Abstract
There are 37 million people globally infected with the Human Immunodeficiency Virus (HIV). People living with HIV can achieve nearly normal lifespans due to the use of antiretroviral drugs (ARVs). However, people living with HIV experience chronic inflammation and increased risk for cardiovascular diseases (CVD) relative to uninfected people. While the cause for this risk is unclear, some ARVs have been associated with CVD, and it is speculated that some ARVs potentiate inflammation in infected individuals. Platelets are a critical link between inflammation and the development and progression of CVD, but the effects of ARVs on platelets are largely understudied. In this study, we examined the effects of ARVs on human platelet function in vitro. Our data show that the ARV ritonavir, a protease inhibitor, severely altered human platelet lipid mediator production (prostaglandin E2 and thromboxane) in both resting and activated platelets. Further characterization revealed that ritonavir altered measures of platelet hemostatic and thrombotic function that included significantly decreased platelet spreading, increased platelet aggregation, and trended toward increased clot strength. These data provide proof-of-principle that ARVs can directly dysregulate human platelets, possibly contributing to inflammation-related comorbidities. These data may provide mechanistic insight into the factors contributing to increased risk of CVD in people living with HIV, and may help guide future development of new HIV agents and ARV regimens that mitigate platelet dysregulation by ARVs.
Keywords: Antiretroviral therapy, platelets, cardiovascular disease
Introduction:
Currently, more than 37 million people globally are infected with the Human Immunodeficiency Virus (HIV). Approximately half of those infected are being treated with antiretroviral drugs (ARVs), with a goal of 30 million people living with HIV to be treated with ARVs by 2020 [1, 2]. Treatment with combination antiretroviral therapies (cART) can suppress viral burden to undetectable levels [3]. However, despite adherence to antiretroviral treatments and undetectable viral loads, people living with HIV experience chronic inflammation and have shorter lifespans compared to the uninfected population [4, 5]. As a result of chronic inflammation, people living with HIV are at increased risk for inflammation-linked comorbidities, including cardiovascular diseases (CVD) [6–8]. CVD is the major cause of death in the United States and similar resource-rich societies, and people living with HIV are at greater risk for CVD, including myocardial infarction, stroke, and atherosclerosis [9–11].
There are many competing theories as to why people living with HIV who are successfully treated with ARVs have increased risk for inflammation-related diseases. One possibility is that low-levels of persistent viral replication cause chronic activation of the immune system. Another factor to be considered is that people living with HIV are at increased risk for behaviors that are known to cause inflammation, such as cigarette smoking. However, these behaviors alone do not account entirely for the increased risk of CVD or other inflammation-linked diseases [12–14]. A separate line of thought is that the ARVs themselves contribute to inflammation. ARVs have many side effects, including dyslipidemia, hepatotoxicity, and lipodystrophy [15, 16]. The role of the different classes of ARVs on the increased risk of CVD is under investigation, with several ARVs suspected of contributing to this risk [17–20]. However, because studies are often conflicting, there is no consensus on the role of ARVs in HIV-associated CVD [18–23].
Platelets are a key cell in the initiation and progression of CVD, yet they have not been adequately investigated in the context of HIV-associated CVD. Platelets are the second most numerous cell in the blood, and are important in systemic inflammation due to their ability to rapidly activate and release inflammatory mediators that include chemokines and cytokines (i.e. soluble CD40 ligand (sCD40L, CD154), interleukin-1 beta (IL-1β), platelet factor 4 (PF4)) and lipids including prostaglandins and thromboxane A2 (TXA2) [24, 25]. People living with HIV have increased markers of platelet activation, which may contribute to chronic inflammation and CVD risk [26–28]. Despite the relationship of ARVs to increased CVD risk, the critical importance of platelets in CVD and thrombosis, and the increase in activated platelets observed in people living with HIV, there are few studies on the direct effects of ARVs on platelet activation and function [29, 30]. Thus, it is important to further characterize the effects of ARVs on platelet inflammatory and hemostatic functions.
In our study, we address this important knowledge gap of the effects of ARVs on human platelets. We screened ARVs for effects on human platelet function in vitro and found that ritonavir, a protease inhibitor, dysregulates platelet inflammatory and hemostatic functions. The findings herein support the hypothesis that ARVs can dysregulate platelets, implicating them in the chronic inflammation that drives HIV-associated CVD.
Methods:
Blood collection:
Peripheral blood was collected from healthy human donors who had not taken platelet-inhibiting drugs for at least 2-weeks and who were consented in accordance with the Helsinki declaration under a protocol approved by the University of Rochester Institutional Review Board. Up to 100 mL of venous blood was drawn from the forearm of the consenting donor via venipuncture using a 19 gauge butterfly needle into vacutainer tubes. 18 donors were recruited for this study. Donor age ranged from 24 to 65, with an average age of 37 and median age of 30. The donors were 22% (4/18) male, 78% (14/18) female, 89% (16/18) Caucasian, and 11% (2/18) non-Caucasian.
Isolation of human platelets:
Whole blood was collected in ACD solution A vacutainer tubes (BD, Franklin Lakes, NJ). Platelet-rich plasma (PRP) was prepared via centrifugation of whole blood at 200 x g for 15 minutes at 21°C with no brake. PRP was collected carefully with a wide-bore pipette from the top third of the centrifuged blood to avoid disrupting the buffy coat. Washed platelets were prepared from PRP as previously described [31]. Briefly, 1 μg/mL of prostacyclin (PGI2) (Cayman Chemical) was added to PRP and mixed gently via inversion. PRP was centrifuged for 10 minutes at 1,000 x g at 21°C. The supernatant was aspirated and the top portion of the platelet pellet was gently resuspended in Tyrode’s Salt Solution (TSS, Sigma-Aldrich): acid-citrate-dextrose (25:3) containing 0.1 μg/mL PGI2 using a wide bore pipet. The resuspended solution was centrifuged at 1,000 x g for 10 minutes at 21°C. The supernatant was aspirated and the top two-thirds of the platelet pellet was gently resuspended in TSS using a wide-bore pipet, leaving the bottom third of the pellet to ensure the platelet resuspension is not contaminated with other cell types. The platelet concentration was measured using a Sysmex automated blood cell counter. Platelets were diluted to the desired concentration in TSS. Unless otherwise noted, platelets were used within 2–3 hours of the blood draw.
In vitro antiretroviral drug screening:
Washed human platelets were diluted to 1.1×108 platelets/mL in TSS and 300 μL aliquots were plated into each well of a 96-well plate. Platelets were treated with ARVs or vehicle (PBS or <0.1% DMSO) for 30 minutes at 37°C as follows: ritonavir 5 μM, abacavir 20 μM, darunavir 10 μM, raltegravir 20 μM, lamivudine 20 μM, tipranavir 25 μM, and atazanavir 5 μM. At 30 minutes, the 96-well plates were centrifuged at 1,200 x g for 10 minutes. Platelet supernatants were collected into a separate 96-well plate for storage and stored at −20°C until used to detect TXB2 and PGE2 using enzyme immunoassays (Cayman Chemical). The following ARV reagents were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: ritonavir (Cat. # 4622), darunavir ethanolate (Cat. # 11447), abacavir (Cat. # 4680), lamivudine (Cat. # 8146), and raltegravir (Cat. # 11680); tipranavir (Cayman Cat. #21028) and atazanavir (Cayman Cat. # 11733) were purchased from Cayman.
In vitro ritonavir treatments.
Human platelets were isolated and washed, then diluted to 1.1×108 platelets/mL in TSS. The platelets were then treated with ritonavir (0.5, 5, 10, 20 μM) or vehicle (<0.1%DMSO) for 30 minutes at 37°C, or for 15 minutes followed by a 15 minute incubation with the platelet agonists ADP and thrombin (2.5 μM and 0.1 U/mL respectively). These washed human platelets were then tested for function as described below.
Platelet Spreading:
After treatments, platelets were spread on fibrinogen-coated coverslips as a surrogate measure of wound healing, as previously described [32]. Coverslips were coated with 50 μL of 250 μg/mL fibrinogen (Sigma-Aldrich, St Louis, MO) for 2 hours at room temperature, then blocked with 0.5% bovine serum albumin (BSA; Sigma-Aldrich, St Louis, MO) in 1 X phosphate buffered saline (PBS) for up to 1.5 hours. Washed human platelets were diluted to 3.3 × 107 platelets/mL in TSS and 300 μL plated onto the fibrinogen coated coverslips, then incubated at 37°C for 45 minutes to spread. The coverslips were then washed with PBS three times, and fixed with 4% paraformaldehyde in PBS for 15 minutes. After fixing, coverslips were washed once with PBS, then mounted onto slides using Fluoromount G mounting media (Southern Biotech). Images were taken at 100X with an Olympus BX51 microscope (Olympus, Melville, NY) using differential interference contrast microscopy (DIC), and at least 5 fields of view were captured for each coverslip. Images were edited using SPOT software to enhance platelet visibility. Platelet spreading was quantified by manual counting, after blinding via randomizing images and obscuring sample information.
Immunoassays:
After treatments, platelets were centrifuged at 1,200 x g for 10 minutes at 21°C. Platelet supernatants were used to detect proinflammatory-mediators. As TXA2 is unstable and quickly converted to the less bioactive Thromboxane B2 (TXB2), we used TXB2 as a surrogate measure. TXB2 and prostaglandin E2 (PGE2) concentrations were determined using enzyme immunoassays (Cayman Chemical). PF4 was assayed using enzyme-linked immunosorbent assay kits (R&D Systems). sCD40L was assayed by ELISA using a mouse anti-human sC40L antibody clone MK13A4 (kind gift from Marilyn Kerry), as previously described [33].
Flow cytometry:
1×106 washed human platelets were fixed with 1.1% PFA for 15 minutes and washed once with TSS. Platelets were centrifuged at 1,200 x g for 10 minutes, supernatant was aspirated, and the platelet pellet was resuspended in human Fc blocking reagent to a final concentration of 1×106 platelets/mL. Platelets were blocked for 15 minutes, then stained with mouse anti-human CD62P-AF647 (Biolegend Cat. # 304918) for 30 minutes at 21°C and analyzed using an Accuri C6 flow cytometer (BD, Franklin Lakes, NJ, USA). Platelets were identified based on size and granularity by forward- and side-scatter on a log-scale; Megamix beads (Stago Cat. # 00420-RUO) were used for gate sizing. The CD62P gate was set using isotype control (Santa Cruz Cat. # 3890).
Aggregometry:
Aggregation was performed using the turbidimetric method using a Chrono-Log Lumi-aggregometer with AGGRO/LINK software (Chrono-Log Corp., Havertown, PA), as described previously [31, 32]. Blood was collected from healthy human donors via venipuncture into buffered sodium citrate vacutainer tubes (BD, Franklin Lakes, NJ). The blood was centrifuged at 21°C at 100 x g for 15 minutes with moderate acceleration and no brake. PRP was collected without disturbing the buffy coat or red blood cells. 700 μL of PRP was collected and centrifuged at 1,200 x g for 10 minutes to generate platelet-poor-plasma (PPP). At least 500 μL of PRP was treated with the indicated concentrations of ritonavir for 30 minutes. After treatment, 450 μL of PRP was added to a cuvette containing a siliconized magnetic stir-bar. 500 μL of PPP was added to a separate cuvette to act as a blank (100% light transmission). The PRP and PPP were placed in the aggregometer. 50 μL of Chrono-lume reagent (Chrono-Log Corp., Havertown, PA) was incubated with PRP for at least 1 minute with stirring before activation. 5 μM ADP was added to the PRP to activate aggregation. Aggregation tracing was measured for 6.5 minutes. Percent aggregation was determined for all tracings at 6.5 minutes.
Thromboelastography:
Whole blood was collected into ACD solution A vacutainer tubes. 1mL of whole blood was treated with vehicle (0.1% DMSO), or ritonavir (0.5, 5, and 10 μM) for 30 minutes at 37°C. Thromboelastography (TEG) was performed within 2 hours of blood collection using a TEG hemostasis system (Haemoscope Corp, Niles, IL, USA) and citrated Kaolin as previously described [31, 34].
Statistics:
Data were analyzed using GraphPad Prism software. Paired two-tailed t-tests were used for statistical analysis except where otherwise indicated. A 2-way ANOVA with Bonferroni post-hoc correction for multiple comparisons was used to compare activated to resting platelets. All error bars represent the standard error of the mean. P-values of < 0.05 were considered significant.
Results:
ARVs dysregulate human platelet function in vitro:
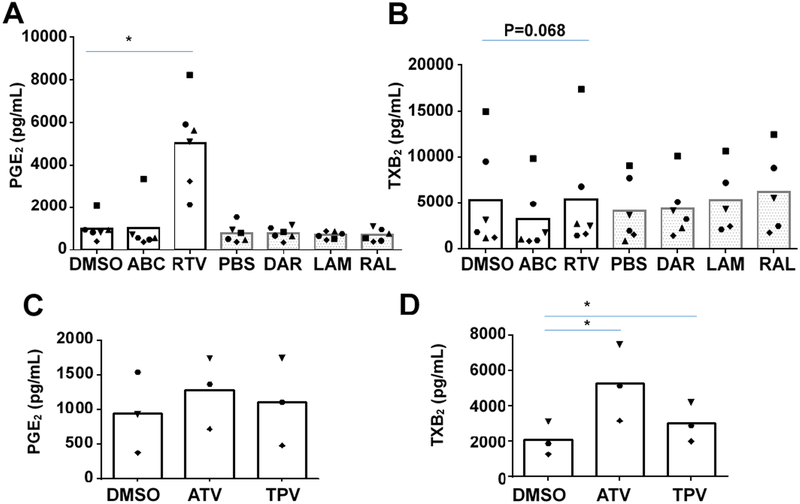
To determine the direct effects of ARVs on human platelet function, we screened commonly used ARVs for effects on human platelet production of the pro-inflammatory lipid-mediators thromboxane (measured via the stable metabolite TXB2) and PGE2 in vitro. Washed human platelets from 3–6 donors were treated with several different classes of ARVs at pharmacologically achievable doses, including the integrase inhibitor raltegravir, nucleoside reverse transcriptase inhibitors lamivudine and abacavir, and the protease inhibitors darunavir and ritonavir (Figure 1). Ritonavir significantly increased production of PGE2 (Figure 1A), and caused a slight decrease in production of thromboxane (Figure 1B). The protease inhibitors atazanavir and tipranavir did not affect PGE2, and modestly but significantly increased TXB2 production (Figure 1C and D). No other ARVs in the screen significantly altered platelet production of thromboxane or PGE2. These data suggest that ritonavir dysregulates platelet function in vitro. As ritonavir had a dramatic effect on PGE2 production, and as tipranavir and atazanavir are often boosted with ritonavir, the remainder of this study focuses on characterizing the direct effects of ritonavir on human platelet function.
Figure 1. Screening ARVs on platelet function in vitro reveals dysregulation by ritonavir.
1.1×108/mL of washed human platelets were treated with the indicated antiretroviral drugs (ARVs) for 30 minutes with either 0.1% DMSO or PBS as vehicle. The following ARVs were tested at pharmacologically relevant concentrations: abacavir (ABC) 20 μM; ritonavir (RTV) 5 μM; tipranavir (TPV) 25 μM; atazanavir (ATV) 5 μM; darunavir (DAR) 5 μM; raltegravir (RAL) 20 μM; lamivudine (LAM) 20 μM. After treatment, platelet supernatant was analyzed for the lipid mediators A&C Prostaglandin E2 (PGE2), and B&D Thromboxane (TXB2). Statistics were determined with paired two-tailed T-tests. n≥3 *p<0.05. Symbols represent individual donors. Solid columns indicate the vehicle was DMSO, and gray dotted columns indicate vehicle was PBS.
Ritonavir dysregulates human platelet production of pro-inflammatory mediators in vitro:
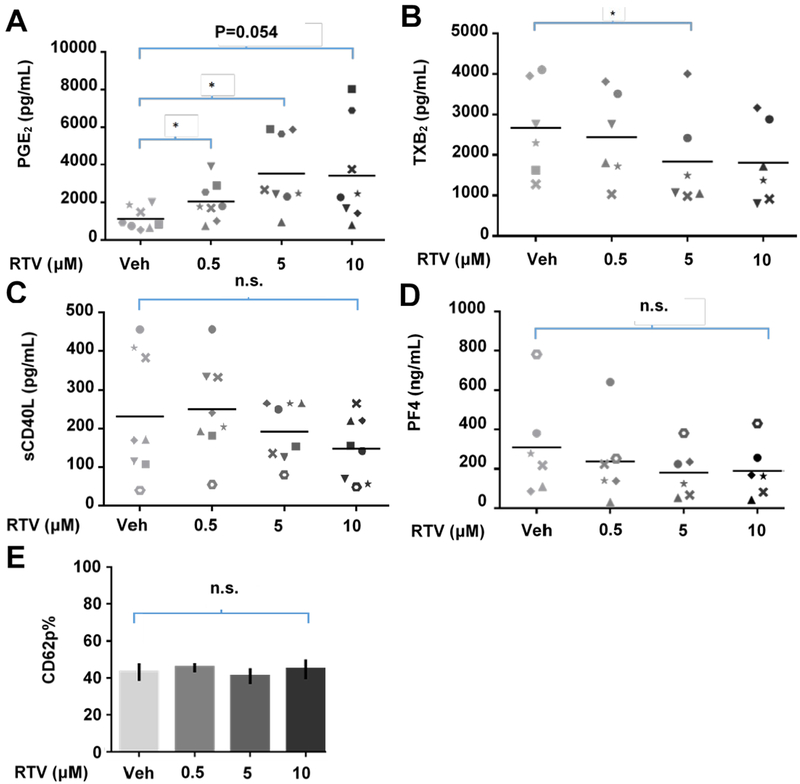
In order to further characterize the effects of ritonavir on human platelet function, we treated human platelets with a range of pharmacologically relevant doses of ritonavir as indicated (Figure 2), and observed changes in platelet mediator production and granule release. Platelet supernatants were analyzed for levels of the pro-inflammatory mediators PGE2, TXB2, sCD40L, and PF4 via immunoassay. Platelet production of PGE2 increased significantly in a dose-responsive manner (Figure 2A). Additionally, platelet production of TXB2 decreased in a dose-responsive manner (Figure 2B). Platelet supernatant concentrations of sCD40L and PF4 did not significantly change (Figure 2C, D). Platelet surface expression of CD62P, associated with alpha-granule release, was unaffected by ritonavir treatment (Figure 2E).
Figure 2: The protease inhibitor ritonavir dysregulates human platelet proinflammatory mediator production and release in vitro.
1.1×108/mL of washed human platelets were treated with vehicle (0.1% DMSO) or the indicated doses of ritonavir. Supernatant concentrations of A. prostaglandin E2 (PGE2), B. the thromboxane A2 metabolite thromboxane B2 (TXB2), C. soluble CD40L (sCD40L), and D. Platelet factor 4 (PF4) were determined via enzyme immunoassay. E. 1.1 × 106 platelets/mL were stained for CD62P. Platelets were gated on based on their size and granularity via forward and side scatter on a log scale. n≥6 for each immunoassay. n≥3 for flow cytometry. Symbols in A–D represent unique donors. Error bars indicate SEM. Statistics were performed using paired two-tailed t-tests. *p<0.05.
Ritonavir exacerbates human platelet activation in vitro.
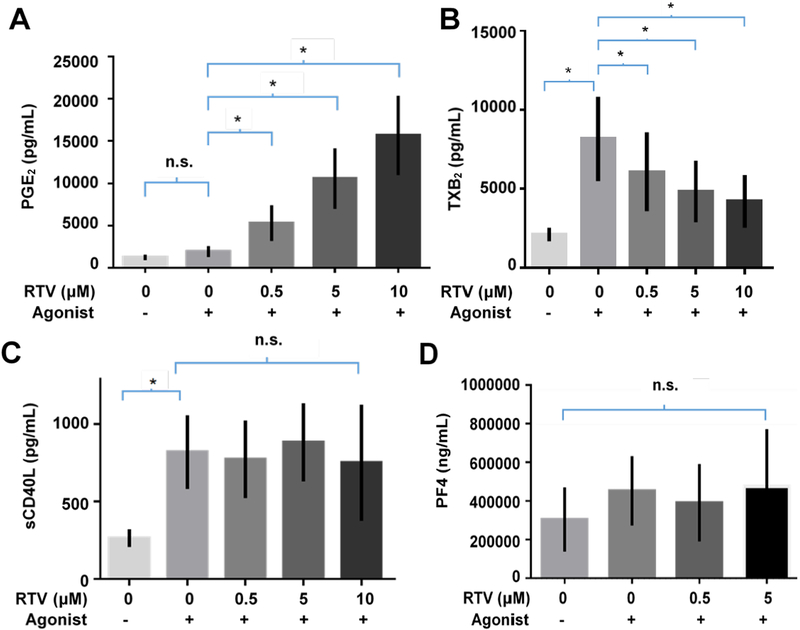
To determine if ritonavir dysregulated platelet activation, we pre-treated human platelets with ritonavir for 15 minutes, then activated platelets for an additional 15 minutes with a combination of the platelet agonists, thrombin and ADP (0.1 U/mL and 2.5 μM). We found that platelets treated with ritonavir before activation produced more PGE2 than untreated activated platelets or resting ritonavir treated platelets(Figure 3A). Conversely, activated platelet production of thromboxane was significantly decreased when pre-treated with ritonavir (Figure 3B). Interestingly, there were still significant differences between production of TXB2 in platelets pretreated with ritonavir and activated or left unactivated, indicating a blunting of thromboxane production, but not complete inhibition (Figure 3B). PF4 and sCD40L did not significantly change when platelets were treated with ritonavir and then activated (Figure 3C, D). These data indicate that platelet proinflammatory mediator production is differentially dysregulated by ritonavir and this dysregulation is exacerbated by platelet activation.
Figure 3. Effects of ritonavir on activated platelet function inflammatory mediators with activation.
1.1×108/mL platelets were pre-treated with the indicated doses of ritonavir then activated. Platelet supernatant concentrations of A. PGE2, B. TXB2, C. sCD40L, and D. PF4 were determined via enzyme immunoassay. n=3–5 for each immunoassay. Error bars indicate standard error of the mean. Statistics were performed with a Two-way ANOVA with post-hoc multiple comparisons with Bonferroni correction. *p<0.05.
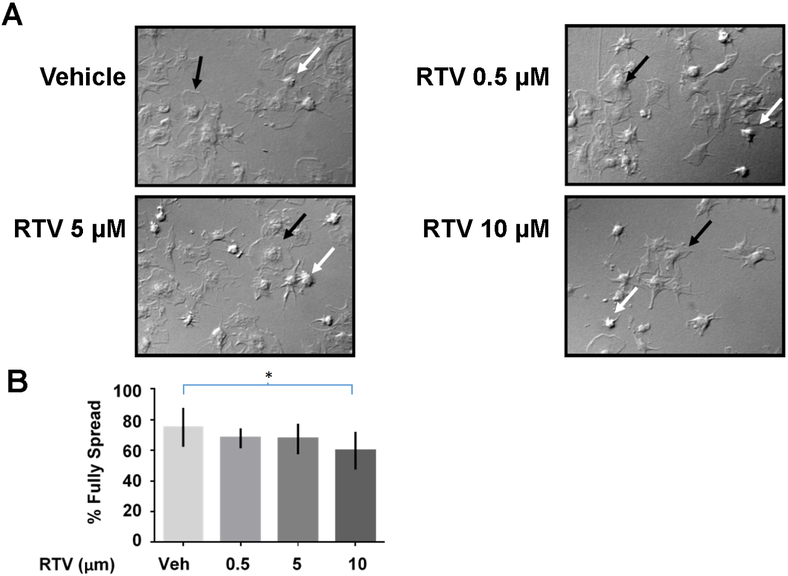
Platelet spreading is inhibited by increasing doses of ritonavir in vitro:
In order to determine how ritonavir affects platelet hemostatic function, we next examined platelet spreading. During injury and wound healing, platelets activate and morph from a discoid shape to a sphere, extend filopodia and lamellipodia, and finally flatten into a thin circle (termed “fully spread”). We used an in vitro measure of spreading by treating washed human platelets with a range of ritonavir concentrations and plating them on fibrinogen coated coverslips. Platelets adhere to and spread on the coverslips, and platelet hemostatic function is measured as the percentage of fully spread platelets. Representative images of a dose-response is shown in Figure 4A. We observed that ritonavir reduced the number of fully spread platelets (black arrows), and increased unspread platelets (white arrows). The quantification of fully spread platelets is shown in Figure 4B. We found that with increasing doses of ritonavir, the relative percentage of fully spread platelets decreased by ~20%, supporting a role for ritonavir in platelet dysregulation.
Figure 4. Ritonavir inhibits platelet spreading.
1.1×108 washed platelets/mL were treated with the indicated doses of ritonavir or vehicle (0.1% DMSO). Platelets were imaged at 100 X magnification using differential interference contrast (DIC) microscopy. At least 5 field of view were taken for each coverslip, and platelets were manually counted. A. Representative images of platelet spreading after treatment. Black arrows show a typical fully spread platelet, and white arrows show a typical unspread platelet. B. Quantification of fully spread platelets after each treatment. *p<0.05 using a paired two-tailed T-tests.
Pre-treatment of platelets with ritonavir results in increased platelet aggregation in vitro:
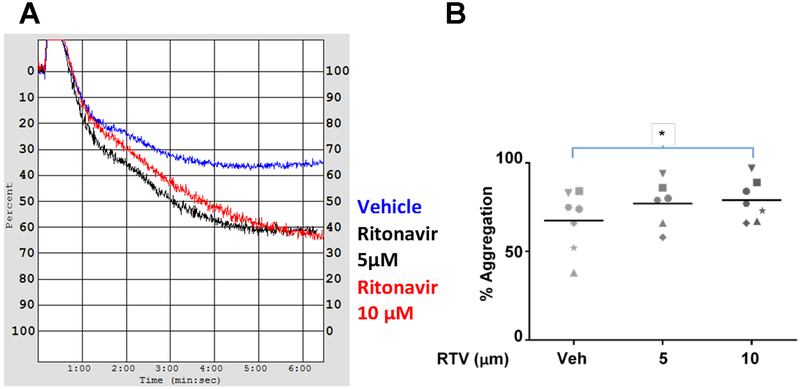
To further characterize ritonavir-induced hemostatic functional defects in human platelets, we performed aggregometry, a clinical measure of platelet clotting ability. We treated freshly collected PRP with ritonavir and measured aggregation in response to the agonist ADP (5 μM). We observed that platelet aggregation increased with ritonavir treatment following activation with ADP (Figure 5). A representative aggregometry profile is shown in Figure 5A, and the quantification of percent aggregation is shown in Figure 5B. These data indicate that ritonavir increases platelet aggregation.
Figure 5. Ritonavir increases platelet aggregation.
PRP was isolated from whole blood and treated with the indicated doses of ritonavir. Aggregometry was performed for 6.5 minutes, with 5 μM ADP being added around 5 seconds into the reading. A. representative aggregometry tracing B. Scatterplot of aggregometry profiles from 6 donors. Symbols represent unique donors. *p<0.05, determined by paired two-tailed t-tests. N≥6.
Ritonavir treatment decreased clot strength as measured by thromboelastography (TEG):
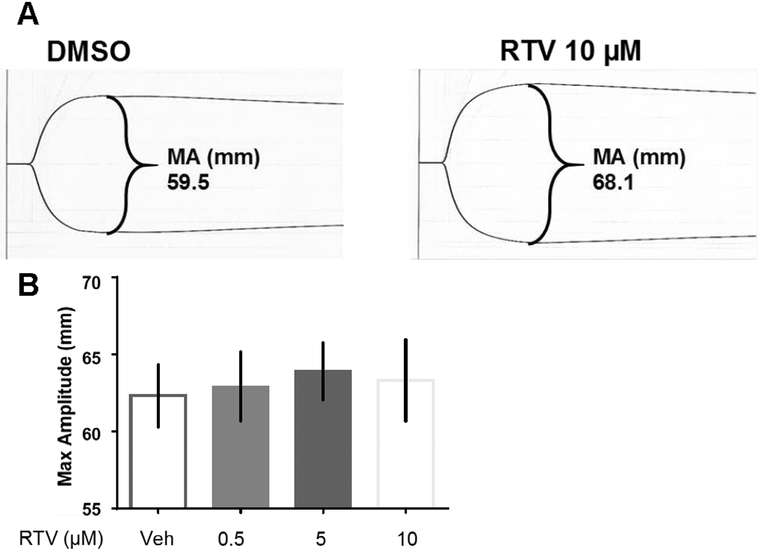
As ritonavir increases aggregation, we next determined if it altered other clotting properties. To test the effects of ritonavir on clot formation, clot strength (a global measure of platelet function) was measured by TEG. We treated whole blood with vehicle or ritonavir, activated with kaolin, and measured clot parameters using TEG. A representative TEG graph from one donor treated with ritonavir is show in Figure 6A, and quantified in Figure 6B. Our data indicate that with increasing doses of ritonavir, clot strength (measured by maximum amplitude (MA)) trended toward an increase, though this did not reach traditional statistical significance. The time to clot formation and clot retraction were unaffected by ritonavir treatment (data not shown).
Figure 6. Ritonavir does not significantly change clot strength.
Thromboelastography was performed using whole human blood collected into ACD tubes. Whole blood was treated with vehicle (0.1% DMSO) or the indicated doses of ritonavir. TEG was measured using a TEG hemostasis system Bars represent SEM. Statistics performed using paired two-tailed T-tests. n=6
Discussion:
The pro-inflammatory role of circulating blood platelets in CVD is well known but understudied in the context of HIV infection [35–38]. Increased platelet activation contributes to the initiation and progression of CVD [39, 40]. People living with HIV have chronic inflammation, increased basal levels of platelet activation, and are at increased risk of CVD [26, 27, 41]. There are few reports on the direct effects of ARVs on platelet function, despite the many known side-effects of ARVs which could contribute to inflammation [42–44]. In our study, we addressed this by screening ARVs for effects on platelet function. We found that the protease inhibitor, ritonavir, dramatically increased platelet production of the proinflammatory mediator PGE2.
Ritonavir was originally used to inhibit HIV proteases, but was repurposed to take advantage of its off-target inhibition of human cytochrome P450 (CYP450), a family of enzymes that metabolize xenobiotics - including ARVs [45]. Ritonavir is now used at a lower dose in combination with other ARVs (e.g. darunavir) to inhibit their degradation and increase their efficacy. Therefore, studies on protease inhibitors often focus on the ARVs co-administered with ritonavir, but not on ritonavir itself. In addition to its use as an ARV, ritonavir is being investigated for novel applications including treatment for hepatitis C virus and some cancer malignancies [46, 47]. As such, it is important to study the effects of ritonavir alone on platelet function.
Protease inhibitors increase coagulation, platelet activation, and are associated with CVD, but there is no consensus on which protease inhibitors are driving these effects [17, 44, 48–52]. As these studies do not focus on ritonavir, its effects on platelet function, coagulation, and hemostasis are largely unknown. Due to this knowledge gap and our finding in this study that ritonavir increased PGE2, we directed our efforts to better understand the direct effects of ritonavir on human platelet function in vitro.
We characterized ritonavir’s effects on platelet inflammatory function by measuring platelet release of alpha granules (containing PF4 and CD62P) and production of pro-inflammatory mediators (PGE2, sCD40L, and TXB2) in response to increasing doses of ritonavir. Platelet alpha-granule release, measured via supernatant concentrations of PF4 and platelet surface expression of CD62P, was unaffected by ritonavir. As with alpha granule release, sCD40L levels were unchanged by ritonavir. However, ritonavir significantly increased production of PGE2, and decreased the relative production of TXB2 in a dose-dependent manner. These data indicated that ritonavir alters platelet lipid mediator production specifically, but does not alter all aspects of platelet function. Additionally, ritonavir alone may not activate platelets, but because PGE2 increases platelet reactivity to agonists, ritonavir may cause platelet hyper-responsiveness. We found that pre-treatment with ritonavir before activation resulted in further increased platelet production of PGE2 and inhibited TXB2, but no change in sCD40L or PF4. While TXB2 was inhibited relative to activated platelets, the levels of TXB2 were still significantly increased compared to resting platelets; platelet functions dependent on thromboxane, such as the second wave of aggregation, may be inhibited but not entirely blunted by ritonavir.
Thromboxane inhibition by ritonavir is consistent with previous observations of decreased urinary thromboxane in a controlled experiment of HIV-negative people taking ritonavir [29]. However, our results were unexpected as both PGE2 and thromboxane are considered proinflammatory mediators; we had expected an increase in both or a decrease in both. One possible explanation for this surprising result is that ritonavir may inhibit the enzyme responsible for thromboxane production, thromboxane synthase (TXAS). If ritonavir inhibited TXAS, arachidonic acid metabolism could be shunted to prostaglandin E2 synthase (PGES), resulting in increased PGE2. To this point, TXAS is a CYP450 enzyme, though of a different family than that targeted by ritonavir. While TXAS has not specifically been tested for inhibition by ritonavir, ritonavir is known to have off-target effects. Determining the mechanism is beyond the scope of our present study, which is focused on characterizing ritonavir’s effects.
To determine ritonavir’s effect on platelet hemostatic function and examine if ritonavir causes platelet hyper-responsiveness, we examined platelet spreading. Platelet spreading is an in vitro, surrogate measure of platelet function in wound healing and hemostasis. When treated with ritonavir, platelet spreading was inhibited. We considered that the reduced percentage of fully spread platelets might be an artifact of decreased platelet adhesion to the coverslips. However, the number of platelets per coverslip was not significantly different between treatments (data not shown). Blunted platelet spreading results from a defect in platelet shape change in response to binding fibrinogen. Thromboxane is a key mediator driving platelet shape change. Thus, ritonavir’s inhibition of thromboxane could drive this inhibited spreading. As ritonavir did not alter platelet sCD62P surface expression or PF4, we conclude that platelet spreading is inhibited independent of platelet granule release.
Our data indicates that ritonavir dysregulates platelet shape change, which impacts platelet hemostatic function. To further elucidate the effects of ritonavir on hemostasis, we tested the effect of ritonavir on ADP-induced aggregation. Aggregation is a clinical assay used to determine platelet adherence to other platelets, with increased aggregation corresponding to a prothrombotic, procoagulant platelet profile in patients. Our data indicate that ritonavir increases ADP-stimulated platelet aggregation. This increased aggregation parallels the increased risk of CVD in people taking protease inhibitors [18, 49], but is surprising given the decrease in platelet spreading observed with ritonavir treatment. However, the spread platelets were not stimulated with ADP, unlike the aggregating platelets. Platelet spreading is heavily driven by thromboxane, but the first wave of aggregation (the only wave present when platelets are stimulated with the dose of ADP used in this study) is not dependent on thromboxane. Additionally, PGE2 increases platelets activation in response to agonists; ritonavir increases PGE2 and thus increases the platelet response to ADP. Consequently, we hypothesized that while ritonavir-treated platelets had inhibited spreading, they were hyper-responsive to agonists. Since ritonavir increased aggregation, it was also possible that it affected other clotting parameters.
To examine the possibility of ritonavir altering other aspects of hemostasis, we studied the effects of ritonavir on whole blood clot formation using thromboelastrography (TEG), a clinical assay of clot formation. TEG measures multiple hemostatic parameters including time to clot initiation and clot strength. Ritonavir did not significantly change time to clot formation (data not shown), but it did modestly increase clot strength, a platelet-dependent parameter, though not significantly. It is important to note that it is believed that slight to modest increases in clot strength, especially over time (as is the case in chronic inflammation), correspond to clinically relevant changes in hemostasis [53]. Thus, the increased clot strength should be further studied in patients taking ritonavir.
Our study determined the direct effect of ritonavir on platelet function using isolated, washed platelets from whole blood, a widely used technique [54, 55]. While platelets are exquisitely sensitive to manipulation, and can be easily activated during isolation, we limited platelet activation during isolation by treating platelets with the short-lived inhibitor prostacyclin, as well as by collecting blood in ACD tubes. These agents are no longer present in platelets following the last wash step. While the use of whole blood could prevent unintentional platelet activation, whole blood contains many other cell types, including leukocytes that would prevent us from studying the direct effects of ritonavir on platelets. Thus, we initially use washed platelets in our studies. A small sample size was analyzed (n=6) for each assay. However, we observed that platelets from these 6 individual donors respond similarly with ritonavir treatment in several measurements of platelet function, indicating a robust effect of ritonavir on human platelet function.
Our study shows that ritonavir dysregulates platelet function by increasing platelet production of PGE2, as well as increasing aggregation and possibly clot strength. Ritonavir may sensitize platelets to agonists via increasing PGE2 levels such that a small amount of another agonist might cause hyper-responsive platelets and increased clotting risk, and when absent of ritonavir platelets would remain quiescent. Our findings support a role for ritonavir-driven platelet dysregulation in contributing to the systemic, chronic inflammation that increases the risk of cardiovascular disease and thrombosis in people living with HIV. Additional studies are required to understand the ritonavir-specific mechanisms that promote platelet dysregulation and to examine how these factors contribute to HIV-associated CVD. Given ritonavir’s potential use for treatment of HCV and cancer, it will be also be of the utmost importance to understand the potential for platelet dysregulation in these patients. This is of special concern in the case of cancer, as many are associated with increased risk of thrombosis.
Acknowledgements:
The authors wish to thank Ann Casey for assistance with phlebotomy and platelet isolation, Hannah McRae and Dr. Majed Refaai for assistance with TEG and use of their TEG hemostasis system, and the Strong Blood Bank for phlebotomy. We also wish to thank Dr. Juilee Thakar for biostatistical advice. Research reported in this publication was supported in part by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Numbers R21HL128129, T32HL066988, T32GM068411, UL1RR024160 and UL1TR000042 from the National Center for Research Resources (NCRR), and the University of Rochester Center for AIDS Research (CFAR) grant P30AI078498 (NIH/NIAID). The content is solely the responsibility of the authors and does not necessarily represent the views of NCRR or NIH.
Footnotes
Conflicts of Interest: Authors have nothing to disclose.
References:
- [1].Global AIDS update 2016, Joint United Nations Programme on HIV/AIDS (UNAIDS), 2016.
- [2].90-90-90 An ambitious treatment target to help end the AIDS epidemic, Joint United Nations Programme on HIV/AIDS (UNAIDS), Geneva, Switzerland, 2014. [Google Scholar]
- [3].Levi J, Raymond A, Pozniak A, Vernazza P, Kohler P, Hill A, Can the UNAIDS 90-90-90 target be achieved? A systematic analysis of national HIV treatment cascades, BMJ global health 1(2) (2016) e000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].C. The Antiretroviral Therapy Cohort, Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies, The Lancet 372(9635) (2008) 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lohse N, Hansen A-BE, Pedersen G, Kronborg G, Gerstoft J, Sørensen HT, Væth M, Obel N, Survival of Persons with and without HIV Infection in Denmark, 1995–2005, Annals of Internal Medicine 146(2) (2007) 87–95. [DOI] [PubMed] [Google Scholar]
- [6].Sudano I, Spieker LE, Noll G, Corti R, Weber R, Lüscher TF, Cardiovascular disease in HIV infection, American Heart Journal 151(6) (2006) 1147–1155. [DOI] [PubMed] [Google Scholar]
- [7].Deeks SG, HIV infection, inflammation, immunosenescence, and aging, Annual review of medicine 62 (2011) 141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Neuhaus J, Jacobs DR, Baker JV, Calmy A, Duprez D, La Rosa A, Kuller LH, Pett SL, Ristola M, Ross MJ, Shlipak MG, Tracy R, Neaton JD, Markers of Inflammation, Coagulation, and Renal Function Are Elevated in Adults with HIV Infection, Journal of Infectious Diseases 201(12) (2010) 1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Feinstein MJ, Bahiru E, Achenbach C, Longenecker CT, Hsue P, So-Armah K, Freiberg MS, Lloyd-Jones DM, Patterns of Cardiovascular Mortality for HIV-Infected Adults in the United States: 1999 to 2013, American Journal of Cardiology 117(2) (2016) 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Triant VA, Lee H, Hadigan C, Grinspoon SK, Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease, The Journal of clinical endocrinology and metabolism 92(7) (2007) 2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, Bidwell Goetz M, Leaf D, Oursler KA, Rimland D, Rodriguez Barradas M, Brown S, Gibert C, McGinnis K, Crothers K, Sico J, Crane H, Warner A, Gottlieb S, Gottdiener J, Tracy RP, Budoff M, Watson C, Armah KA, Doebler D, Bryant K, Justice AC, HIV infection and the risk of acute myocardial infarction, JAMA internal medicine 173(8) (2013) 614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Feldman JG, Minkoff H, Schneider MF, Gange SJ, Cohen M, Watts DH, Gandhi M, Mocharnuk RS, Anastos K, Association of Cigarette Smoking With HIV Prognosis Among Women in the HAART Era: A Report From the Women’s Interagency HIV Study, American Journal of Public Health 96(6) (2006) 1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tesoriero JM, Gieryic SM, Carrascal A, Lavigne HE, Smoking among HIV positive New Yorkers: prevalence, frequency, and opportunities for cessation, AIDS and behavior 14(4) (2010) 824–35. [DOI] [PubMed] [Google Scholar]
- [14].Rasmussen LD, Helleberg M, May MT, Afzal S, Kronborg G, Larsen CS, Pedersen C, Gerstoft J, Nordestgaard BG, Obel N, Myocardial infarction among Danish HIV-infected individuals: population-attributable fractions associated with smoking, Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 60(9) (2015) 1415–23. [DOI] [PubMed] [Google Scholar]
- [15].Gardner K, Hall PA, Chinnery PF, Payne BAI, HIV Treatment and Associated Mitochondrial Pathology: Review of 25 Years of in Vitro, Animal, and Human Studies, Toxicologic Pathology 42(5) (2014) 811–822. [DOI] [PubMed] [Google Scholar]
- [16].Gupta S, Knight AG, Losso BY, Ingram DK, Keller JN, Bruce-Keller AJ, Brain Injury Caused by HIV Protease Inhibitors: role of Lipodystrophy and Insulin Resistance, Antiviral Res 95(1) (2012) 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bavinger C, Bendavid E, Niehaus K, Olshen RA, Olkin I, Sundaram V, Wein N, Holodniy M, Hou N, Owens DK, Desai M, Risk of cardiovascular disease from antiretroviral therapy for HIV: a systematic review, PLoS One 8(3) (2013) e59551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Friis-Moller N, Weber R, Reiss P, Thiebaut R, Kirk O, d’Arminio Monforte A, Pradier C, Morfeldt L, Mateu S, Law M, El-Sadr W, De Wit S, Sabin CA, Phillips AN, Lundgren JD, Cardiovascular disease risk factors in HIV patients--association with antiretroviral therapy. Results from the DAD study, AIDS (London, England) 17(8) (2003) 1179–93. [DOI] [PubMed] [Google Scholar]
- [19].Group DADS, Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration, The Lancet 371(9622) (2008) 1417–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Smith CJ, Levy I, Sabin CA, Kaya E, Johnson MA, Lipman MC, Cardiovascular disease risk factors and antiretroviral therapy in an HIV-positive UK population, HIV medicine 5(2) (2004) 88–92. [DOI] [PubMed] [Google Scholar]
- [21].D.s. group, Combination Antiretroviral Therapy and the Risk of Myocardial Infarction, New England Journal of Medicine 350(9) (2004) 955–955. [DOI] [PubMed] [Google Scholar]
- [22].Filardi P, Paililli S, Marciano C, Loria A, Losco T, Marsico F, Scala O, Ruggiero D, Ferraro S, Chiarello M, Cardiovascular effects of antiretroviral drugs: clinical review., Cardiovasc Hematol Disord Drug Targets 8(4) (2008) 238–244. [DOI] [PubMed] [Google Scholar]
- [23].Baker JV, Huppler Hullsiek K, Bradford RL, Prosser R, Tracy RP, Key NS, Circulating levels of tissue factor microparticle procoagulant activity are reduced with antiretroviral therapy and are associated with persistent inflammation and coagulation activation among HIV-positive patients, Journal of acquired immune deficiency syndromes (1999) 63(3) (2013) 367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Morrell CN, Aggrey AA, Chapman LM, Modjeski KL, Emerging roles for platelets as immune and inflammatory cells, Blood 123(18) (2014) 2759–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Garlichs CD, Cicha I, Raaz D, Meyer L, Stumpf C, Klinghammer L, Yilmaz A, Daniel WG, CD40/CD154 system and pro-inflammatory cytokines in young healthy male smokers without additional risk factors for atherosclerosis, Inflammation research : official journal of the European Histamine Research Society … [et al. ] 58(6) (2009) 306–11. [DOI] [PubMed] [Google Scholar]
- [26].Mayne E, Funderburg NT, Sieg SF, Asaad R, Kalinowska M, Rodriguez B, Schmaier AH, Stevens W, Lederman MM, Increased platelet and microparticle activation in HIV infection: upregulation of P-selectin and tissue factor expression, Journal of acquired immune deficiency syndromes (1999) 59(4) (2012) 340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Singh MV, Davidson DC, Jackson JW, Singh VB, Silva J, Ramirez SH, Maggirwar SB, Characterization of platelet-monocyte complexes in HIV-1-infected individuals: possible role in HIV-associated neuroinflammation, Journal of immunology (Baltimore, Md. : 1950) 192(10) (2014) 4674–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pan A, Testa S, Quiros Roldan E, Tinelli C, Bodini U, Cadeo B, Carnevale G, Martinelli I, Maserati R, Morstabilini P, Seminari E, Signorini L, Carosi G, Haemostatic activation in HIV infected patients treated with different antiretroviral regimens, Curr HIV Res 6(1) (2008) 70–6. [DOI] [PubMed] [Google Scholar]
- [29].Jens JK, Stella A, Joseph S, John PS, Veronika K, Patrick R, Effects of Tipranavir, Darunavir, and Ritonavir on Platelet Function, Coagulation, and Fibrinolysis in Healthy Volunteers, Current HIV Research 9(4) (2011) 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Baum PD, Sullam PM, Stoddart CA, McCune JM, Abacavir increases platelet reactivity via competitive inhibition of soluble guanylyl cyclase, AIDS (London, England) 25(18) (2011) 2243–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lannan KL, Refaai MA, Ture SK, Morrell CN, Blumberg N, Phipps RP, Spinelli SL, Resveratrol preserves the function of human platelets stored for transfusion, British journal of haematology 172(5) (2016) 794–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lannan KL, Spinelli SL, Blumberg N, Phipps RP, Maresin 1 induces a novel pro-resolving phenotype in human platelets, J Thromb Haemost 15(4) (2017) 802–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kaufman J, Spinelli SL, Schultz E, Blumberg N, Phipps RP, Release of biologically active CD154 during collection and storage of platelet concentrates prepared for transfusion, J Thromb Haemost 5(4) (2007) 788–96. [DOI] [PubMed] [Google Scholar]
- [34].Refaai MA, Carter J, Henrichs KF, Davidson DC, Pollock SJ, Casey AE, Spinelli SL, Phipps RP, Francis CW, Blumberg N, Alterations of platelet function and clot formation kinetics following in vitro exposure to anti-A and -B antibodies, Transfusion 53(2) (2013) 382–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shi G, Morrell CN, Platelets as initiators and mediators of inflammation at the vessel wall, Thromb Res 127(5) (2011) 387–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Garraud O, Pathogen sensing, subsequent signalling, and signalosome in human platelets, Thrombosis Research 127(4) (2011) 283–286. [DOI] [PubMed] [Google Scholar]
- [37].Messner B, Bernhard D, Mechanisms of Endothelial Dysfunction and Early Atherogenesis, Arterioscel Thromb Vasc Biol 34(3) (2014) 509–515. [DOI] [PubMed] [Google Scholar]
- [38].Barua R, Ambrose J, Mechanism of Coronary Thrombosis in Cigarette Smoke Exposure, Arterioscel Thromb Vasc Biol 33(7) (2013) 1460–1467. [DOI] [PubMed] [Google Scholar]
- [39].Csordas A, Bernhard D, The biology behind the atherothrombotic effects of cigarette smoke, Nature reviews. Cardiology 10(4) (2013) 219–30. [DOI] [PubMed] [Google Scholar]
- [40].McNicol A, Isreals S, Beyond Hemostasis: The Role of Platelets in Inflammation, Malignancy, and Infection, Cardiovasc Hematol Disord Drug Targets 8 (2008) 99–117. [DOI] [PubMed] [Google Scholar]
- [41].Gresele P, Falcinelli E, Sebastiano M, Baldelli F, Endothelial and platelet function alterations in HIV-infected patients, Thromb Res 129(3) (2012) 301–8. [DOI] [PubMed] [Google Scholar]
- [42].Akay C, Cooper M, Odeleye A, Jensen BK, White MG, Vassoler F, Gannon PJ, Mankowski J, Dorsey JL, Buch AM, Cross SA, Cook DR, Peña M-M, Andersen ES, Christofidou-Solomidou M, Lindl KA, Zink MC, Clements J, Pierce RC, Kolson DL, Jordan-Sciutto KL, Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system, J Neurovirol 20(1) (2014) 39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Giannarelli C, Klein RS, Badimon JJ, Cardiovascular implications of HIV-induced dyslipidemia, Atherosclerosis 219(2) (2011) 384–9. [DOI] [PubMed] [Google Scholar]
- [44].Stein JH, Klein MA, Bellehumeur JL, McBride PE, Wiebe DA, Otvos JD, Sosman JM, Use of human immunodeficiency virus-1 protease inhibitors is associated with atherogenic lipoprotein changes and endothelial dysfunction, Circulation 104(3) (2001) 257–62. [DOI] [PubMed] [Google Scholar]
- [45].Ernest CS, 2nd, S.D. Hall, D.R. Jones, Mechanism-based inactivation of CYP3A by HIV protease inhibitors, The Journal of pharmacology and experimental therapeutics 312(2) (2005) 583–91. [DOI] [PubMed] [Google Scholar]
- [46].Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, Weiland O, Aguilar H, Xiong J, Pilot-Matias T, DaSilva-Tillmann B, Larsen L, Podsadecki T, Bernstein B, Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin, The New England journal of medicine 370(17) (2014) 1594–603. [DOI] [PubMed] [Google Scholar]
- [47].Sato A, Asano T, Isono M, Ito K, Asano T, Ritonavir acts synergistically with panobinostat to enhance histone acetylation and inhibit renal cancer growth, Molecular and Clinical Oncology 2(6) (2014) 1016–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Seminari E, Pan A, Voltini G, Carnevale G, Maserati R, Minoli L, Meneghetti G, Tinelli C, Testa S, Assessment of atherosclerosis using carotid ultrasonography in a cohort of HIV-positive patients treated with protease inhibitors, Atherosclerosis 162(2) (2002) 433–8. [DOI] [PubMed] [Google Scholar]
- [49].Friis-Moller N, Reiss P, Sabin CA, Weber R, Monforte A, El-Sadr W, Thiebaut R, De Wit S, Kirk O, Fontas E, Law MG, Phillips A, Lundgren JD, Class of antiretroviral drugs and the risk of myocardial infarction, The New England journal of medicine 356(17) (2007) 1723–35. [DOI] [PubMed] [Google Scholar]
- [50].Kumar P, DeJesus E, Huhn G, Sloan L, Small CB, Edelstein H, Felizarta F, Hao R, Ross L, Stancil B, Pappa K, Ha B, Evaluation of cardiovascular biomarkers in a randomized trial of fosamprenavir/ritonavir vs. efavirenz with abacavir/lamivudine in underrepresented, antiretroviral-naive, HIV-infected patients (SUPPORT): 96-week results, BMC infectious diseases 13 (2013) 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Graff J, von Hentig N, Kuczka K, Angioni C, Gute P, Klauke S, Babacan E, Harder S, Significant effects of tipranavir on platelet aggregation and thromboxane B2 formation in vitro and in vivo, Journal of Antimicrobial Chemotherapy 61(2) (2008) 394–399. [DOI] [PubMed] [Google Scholar]
- [52].Lee GA, Seneviratne T, Noor MA, Lo JC, Schwarz JM, Aweeka FT, Mulligan K, Schambelan M, Grunfeld C, The metabolic effects of lopinavir/ritonavir in HIV-negative men, Aids 18(4) (2004) 641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yee DL, Edwards RM, Mueller BU, Teruya J, Thromboelastographic and hemostatic characteristics in pediatric patients with sickle cell disease, Archives of pathology & laboratory medicine 129(6) (2005) 760–5. [DOI] [PubMed] [Google Scholar]
- [54].Moraes LA, Spyridon M, Kaiser WJ, Jones CI, Sage T, Atherton REL, Gibbins JM, Non-genomic effects of PPARγ ligands: inhibition of GPVI-stimulated platelet activation, Journal of Thrombosis and Haemostasis 8(3) (2010) 577–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Davidson DC, Hirschman MP, Spinelli SL, Morrell CN, Schifitto G, Phipps RP, Maggirwar SB, Antiplatelet activity of valproic acid contributes to decreased soluble CD40 ligand production in HIV type 1-infected individuals, Journal of immunology (Baltimore, Md. : 1950) 186(1) (2011) 584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]