsuan J.; Saklatvala J. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell 78:1039–1049; 1994. [DOI] [PubMed] [Google Scholar]
- 11. Fukuda M.; Asano S.; Nakamura T.; Adachi M.; Yoshida M.; Yanagida M.; Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature 390:308–311; 1997. [DOI] [PubMed] [Google Scholar]
- 12. Fukuda M.; Gotoh I.; Adachi M.; Gotoh Y.; Nishida E. A novel regulatory mechanism in the mitogen-activated protein (MAP) kinase cascade Role of nuclear export signal of MAP kinase kinase. J. Biol. Chem. 272:32642–3268; 1997. [DOI] [PubMed] [Google Scholar]
- 13. Fukuda M.; Gotoh I.; Gotoh Y.; Nishida E. Cytoplasmic localization of mitogen-activated protein kinase kinase directed by its NH2-terminal, leucine-rich short amino acid sequence, which acts as a nuclear export signal. J. Biol. Chem. 271:20024–20028; 1996. [DOI] [PubMed] [Google Scholar]
- 14. Fukuda M.; Gotoh Y.; Nishida E. Interaction of MAP kinase with MAP kinase kinase: Its possible role in the control of nucleocytoplasmic transport of MAP kinase. EMBO J. 16:1901–1908; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gaits F.; Degols G.; Shiozaki K.; Russell P. Phosphorylation and association with the transcription factor Atfl regulate localization of Spc1/Sty1 stress-activated kinase in fission yeast. Genes Dev. 12: 1464–1473; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Galcheva-Gargova Z.; Derijard B.; Wu I. H.; Davis R. J. An osmosensing signal transduction pathway in mammalian cells. Science 265:806–808; 1994. [DOI] [PubMed] [Google Scholar]
- 17. Gille H.; Sharrocks A. D.; Shaw P. E. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature 358:414–417; 1992. [DOI] [PubMed] [Google Scholar]
- 18. Gonzalez F. A.; Seth A.; Raden D. L.; Bowman D. S.; Fay F. S.; Davis R. J. Serum-induced translocation of mitogen-activated protein kinase to the cell surface ruffling membrane and the nucleus. J. Cell Biol. 122: 1089–1101; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Görner W.; Durchschlag E.; Martinez-Pastor M. T.; Estruch F.; Ammerer G.; Hamilton B.; Ruis H.; Schuller C. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 12:586–597; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Görlich D. Transport into and out of the cell nucleus. EMBO J. 17:2721–2727; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hipskind R. A.; Baccarini M.; Nordheim A. Transient activation of RAF-1, MEK, and ERK2 coincides kinetically with ternary complex factor phosphorylation and immediate-early gene promoter activity in vivo. Mol. Cell. Biol. 14:6219–6231; 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jaaro H.; Rubinfeld H.; Hanoch T.; Seger R. Nuclear translocation of mitogen-activated protein kinase kinase (MEK1) in response to mitogenic stimulation. Proc. Natl. Acad. Sci. USA 94:3742–3747; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khokhlatchev A. V.; Canagarajah B.; Wilsbacher J.; Robinson M.; Atkinson M.; Goldsmith E.; Cobb M. H. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell 93:605–615; 1998. [DOI] [PubMed] [Google Scholar]
- 24. Kyriakis J. M.; Banerjee P.; Nikolakaki E.; Dai T.; Rubie E. A.; Ahmad M. F.; Avruch J.; Woodgett J. R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature 369:156–160; 1994. [DOI] [PubMed] [Google Scholar]
- 25. Lee J. C.; Laydon J. T.; McDonnell P. C.; Gallagher T. F.; Kumar S.; Green D.; McNulty D.; Blumenthal M. J.; Heys J. R.; Landvatter S. W.; et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372:739–746; 1994. [DOI] [PubMed] [Google Scholar]
- 26. Lenormand P.; Brondello J. M.; Brunei A.; Pouyssegur J. Growth factor-induced p42/p44 MAPK nuclear translocation and retention requires both MAPK activation and neosynthesis of nuclear anchoring proteins. J. Cell Biol. 142:625–633; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lenormand P.; Sardet C.; Pages G.; L’Allemain G.; Brunet A.; Pouyssegur J. Growth factors induce nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45mapkk) in fibroblasts. J. Cell Biol. 122:1079–1088; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Madhani H. D.; Fink G. R. The riddle of MAP kinase signaling specificity. Trends Genet. 14:151–155; 1998. [DOI] [PubMed] [Google Scholar]
- 29. Martinez-Pastor M. T.; Marchler G.; Schuller C.; Marchler-Bauer A.; Ruis H.; Estruch F. The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress response element (STRE). EMBO J. 15:2227–2235; 1996. [PMC free article] [PubMed] [Google Scholar]
- 30. Moskvina E.; Schuller C.; Maurer C. T.; Mager W. H.; Ruis H. A search in the genome of Saccharomyces cerevisiae for genes regulated via stress response elements. Yeast 14:1041–1050; 1998. [DOI] [PubMed] [Google Scholar]
- 31. Nigg E. A. Nucleocytoplasmic transport: Signals, mechanisms and regulation. Nature 386:779–787; 1997. [DOI] [PubMed] [Google Scholar]
- 32. Ossareh-Nazari B.; Bachelerie F.; Dargemont C. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science 278:141–144; 1997. [DOI] [PubMed] [Google Scholar]
- 33. Raingeaud J.; Gupta S.; Rogers J. S.; Dickens M.; Han J.; Ulevitch R. J.; Davis R. J. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 270: 7420–7426; 1995. [DOI] [PubMed] [Google Scholar]
- 34. Robinson M. J.; Cobb M. H. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 9:180–186; 1997. [DOI] [PubMed] [Google Scholar]
- 35. Rouse J.; Cohen P.; Trigon S.; Morange M.; Alonso-Llamazares A.; Zamanillo D.; Hunt T.; Nebreda A. R. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell 78: 1027–1037; 1994. [DOI] [PubMed] [Google Scholar]
- 36. Shiozaki K.; Russell P. Conjugation, meiosis, and the osmotic stress response are regulated by Spc1 kinase through Atf1 transcription factor in fission yeast. Genes Dev. 10:2276–2288; 1996. [DOI] [PubMed] [Google Scholar]
- 37. Stade K.; Ford C. S.; Guthrie C.; Weis K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell 90:1041–1050; 1997. [DOI] [PubMed] [Google Scholar]
- 38. Su B.; Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr. Opin. Immunol. 8:402–411; 1996. [DOI] [PubMed] [Google Scholar]
- 39. Toone W. M.; Jones N. Stress-activated signalling pathways in yeast. Genes Cells 3:485–198; 1998. [DOI] [PubMed] [Google Scholar]
- 40. Traverse S.; Gomez N.; Paterson H.; Marshall C.; Cohen P. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem. J. 288:351–355; 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Treisman R. Regulation of transcription by MAP kinase cascades. Curr. Opin. Cell Biol. 8:205–215; 1996. [DOI] [PubMed] [Google Scholar]
- 42. Wilkinson M. G.; Samuels M.; Takeda T.; Toone W. M.; Shieh J. C.; Toda T.; Millar J. B.; Jones N. The Atf1 transcription factor is a target for the Sty1 stress-activated MAP kinase pathway in fission yeast. Genes Dev. 10:2289–2301; 1996. [DOI] [PubMed] [Google Scholar]
- 43. Wurgler-Murphy S. M.; Maeda T.; Witten E. A.; Saito H. Regulation of the Saccharomyces cerevisiae HOG1 mitogen-activated protein kinase by the PTP2 and PTP3 protein tyrosine phosphatases. Mol. Cell. Biol. 17:1289–1297; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


