Abstract
A growing number of experimental observations reveal that the cell nucleus is functionally compartmentalized yet organized to ensure a dynamic response to events that influence nuclear activities. The cellular and molecular response to physiological and environmental stress induces a rapid and transient change in gene expression associated with major changes in nuclear architecture that impacts on signals involved in cell growth. In this review, we will address the effects of stress on the functional compartmentation of the cell nucleus and the dynamic reorganization of nuclear stuctures and function.
Keywords: Heat shock, HSFs, HSPs, Nuclear structure, Nuclear function
THE CELLULAR RESPONSE TO STRESS
Cells have developed an evolutionary conserved mechanism to cope with potentially deleterious stress conditions. The cellular response to stress can be induced by a variety of environmental and physiological conditions including heat shock, alcohol, heavy metals, cell cycle, aging, inflammation, or cancer [for reviews see (25,39,40)]. Stress-inducing agents are characterized by their ability to cause the appearance of misfolded proteins whose accumulation and aggregation can be damaging to the cell. Cells respond to stress by the synthesis of large amounts of specialized proteins known as heat shock proteins (HSPs), whose primary functions are to associate with misfolded proteins to prevent their aggregation and to facilitate their refolding or degradation [for reviews see (17,82)]. HSPs also function as chaperones in unstressed cells, to assist in the folding of newly synthesized proteins and assembly of macromolecular complexes. Moreover, the major heat shock protein HSP90 functions as a capacitor for morphological evolution in Drosophila (60). Indeed, the fruit fly genome contains a hidden reservoir of mutations whose phenotype is undetected in “wild-type” animals expressing normal levels of HSP90 and revealed under conditions when the supply of HSP90 is reduced, thus endowing the organism with the capacity to evolve rapidly when external conditions change. HSPs are thus essential to maintain protein homeostasis at all stages of cell life.
The cellular response to stress has been widely employed as a tool to investigate mechanistic and dynamic aspects of gene expression. The activation of hsp gene transcription is mediated by a family of heat shock transcription factors (HSFs) [for reviews see (41,84)]. Among the various cloned mammalian HSF genes, there is an overall sequence conservation of 40%. Three different HSFs have been identified in humans; HSF1 is responsible for the stress-induced expression of hsp genes (51,61) whereas HSF2 is activated during specific stages of development (64), upon hemin-induced cell differentiation (68), and during inhibition of the ubiquitin-dependent proteasome (29). The function of HSF4 is less well understood and is expressed in a tissue-specific manner with constitutively DNA binding activity, although lacking transcriptional activity, perhaps representing a new class of HSF repressors (45). Active trimeric HSFs bind to heat shock promoter elements, characterized as multiple adjacent repeats of the motif 5′-nGAAn-3′, located in the 5′-flanking regions of hsp genes [(50); for reviews see (41,84)].
The regulation of the transcriptional response to stress has been extensively studied and is well documented [for reviews see (25,39,40)]. In this review, we will focus on the effects of stress on the functional compartmentation of the cell nucleus and on the use of this model system to investigate the dynamic organization of nuclear stuctures and functions, in particular those related to mRNA metabolism.
STRESS AS A MODEL TO STUDY NUCLEAR ORGANIZATION
Understanding how the interphase nucleus is organized and how the internal structure of the nucleus may influence gene replication and activity has become a subject of intense studies in the last decade [for reviews see (23,35,36,67)]. The complexity of the interior of the nucleus is strongly influenced by the high concentrations and organization of proteins and nucleic acids, consequently the regulation of DNA replication and transcription or splicing activities must rely upon different levels of functional compartmentation to ensure access, in an orderly manner, to specific DNA sequences and regulatory proteins at each of the stages in the life of the cell.
Investigating the Organization of the Nucleus
Molecular biology has revealed essential features of the basic molecular mechanisms of gene expression. However, as many of these studies were performed using in vitro assays, the influence of the three-dimensional organization of the nucleus had not been incorporated into the thinking. As recently as 20 years ago, the cell nucleus was still viewed as a container where chromosomes and proteins were floating in a poorly characterized nucleoplasm. Electron microscopic observations subsequently revealed the presence of granular and fibrous structures in the nucleus, but they were usually considered as transient complexes that formed and dispersed as a result of nuclear activities. Progress in the understanding of nuclear organization and its influence on nuclear activity has come from the application of molecular cloning reagents and from the development of new in situ methodologies and high-resolution cell imaging techniques, which have allowed investigations on the localization and function of genes, RNAs, and proteins (70). The discovery and widespread use of the green fluorescent protein (GFP), a very stable green light-emitting autofluorescent protein from the jellyfish Aequorea victoria, allows the analysis of realtime movements of proteins in living cells [(6); for reviews see (33,74)].
Important findings on the organization of chromosomes and nuclear activities have also come from studies using transgenes or viral constructs expressed transiently or stably in human cells and from the analysis of endogenous gene expression. The influence of the integration site of exogenous DNAs on transcriptional activity has been well documented and represents a potential caveat when such constructs are used to investigate nuclear organization with relation to gene expression (63). This “position effect” is particularly remarkable when active genes are translocated to transcriptionally silent heterochromatic regions (13,19). For these and other reasons, it has been important to also examine changes in nuclear events associated with the inducible transcription of hsp genes as they provide a wide dynamic range of expression levels under physiologically relevant conditions.
Stress and Nuclear Function
Transcription
The cellular response to stress represents a rapid and precisely regulated process for inducible gene expression. Multiple levels of control ensure the rapid expression of HSPs and minimize the synthesis of aberrant mRNAs and proteins. Analysis of Drosophila cells has revealed that RNA polymerase II is paused 20 to 40 nucleotides downstream from the transcription start site of the heat shock genes hsp70, hsp27, and hsp26 when the genes are not transcriptionally active (47,53,59). The paused RNA polymerases on the promoters of heat shock genes are released upon binding of active trimers of HSFs to the proximal HSEs, thus resulting in transcriptional activation. At the elevated temperatures of heat shock, the transcription of most non-hsp genes, including ribosomal genes, is arrested with kinetics of repression that are inversely linked with the activation of hsp genes [for reviews see (18,25,39,40,76)]. How these changes are coordinated within minutes upon temperature elevation remains unaddressed.
mRNA Processing
Heat shock also blocks splicing activity in Drosophila, either partially or completely depending on the intensity and duration of the stress (85). Splicing activities seem particularly sensitive to elevated temperatures in many organisms (3,86). Heat shock affects 5′ splice site selection and cleavage of some pre-mRNA (32,71) and can affect alternative splicing (16,66). The thermotolerant state in Drosophila cells, induced by a short treatment at intermediate elevated temperatures, prevents the heat shock-induced disruption of splicing during a subsequent severe heat exposure (7,85). Similarly, HSP104 and HSP70 were shown to reactivate mRNA splicing after heat inactivation in yeast (77). How splicing is disrupted upon stress is not clear, and one hypothesis posits that mRNA processing involves thermolabile factors that become inactivated by stress.
The absence of introns in certain hsp genes such as hsp70 is thought to represent an evolutionary mechanism employed to accelerate the production of mature transcripts and to escape splicing arrest. However, other heat shock genes such as hsp90 are intron containing, which suggests that at least certain intron-containing hsp transcripts can be correctly spliced during stress (31,44,48). A possible explanation is that the splicing of hsp transcripts might be favored if they share a specific sequence for recognition by splicing components. Transcripts lacking this sequence would not be spliced, or would occur at a much lower rate than for hsp transcripts. The poly(A) tail of mRNAs could represent a recognition motif for the splicing machinery, as it has been shown that heat shock increases poly(A) tail length of some hsp transcripts (37,48).
mRNA Transport and Translation
Another means to ensure the continued synthesis of HSPs during stress is the selective transport of hsp mRNAs to the cytoplasm, as observed for hsp70 mRNA in heat-treated yeast (26,38), associated with the inhibition of poly(A) RNA export (26,72). Hsp mRNAs are preferentially translated during stress, and the translation of non-hsp mRNAs is repressed [for reviews see (5,25,39)]. Both mechanisms suggest specific recognition motifs in components of the translational machinery or the nuclear pore complexes and RNAs. Newly synthesized hsp70 transcripts are retained for a period of approximately 15 min after their completion during heat treatment of normal human fibroblasts at 42°C or 45°C (21), which would seem inconsistent with the proposal that hsp genes are intronless as a means to accelerate mRNAs synthesis. The retention of full-length hsp transcripts at the site of transcription may correspond to yet another step of control in mRNA synthesis, regulating the amount of transcripts delivered until a threshold concentration is reached. Alternatively, this retention may be related to the selective export of hsp70 transcripts to the cytoplasm, perhaps reflecting a role for the transcriptional machinery in mRNA export through association between the active transcription site and nuclear pore complexes. It is noteworthy that active hsp genes are preferentially distributed at the nuclear periphery in different cell types [(89); C. Jolly, unpublished observation]. A similar role in mRNA transport has already been suggested for nucleoli based on the nucleolar accumulation of poly(A) RNA in heat-treated yeast cells (72).
Cell Cycle and Cell Life
Exposure to heat shock and other forms of stress negatively impacts on cell proliferation. HSPs are cell cycle and growth regulated and have been shown to have a role in cell cycle control in many organisms [for review see (62)]. Heat shock delays cell cycle progression at the G1/S and S/G2 boundaries (46,78); this may represent a defense mechanism to prevent cells from entering mitosis, a stage when cells are particularly thermosensitive (83). During mitosis, heat shock gene expression cannot be induced despite complete activation of HSF1 (27). When irreparable heat-induced damage occurs, cell death either by apoptosis or necrosis ensues [(1); for review see (11)]. Stress-induced cell death is thought to be due in part to protein aggregation in the nuclear matrix (58). HSPs, in particular HSP70, appear to be essential for the protection against stress-induced apoptosis (15,43). The heat shock response may also be critical in cell aging as aged cells exhibit a dampened response to stress [for review see (55)]. Repeated exposure to mild heat shocks has been shown to delay in vitro aging in human normal fibroblasts (54) and to extend life span in yeast (65). In this regard, the stress response could represent an interesting model system to understand the role of maintenance and repair mechanisms in cell aging.
Stress and Nuclear Structures
Chromosomes
Individual chromosomes occupy discrete regions of the interphase nucleus referred to as chromosome territories [for reviews see (9,23)]. The interchromosomal space is thought to generate a three-dimensional network of channels throughout the nucleoplasm called interchromosomal domain (ICD) where the factors for mRNA synthesis concentrate [(89); for review see (23)]. Evidence for the existence of such an interchromosomal compartment has recently come from the use of a nuclear-targeted vimentin, which polymerizes into a network of channels localized outside of chromosome territories, disassembling during mitosis and reassembling into filaments in anaphase–telophase (4). Active genes would be preferentially localized at the periphery of chromosome territories, such that nascent transcripts can be released into the ICD where they can be immediately processed (22,89). This model suggests that transcriptionally active chromosomes should have more surface area in contact with the ICD. This has already been confirmed for the X chromosomes. Both the active and inactive X chromosomes occupy the same nuclear volume and display similar levels of condensation; however, the inactive X, which contains fewer active genes, has a much reduced surface area compared to the active X (12). Based on this model, one would expect to see a dramatic change in chromosome domain organization during stress. Because stress results in a general decrease in the transcriptional activity of non-hsp genes, the surface area of most chromosomes may be reduced during exposure to stress.
Nuclear Matrix
The discovery of the nuclear matrix, a meshwork of insoluble proteins that remains after extraction of nucleic acids and nuclear soluble proteins, revealed an initial insight into the organization of the nucleus (2). Although the existence of the nuclear matrix remains controversial, a role in maintaining nuclear architecture, regulating key reactions in the nucleus such as replication or transcription, and intranuclear transport has been suggested [for review see (49)]. A consequence of heat shock is a general increase in the mass of nuclear matrix proteins and modifications in the association of specific proteins with the matrix (75,79). Because stress results in disruption of nuclear matrix-associated functions and cell death at high temperatures, the matrix has been suggested to be a target for the stress-induced modifications in nuclear activity and nuclear structures (57). Some of these events may be associated with the stress-induced changes in transcriptional activity by altering essential matrix attachment sites and modifying the association of specific regulatory proteins with the nuclear matrix. Changes in nuclear matrix network may also act to preserve nuclear architecture during stress. Similarly, a collapse and aggregation of vimentin-containing intermediate filaments is also observed in the perinuclear region of the cytoplasm, perhaps as a means to preserve nuclear architecture (81).
Nucleoli
Stress also affects other aspects of the internal structure of the nucleus, of which the nucleolus represents one of the most studied and best understood subnuclear compartments. The nucleolus is a paradigm for nuclear compartmentation [for review see (23)]. Stress causes a general relaxation in the condensation state of nucleoli, changes in both the number and size of the granular ribonucleoprotein components, and a reorganization of the nucleolar fibrillar reticulum (26,72,81). These changes correlate with a dramatic reduction in ribosomal gene transcription (18), and may represent a mechanism to protect the cells from producing aberrant rRNAs and ribosomes. HSP70 and HSP110 accumulate in nucleoli following heat shock, and may have a role in the recovery of nucleolar architecture after stress (80,81). Heat shock also induces the appearance in the nucleoplasm of rod-shaped bodies comprised primarily of actin filaments (81). It is not clear if these nuclear filaments are a mere consequence of dramatic cellular and nuclear reorganization or if they have a structural or functional role in the nucleus of stressed cells.
Nuclear Structures Enriched in Splicing Components
Since the 1970s, an increasing number of nuclear compartments have been described; however, for many of these their function remains unclear. These include the coiled bodies, also known as sphere organelles in amphibians. First observed in 1903 by Ramon y Cajal (52), coiled bodies contain several splicing-related proteins and are associated with some of the genes encoding their constituants [for reviews see (28,56)]. Another example are nuclear speckles enriched in splicing factors such as snRNPs, SC35, or U2B″, poly(A) RNA, and subpopulations of RNA Pol II. While these speckles correspond to 20–40 nuclear sites that exhibit a dynamic reorganization in response to gene activation (34,88), their role in splicing remains an open issue. Several observations demonstrate a spatial association of intron-containing active genes with speckles, suggesting an involvement in both transcription and splicing activities. However, sites of nascent transcription revealed by BrUTP or tritiated uridine incorporation or the distribution of active RNA Pol II are randomly distributed relative to the speckles, suggesting that transcription and splicing occur throughout the nucleoplasm [for reviews see (23,35,36,67)].
The heat shock system has proved to be a powerful model to investigate the role of these nuclear speckles in transcription and splicing. Effect of heat shock on the intranuclear localization of RNA Pol II is not clear, as different anti-Pol II antibodies yield distinct subcellular patterns, perhaps reflecting the differential distribution of RNA Pol II subpopulations. Heat shock was shown to induce the redistribution of active RNA Pol II from a dispersed nucleoplasmic staining to speckles (88), whereas another study, using three different antibodies, revealed no significant changes in Pol II distribution upon heat shock (C. Jolly, C. Vourc’h, M. Robert-Nicoud, and R. I. Morimoto, submitted) (Fig. 1). In contrast, heat shock clearly induces the disruption of snRNPs from the nuclear speckles enriched in splicing factors (69) (Fig. 1). Surprisingly, the nuclear distribution of other splicing factors such as SC35 or U2B″ is not dramatically affected by heat exposure, as well as coiled bodies. Depending on the heat shock conditions and on the cell type considered, only a slight morphological change to a rounded shape of SC35 foci associated with a loss of interconnections between speckles can be observed (69,88) (Fig. 1), whereas the distribution of U2B″ is not affected by stress in human primary fibroblasts (C. Jolly, C. Vourc’h, M. Robert-Nicoud, and R. I. Morimoto, submitted). Although snRNPs no longer concentrate in the speckles in heat-shocked cells, RNA processing still occurs correctly at least for the hsp transcripts, thus demonstrating that high concentrations of snRNPs within these structures are not required for splicing activity. In addition, the intronless hsp70 gene was associated with speckles only when it was activated by stress, demonstrating that subnuclear complexes containing splicing factors function in association with the dynamics of transcription, independent of the presence of introns (C. Jolly, C. Vourc’h, M. Robert-Nicoud, and R. I. Morimoto, submitted). Why splicing factors should associate with intronless nascent transcripts is not clear. This observation supports the proposal that splicing factors are co-recruited with RNA Pol II to new sites of transcription and is supported by numerous observations revealing a physical interaction between splicing factors and the hyperphosphorylated carboxy-terminal domain (CTD) of Pol II (10,30,42,87) (Fig. 2).
FIG. 1.
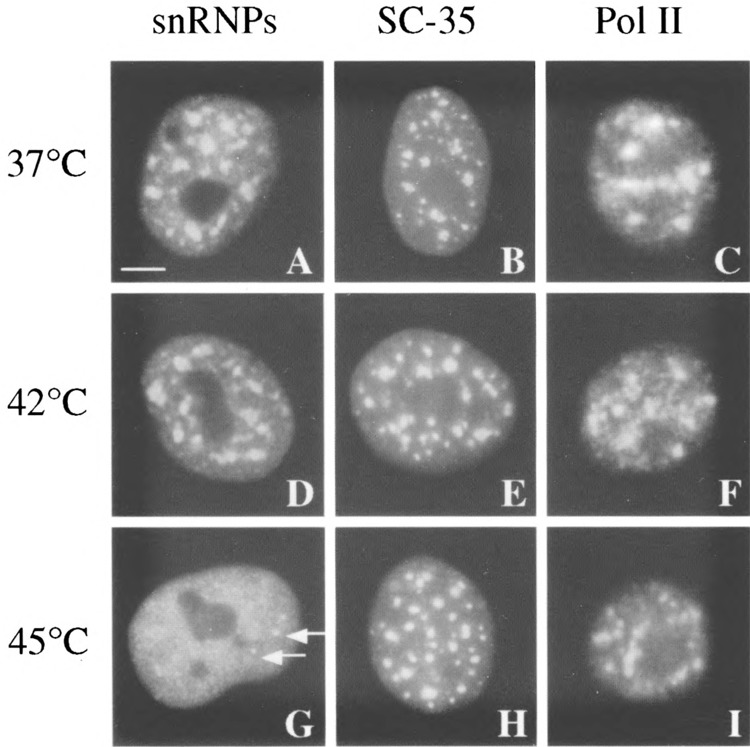
Effect of heat shock on the intranuclear distribution of splicing factors and RNA polymerase II. SC35 (antibody from Sigma) (14) and snRNPs splicing factors (Y12 antibody) (24) as well as a subpopulation of active RNA Pol II (CC-3 antibody) (73) concentrate in the nucleus of human normal fibroblasts in 20–40 nuclear speckles in addition to a nucleoplasmic staining (A–C). Heat shock at 42°C does not affect the distribution of the three proteins (D–F). Heat shock at 45°C disrupts the association of snRNPs with the speckles (G). Only a few dots persist (arrows), perhaps corresponding to coiled bodies. In contrast, the distribution of SC35 and RNA Pol II is not dramatically altered (H–I). Only a slight morphological change in SC35 speckles to a rounded shape is detectable. Bar: 5 μm.
FIG. 2.
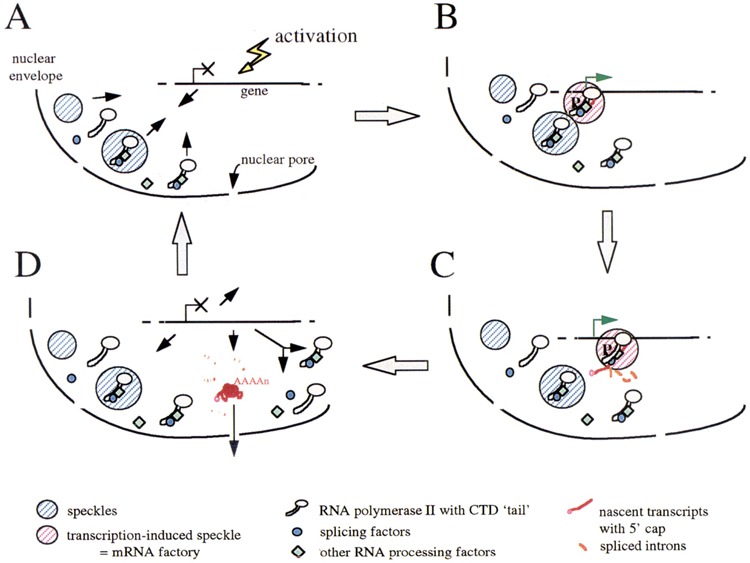
A model of nuclear compartmentation of transcription, splicing, and RNA processing activities in spatially organized mRNA “factories.” Part or all of the RNA processing factors present in the speckles would be bound to the CTD of RNA Pol II even when the enzyme is not active. Some factors, in complexes or alone, could also be present as a diffuse population in the nucleoplasm (A). When a gene is activated, the required amount of RNA Pol II and processing factors would be recruited to this gene, together or alone, independent of the presence of introns. Alternatively, the gene itself could move towards a neighboring speckle, or both mechanisms could occur coordinately to accelerate the process (B). Nascent transcripts would be spliced and polyadenylated co- or posttranscriptionally at the site of transcription, within this mRNA “factory” (pink hatched area) (C). Mature transcripts would then be released in the nucleoplasm and be exported to the cytoplasm. When transcription is attenuated, the polymerase and processing factors would be released from the site of transcription as single molecules or as complexes that can then be recycled (D).
HSF1 Stress Granules
Stress causes the drastic redistribution of HSF1 from a diffuse nucleoplasmic pattern to large nuclear stress granules (8,20,61) (Fig. 3). These nuclear structures were found to be highly dynamic, transient, and reversible, appearing within seconds upon exposure of HeLa cells to 42°C and disappearing within minutes during recovery (C. Jolly, Y. Usson, and R. I. Morimoto, submitted). These observations reveal new features of the dynamics of nuclear organization and demonstrate that the cell nucleus is able to reorganize its structure within seconds in response to changes in environmental conditions and/or in transcriptional activities. They also reveal the existence of signaling pathways precisely regulating the intranuclear trafficking of HSF1 upon stress exposure. The function of the HSF1 stress granules is not known; however, they correspond to the hyperphosphorylated, trimeric DNA binding state of HSF1. Their appearance correlates temporally with the inducible transcription of heat shock genes, but they are distinct from the sites of transcription of the major hsp genes (20). Their presence in heat-shocked mitotic cells, which are devoid of transcription, clearly confirms that they are involved in a function distinct from transcription (C. Jolly, Y. Usson, and R. I. Morimoto, submitted). HSF1 stress granules could represent a novel nuclear compartment that may function to coordinately regulate the multiple states of HSF1 activity during events of inducible phosphorylation and dephosphorylation, association with trans-regulatory chaperones and negative regulators, and changes in oligomeric state [for review see (41)] (Fig. 3).
FIG. 3.
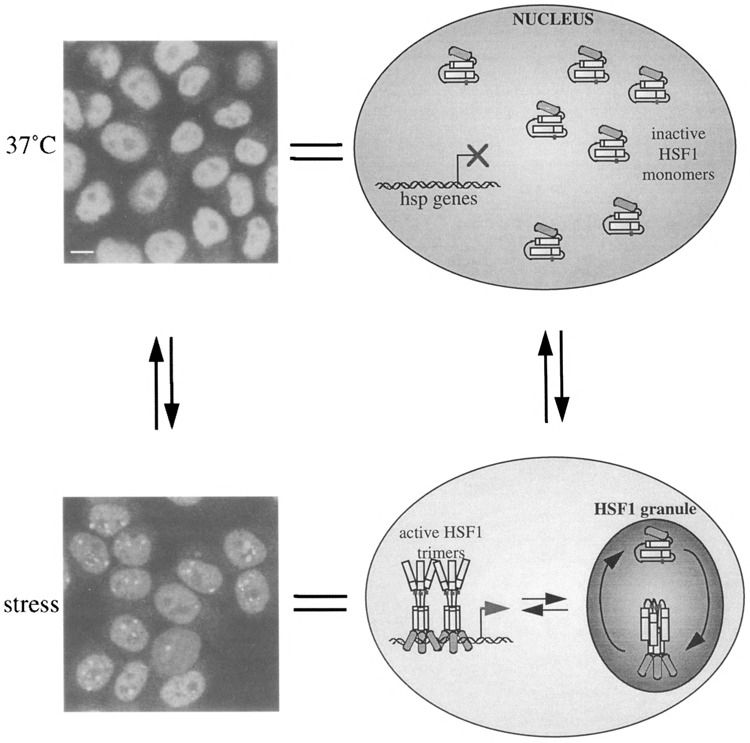
HSF1 stress granules as a highly dynamic, reversible, and transient compartment for the regulation of HSF1 activity. Under normal growth conditions, HSF1 detected with a rat monoclonal antibody (8) is present in the nucleus as a diffuse population excluded from nucleoli and corresponding to inactive monomers (top panel). Upon stress, HSF1 stress granules form rapidly in discrete regions of the nucleus distinct from the location of hsp genes. These granules may represent central “depots” where the multistep activation of HSF1 including trimerization, phosphorylation, and acquisition of DNA binding activity takes place, and from which transcriptionally competent HSF1 trimers can be dispensed to hsp genes (bottom panel). Bar: 5 μm.
CONCLUSIONS
The cell nucleus is a highly dynamic organelle in which compartments organize in response to metabolic requirements as a means to bring together molecules of related function and thus enhance the efficiency of nuclear activities. Because stress induces a rapid and transient change in gene expression associated with major changes in nuclear architecture, it clearly represents a powerful tool to investigate structure–function relationships in the nucleus. Of particular interest will be to utilize the stress response to investigate how the nucleus reorganizes in a stress-dependent manner to integrate signals for protection, recovery, and cell death.
ACKNOWLEDGMENTS
We are grateful to Dr. M. Vincent (University of Laval, Quebec, Canada) and to Dr. J. A. Steitz (Yale University, CT) for providing us with the CC-3 and Y12 antibodies, respectively, and to Robert A. Lamb for providing access to the light microscopy facilities. R. I. Morimoto was supported by the National Institute of Health grant #GM38109.
REFERENCES
- 1. Barry M. A.; Behnke C. A.; Eastman A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem. Pharmacol. 40:2353–2362; 1990. [DOI] [PubMed] [Google Scholar]
- 2. Berezney R.; Coffey D. S. Identification of a nuclear protein matrix. Biochem. Biophys. Res. Commun. 60:1410–1417; 1974. [DOI] [PubMed] [Google Scholar]
- 3. Bond U. Heat shock but not other stress inducers leads to the disruption of a sub-set of snRNPs and inhibition of in vitro splicing in HeLa cells. EMBO J. 7:3509–3518; 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bridger J. M.; Herrmann H.; Munkel C.; Lichter P. Identification of an interchromosomal compartment by polymerization of nuclear-targeted vimentin. J. Cell Sci. 111:1241–1253; 1998. [DOI] [PubMed] [Google Scholar]
- 5. Brostom C. O.; Brostom M. A. Regulation of translational initiation during cellular responses to stress. Prog. Nucleic Acid Res. Mol. Biol. 58:79–125; 1998. [DOI] [PubMed] [Google Scholar]
- 6. Chalfie M.; Tu Y.; Euskirchen G.; Ward W. W.; Prascher D. C. Green fluorescent protein as a marker for gene expression. Science 263:802–805; 1994. [DOI] [PubMed] [Google Scholar]
- 7. Corell R. A.; Gross R. H. Splicing thermotolerance maintains pre-mRNA transcripts in the splicing pathway during severe heat shock. Exp. Cell Res. 202:233–242; 1992. [DOI] [PubMed] [Google Scholar]
- 8. Cotto J. J.; Fox S. G.; Morimoto R. I. HSF1 granules: A novel stress-induced nuclear compartment of human cells. J. Cell Sci. 110:2925–2934; 1997. [DOI] [PubMed] [Google Scholar]
- 9. Cremer T.; Kurz A.; Zirbel R.; Dietzel S.; Rinke B.; Schrock E.; Speicher M. R.; Mathieu U.; Jauch A.; Emmerich P.; Scherthan H.; Ried T.; Cremer C.; Lichter P. Role of chromosome territories in the functional compartmentalization of the cell nucleus. Cold Spring Harbor Symp. Quant. Biol. 58:777–792; 1993. [DOI] [PubMed] [Google Scholar]
- 10. Du L.; Warren S. L. A functional interaction between the carboxy-terminal domain of RNA polymerase II and pre-mRNA splicing. J. Cell Biol. 136:5–18; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Edwards M. J. Apoptosis, the heat shock response, hyperthermia, birth defects, disease and cancer. Where are the common links? Cell Stress Chaperones 3:213–220; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eils R.; Dietzel S.; Benin E.; Schrock E.; Speicher M. R.; Ried T.; Robert-Nicoud M.; Cremer C.; Cremer T. Three-dimensional reconstruction of painted human interphase chromosomes: Active and inactive X chromosome territories have similar volumes but differ in shape and surface structure. J. Cell Biol. 135:1427–1440; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Elgin S. C. Heterochromatin and gene regulation in Drosophila . Curr. Opin. Genet. Dev. 6:193–202; 1996. [DOI] [PubMed] [Google Scholar]
- 14. Fu X.-D.; Maniatis T. Factor required for mammalian spliceosome assembly is localized to discrete regions in the nucleus. Nature 343:437–441; 1990. [DOI] [PubMed] [Google Scholar]
- 15. Gabai V. L.; Meriin A. B.; Yaglom J. A.; Volloch V. Z.; Sherman M. Y. Role of Hsp70 in regulation of stress-kinase JNK: Implications in apoptosis and aging. FEBS Lett. 438:1–4; 1998. [DOI] [PubMed] [Google Scholar]
- 16. Gattoni R.; Mahe D.; Mahl P.; Fischer N.; Mattei M. G.; Stevenin J.; Fuchs J. P. The human hnRNP-M proteins: Structure and relation with early heat shock-induced splicing arrest and chromosome mapping. Nucleic Acids Res. 24:2535–2542; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Georgopoulos C.; Welch W. J. Role of the major heat shock proteins as molecular chaperones. Annu. Rev. Cell Biol. 9:601–634; 1993. [DOI] [PubMed] [Google Scholar]
- 18. Ghoshal K.; Jacob S. T. Heat shock selectively inhibits ribosomal RNA gene transcription and down-regulates E1BF/Ku in mouse lymphosarcoma cells. Biochem. J. 317:689–695; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gottschling D. E.; Aparicio O. M.; Billington B. L.; Zakian V. A. Position effect of S. cerevisiae telomeres: Reversible repression of Pol II transcription. Cell 63:751–762; 1990. [DOI] [PubMed] [Google Scholar]
- 20. Jolly C.; Morimoto R. I.; Robert-Nicoud M.; Vourc’h C. Nuclear foci enriched in HSF1 transcription factor: Relationship with heat shock gene transcription sites. J. Cell Sci. 110:2935–9241; 1997. [DOI] [PubMed] [Google Scholar]
- 21. Jolly C.; Robert-Nicoud M.; Vourc’h C. Contribution of the growing RNA molecules to the nuclear transcripts foci observed by FISH. Exp. Cell Res. 238:299–304; 1998. [DOI] [PubMed] [Google Scholar]
- 22. Kurz A.; Lampel S.; Nickolenko J. E.; Bradl J.; Benner A.; Zirbel R. M.; Cremer T.; Lichter P. Active and inactive genes localize preferentially in the periphery of chromosome territories. J. Cell Biol. 135:1195–1205; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lamond A. I.; Eamshaw W. C. Structure and function in the nucleus. Science 280:547–553; 1998. [DOI] [PubMed] [Google Scholar]
- 24. Lerner E. A.; Lerner M. R.; Janeway C. A. J.; Steitz J. A. Monoclonal lntibodies st nucleic acid-containing cellular constituents: Probes for molecular biology and autoimmune disease. Proc. Natl. Acad. Sci. USA 78:2737–2741; 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lindquist S. The heat-shock response. Annu. Rev. Biochem. 55:1151–11191; 1986. [DOI] [PubMed] [Google Scholar]
- 26. Liu Y.; Liang S.; Tartakoff A. M. Heat shock disassembles the nucleolus and inhibits nuclear protein import and poly(A)+ export. EMBO J. 15:6750–6757; 1996. [PMC free article] [PubMed] [Google Scholar]
- 27. Martinez-Balbás M. A.; Dey A.; Rabindran S.; Ozato K.; Wu C. Displacement of sequence-specific transcription factors from mitotic chromatin. Cell 83:29–38; 1995. [DOI] [PubMed] [Google Scholar]
- 28. Matera A. G. Of coiled bodies, gems, and salmon. J. Cell. Biochem. 70:181–192; 1998. [PubMed] [Google Scholar]
- 29. Mathew A.; Mathur S. K.; Morimoto R. I. Heat shock response and protein degradation: Regulation of HSF2 by the ubiquitin-proteasome pathway. Mol. Cell. Biol. 18:5091–5098: 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McCracken S.; Fong N.; Yankulov K.; Ballantyne S.; Pan G.; Greenblatt J.; Patterson S. D.; Wickens M.; Bentley D. L. The C-terminal domain of RNA polymerase II couples mRNA processing to transcription. Nature 385:357–361; 1997. [DOI] [PubMed] [Google Scholar]
- 31. Minchiotti G.; Gargano S.; Maresca B. The introncontaining hsp82 gene of the dimorphic pathogenic fungus Histoplasma capsulatum is properly spliced in severe heat shock conditions. Mol. Cell. Biol. 11:5624–5630; 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miriami E.; Sperling J.; Sperling R. Heat shock affects 5′ splice site selection, cleavage and ligation of CAD pre-mRNA in hamster cells, but not its packaging in InRNP particles. Nucleic Acids Res. 22:3084–3091; 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Misteli T.; Spector D. L. Applications of the green fluorescent protein in cell biology and biotechnology. Nature Biotechnol. 15:961–964; 1997. [DOI] [PubMed] [Google Scholar]
- 34. Misteli T.; Caceres J. F.; Spector D. L. The dynamics of a pre-mRNA splicing factor in living cells. Nature 387:523–527; 1997. [DOI] [PubMed] [Google Scholar]
- 35. Misteli T.; Spector D. L. The cellular organization of gene expression. Curr. Opin. Cell Biol. 10:322–331; 1998. [DOI] [PubMed] [Google Scholar]
- 36. Moen P. T. Jr.; Smith K. P.; Lawrence J. B. Compartmentalization of specific pre-mRNA metabolism: An emerging view. Hum. Mol. Genet. 4:1779–1789; 1995. [DOI] [PubMed] [Google Scholar]
- 37. Moerman A. M.; de Maria A. C.; Gomes S. L.; Klein C. Heat shock alters poly(A) tail length of Dictyostelium discoideum hsp32 RNA. DNA Cell Biol. 17:635–641; 1998. [DOI] [PubMed] [Google Scholar]
- 38. Moore M.; Schaack J.; Baim S. B.; Morimoto R. I.; Shenk T. Induced heat shock mRNAs escape the nucleocytoplasmic transport block in adenovirus-infected HeLa cells. Mol. Cell. Biol. 7:4505–4512; 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Morimoto R. I. Cells in stress: Transcriptional activation of heat shock genes. Science 259:1409–1410; 1993. [DOI] [PubMed] [Google Scholar]
- 40. Morimoto R. I.; Kroeger P. E.; Cotto J. J. The transcriptional regulation of heat shock genes: A plethora of heat shock factors and regulatory conditions. In: Morimoto R. I.; Feige U.; Yahara I.; Polla B. S.; eds. Stress inducible cellular responses. Basel: Birkhaüser Verlag; 1996:139–163. [DOI] [PubMed] [Google Scholar]
- 41. Morimoto R. I. Regulation of the heat shock transcriptional response: Cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 12:3788–3796; 1998. [DOI] [PubMed] [Google Scholar]
- 42. Mortillaro M. J.; Blencowe B. J.; Wei X.; Nakayasu H.; Du L.; Warren S. L.; Sharp P. A.; Berezney R. A hyperphosphorylated form of the large subunit of RNA polymerase II is associated with splicing complexes and the nuclear matrix. Proc. Natl. Acad. Sci. USA 93:8253–8257; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mosser D. D.; Caron A. W.; Bourget L.; Denis-Larose C.; Massie B. Role of the human heat shock protein hsp70 in protection against stress-induced apop-tosis. Mol. Cell. Biol. 17:5317–5327; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Muhich M. L.; Boothroyd J. C. Synthesis of trypanosome hsp70 mRNA is resistant to disruption of trans-splicing by heat shock. J. Biol. Chem. 264:7107–7110; 1989. [PubMed] [Google Scholar]
- 45. Nakai A.; Tanabe M.; Kawazoe Y.; Inazawa J.; Morimoto R. I.; Nagata K. HSF4, a new member of the human heat shock factor family which lacks properties of a transcriptional activator. Mol. Cell. Biol. 17:469–481; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nitta M.; Okamura H.; Aizawa S.; Yamaizumi M. Heat shock induces transient p53-dependent cell cycle arrest at G1/S. Oncogene 15:561–568; 1997. [DOI] [PubMed] [Google Scholar]
- 47. O’Brien T.; Lis J. T. RNA polymerase II pauses at the 5′ end of the transcriptionally induced Drosophila hsp70 gene. Mol. Cell. Biol. 11:5285–5290; 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Osteryoung K. W.; Sundberg H.; Vierling E. Poly(A) tail length of a heat shock protein RNA is increased by severe heat stress, but intron splicing is unaffected. Mol. Gen. Genet. 239:323–333; 1993. [DOI] [PubMed] [Google Scholar]
- 49. Pederson T. Thinking about a nuclear matrix. J. Mol. Biol. 277:147–159; 1998. [DOI] [PubMed] [Google Scholar]
- 50. Pelham H. R. B. A regulatory upstream promoter element in the Drosophila hsp70 heat-shock gene. Cell 30:517–528; 1982. [DOI] [PubMed] [Google Scholar]
- 51. Rabindran S. R.; Giorgi G.; Clos J.; Wu C. Molecular cloning and expression of a human heat shock factor, HSF1. Proc. Natl. Acad. Sci. USA 88:6906–6910; 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ramon y Cajal S. Un sencillo metodo de coloracion selectiva del reticulo protoplasmico y sus efectos en los diversos organos nerviosos. Trad. Lab. Invest. Biol. 2:129–221; 1903. [Google Scholar]
- 53. Rasmussen E. B.; Lis J. T. In vivo transcriptional pausing and cap formation on three Drosophila heat shock genes. Proc. Natl. Acad. Sci. USA 90:7923–7927; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rattan S. I. Repeated mild heat shock delays ageing in cultured human skin fibroblasts. Biochem. Mol. Biol. Int. 45:753–759; 1998. [DOI] [PubMed] [Google Scholar]
- 55. Richardson A.; Holbrook N. J. Aging and the cellular response to stress: Reduction in the heat shock response. In: Holbrook N. J.; Martin G. R.; Lockshin R. A., eds. Cellular aging and cell death. New York: Wiley-Liss; 1996:67–80. [Google Scholar]
- 56. Roth M. B. Spheres, coiled bodies and nuclear bodies. Curr. Opin. Cell Biol. 7:325–328; 1995. [DOI] [PubMed] [Google Scholar]
- 57. Roti Roti J. L.; Wright W. D.; vanderWaal R. The nuclear matrix: A target for heat shock effects and a determinant for stress response. Crit. Rev. Eukaryot. Gene Expr. 7:343–360; 1997. [DOI] [PubMed] [Google Scholar]
- 58. Roti Roti J. L.; Kampinga H. H.; Malyapa R. S.; Wright W. D.; vanderWaal R. P.; Xu M. Nuclear matrix as a target for hyperthermic killing of cancer cells. Cell Stress Chaperones 3:245–255; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rougvie A. E.; Lis J. T. The RNA polymerase II molecule at the 5′ end of the uninduced hsp70 gene of D. melanogaster is transcriptionally engaged. Cell 54:795–804; 1988. [DOI] [PubMed] [Google Scholar]
- 60. Rutherford S. L.; Lindquist S. Hsp90 as a capacitor for morphological evolution. Nature 396:336–342; 1998. [DOI] [PubMed] [Google Scholar]
- 61. Sarge K. D.; Murphy S. P.; Morimoto R. I. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol. Cell. Biol. 13:1392–1407; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sato N.; Torigoe T. The molecular chaperones in cell cycle control. Ann. NY Acad. Sci. 851:61–66; 1998. [DOI] [PubMed] [Google Scholar]
- 63. Schaffer C. D.; Wallrath L. L.; Elgin S. C. R. Regulating genes by packaging domains: Bits of heterochromatin in euchromatin. Trends Genet. 9:3537; 1993. [DOI] [PubMed] [Google Scholar]
- 64. Schuetz T. J.; Gallo G. J.; Sheldon L.; Tempst P.; Kingston R. E. Isolation of a cDNA for HSF2: Evidence for two heat shock factor genes in humans. Proc. Natl. Acad. Sci. USA 88:6911–6915; 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shama S.; Lai C. Y.; Antoniazzi J. M.; Jiang J. C.; Jazwinski S. M. Heat stress-induced life span extension in yeast. Exp. Cell Res. 245:379–388; 1998. [DOI] [PubMed] [Google Scholar]
- 66. Shen J.; Beall C. J.; Hirsh J. Tissue-specific alternative splicing of the Drosophila dopa decarboxylase gene is affected by heat shock. Mol. Cell. Biol. 13:4549–4555; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Singer R. H.; Green M. R. Compartmentalization of eukaryotic gene expression: Causes and effects. Cell 91:291–294; 1997. [DOI] [PubMed] [Google Scholar]
- 68. Sistonen L.; Sarge K. D.; Phillips B.; Abravaya K.; Morimoto R. I. Activation of heat shock factor 2 during hemin-induced differentiation of human erythro-leukemia cells. Mol. Cell. Biol. 12:4104–4111; 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Spector D. L.; Fu X.-D.; Maniatis T. Associations between distinct pre-mRNA splicing components and the cell nucleus. EMBO J. 10:3467–3481; 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Spector D. L.; Goldman R. D.; Leinwand L. A. Cells: A laboratory manual. New York: Cold Spring Harbor Laboratory Press; 1998. [Google Scholar]
- 71. Takechi H.; Hosokawa N.; Hirayoshi K.; Nagata K. Alternative 5′ splice site selection induced by heat shock. Mol. Cell. Biol. 14:567–575; 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tani T.; Derby R. J.; Hiraoka Y.; Spector D. L. Nucleolar accumulation of poly(A)+ RNA in heat-shocked yeast cells: Implication of nucleolar involvement in mRNA transport. Mol. Biol. Cell 7:173–192; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Thibodeau A.; Vincent M. Monoclonal antibody CC-3 recognizes phosphoproteins in interphase and mitotic cells. Exp. Cell Res. 195:145–153; 1991. [DOI] [PubMed] [Google Scholar]
- 74. Tsien R. Y. The green fluorescent protein. Annu. Rev. Biochem. 67:509–544; 1998. [DOI] [PubMed] [Google Scholar]
- 75. vanderWaal R.; Thampy G.; Wright W. D.; Roti Roti J. L. Heat-induccd modificattons in the association of specific proteins with the nuclear matrix. Radiat. Res. 145:746–753; 1996. [PubMed] [Google Scholar]
- 76. Vazquez J.; Pauli D.; Tissieres A. Transcriptional regulation in Drosophila during heat shock: A nuclear run-on analysis. Chromosoma 102:233–248; 1993. [DOI] [PubMed] [Google Scholar]
- 77. Vogel J. L.; Parsell D. A.; Lindquist S. Heat-shock proteins Hsp104 and Hsp70 reactivate mRNA splicing after heat inactivation. Curr. Biol. 5:306–617; 1995. [DOI] [PubMed] [Google Scholar]
- 78. Walsh D.; Li K.; Wass J.; Dolnikov A.; Zeng F.; Zhe L.; Edwards M. Heat-shock gene expression and cell cycle changes during mammalian embryonic development. Dev. Genet. 14:127–136; 1993. [DOI] [PubMed] [Google Scholar]
- 79. Warters R. L.; Brizgys L. M.; Sharma R.; Roti Roti J. L. Heat shock (45 degrees C) results in an increase of nuclear matrix protein mass in HeLa cells. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 50:253–268: 1986. [DOI] [PubMed] [Google Scholar]
- 80. Welch W. J.; Feramisco J. R. Nuclear and nucleolar localization of the 72,000-dalton heat shock protein in heat-shocked mammalian cells. J. Biol. Chem. 259:4501–4513; 1984. [PubMed] [Google Scholar]
- 81. Welch W. J.; Suhan J. P. Morphological study of the mammalian stress response: Characterization of changes in cytoplasmic organelles, cytoskeleton, and nucleoli, and appearance of intranuclear actin filaments in rat fibroblasts after heat-shock treatments. J. Cell Biol. 101:1198–1211; 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Welch W. J.; Gambetti P. Chaperoning brain diseases. Nature 392:23–24; 1998. [DOI] [PubMed] [Google Scholar]
- 83. Westra A.; Dewey W. C. Variation in sensitivity to heat shock during the cell-cycle of Chinese hamster cells in vitro . Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 19:467–477; 1971. [DOI] [PubMed] [Google Scholar]
- 84. Wu C. Heat shock transcription factors: Structure and regulation. Annu. Rev. Cell. Dev. Biol. 11:441–469; 1995. [DOI] [PubMed] [Google Scholar]
- 85. Yost H. J.; Lindquist S. RNA splicing is interrupted by heat shock and is rescued by heat shock protein synthesis. Cell 45:185–193; 1986. [DOI] [PubMed] [Google Scholar]
- 86. Yost H. J.; Lindquist S. Heat shock proteins affect RNA processing during the heat shock response of Saccharomyces cerevisiae . Mol. Cell. Biol. 11:1062–1068; 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yuryev A.; Patturajan M.; Litingtung Y.; Joshi R. V.; Gentile C.; Gebara M.; Corden J. L. The C-terminal domain of the largest subunit of RNA polymerase II interacts with a novel set of serine/arginine-rich proteins. Proc. Natl. Acad. Sci. USA 93:6975–6980; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zeng C. Q.; Kim E.; Warren S. L.; Berget S. M. Dynamic relocation of transcription and splicing factors dependent upon transcriptional activity. EMBO J. 16:1401–1412; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zirbel R. M.; Mathieu U. R.; Cremer T.; Lichter P. Evidence for a nuclear compartment of transcription and splicing located at chromosome domain boundaries. Chrom. Res. 1:93–106; 1993. [DOI] [PubMed] [Google Scholar]


